Abstract
Ardeid birds and pigs are known as major amplifying hosts for Japanese encephalitis virus, and ducklings and chickens have been considered to play at best a minor role in outbreaks because of their low or absent viremia. We hypothesized that viremia of sufficient magnitude would develop in young ducklings (Anas platyrhynchos) and chicks (Gallus gallus) for them to serve as reservoir hosts and thereby contribute to the transmission cycle. Infection was associated with reduced weight gain in both species, and ducklings infected at 10 days of age or less showed overt clinical signs of disease. The mean peak viremia in birds of both species decreased as the age at infection increased from 2 to 42 days, indicating the importance of age of infection on magnitude of viremia in birds from both species, and suggesting that young poultry may be amplifying hosts of importance in disease-endemic regions.
Introduction
Japanese encephalitis virus (JEV) is a mosquito-borne member of the genus Flavivirus and it remains a prominent cause of epidemic viral encephalitis in the world despite available vaccines.1–3 A total of 30,000–50,000 human clinical cases of Japanese encephalitis are reported annually, and most cases occur in disease-endemic regions in Asia.1 Clinical manifestations of the disease in humans include fever, headache, tremors, vomiting, convulsions, and, in some cases, death; a significant fraction of survivors display lasting Parkinsonian-like neurologic deficits.4 Clinical disease is particularly prevalent in children, although most persons in disease-endemic areas undergo subclinical infections during childhood.5 The virus continues to spread to new areas previously not affected, including Australia and new regions in India, and there is concern for its incursion into the Americas and Europe.6–8
Japanese encephalitis virus is maintained in a mosquito–bird or mosquito–bird–pig transmission cycle in which the vertebrate amplifying host develops a high-titer viremia but rarely manifests disease.9 Morbidity and mortality are observed in humans and horses, both of which are considered dead-end hosts.10 In a series of studies conducted in Japan during the 1950s, Culex tritaeniorhynchus was implicated as the major arthropod vector of JEV and ardeid birds (egrets and herons) or pigs as the principle vertebrate amplifying hosts.11–15 Subsequent studies established that mosquitoes transmit JEV from a number of different species and reinforced the concept that JEV transmission is especially prevalent in areas where humans live near water birds or domestic pigs, and during the seasons when mosquito vector populations are high.15–17 Birds from a variety of different species have been shown to be susceptible to infection with JEV, but are considered to play a minor role in virus transmission in comparison to egrets, herons, and pigs.11,13,18 Adult ducks and chickens are among birds that develop a low magnitude viremia, unlikely to result in substantial transmission to feeding mosquitoes.19,20 However, it has been reported that recently hatched chicks develop much higher viremia than adult birds,21 and in JEV-endemic regions, large numbers of free-ranging chickens and ducks live and breed near humans, providing an abundant pool of potential amplifying hosts for JEV. With this transmission scenario in mind, the goal of this study was to characterize the relationship between age and magnitude of viremia in chicks and ducklings infected with JEV and to evaluate clinical manifestations of disease associated with infection in these two species.
Materials and Methods
Animals and husbandry:
Indian Runner ducklings (Anas platyrhynchos) and white leghorn chicks (Gallus gallus) were obtained from commercial farms and received at the age of two days post-hatch. They were housed in an animal biosafety level 3 facility in round metal tanks two meters in diameter. Each tank contained wood shavings on the floor, two heating lamps, a small pool with fresh water, and poultry starter feed. Tanks containing uninfected birds were housed in one room, and groups of birds were moved to similarly furnished tanks in a different room on the day of infection. Birds inoculated on day 2 post-hatch were allowed to rest for eight hours after arrival before injection of virus. When ducklings reached two weeks of age they were removed from the tanks and allowed to move freely around the room (5.5 × 3.7 meters). Chicks remained in their pens throughout the experiment.
Viruses.
Two strains of JEV were used. The first strain (JE-VN) was isolated from a pool of Cx. tritaeniorhynchus mosquitoes collected in Vietnam in 2003 (Miller B, unpublished data) and had been passaged once in suckling mice and twice in Vero cells obtained from the American Type Culture Collection (Manassas, VA). The second strain of virus (JE-P3) was isolated from mosquitoes in China in 1949 and had been passaged extensively in suckling mice and cultured cells.22 Molecular sequencing of the pre-membrane region of both viruses confirmed that the viruses represented genotypes I (JE-VN) and III (JE-P3) respectively.
Infection and virus titration.
Groups of three ducklings and three chicks where inoculated subcutaneously with each strain of JEV on days 2, 4, 7, 10, 14, 21, 28, and 42 after hatching. The dose received in all cases was 15,000–25,000 plaque-forming units (PFU) as determined by backtitration of the inocula. Four ducklings and four chickens were used as non-infected controls during the study. Samples of whole blood (100 μL) were collected from all birds on days 1 through 5 and on day 7 post-infection, and added to 450 μL of BA1 medium (minimal essential medium salts, 1% bovine serum albumin, 350 mg/L sodium bicarbonate, 100 units/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B in 0.05 M Tris) to provide samples of approximately 10% serum. These samples were allowed to clot for 30 minutes, then centrifuged at 2,000 × g for 3 minutes and the diluted serum was frozen at −80C until tested. On day 10 post-infection, a final blood sample was collected and the animals were euthanized via intravenous injection of pentobarbital. Virus in blood samples was titrated by plaque assay on Vero cells as described.23
Statistical analyses.
Differences in the rate of growth between infected and uninfected birds was determined by t-test, and the general effect of age versus peak viremia titer was assessed by using logarithmic regression. For modeling the influence of age on magnitude of viremia, virus concentrations in serum were transformed by using a power function such that the transformed value (PFUTRANS) was equal to the raw concentration (PFU/mL) raised to the power of 0.15. As articulated previously,24 the power transformation is preferable to a logarithmic transformation because of its ability to deal with the absence of the virus in a specimen. These data were then related to time post-infection (t) and age of bird at the time of infection (AGE) by using the lognormal distribution scaled by bird age. The parameters were estimated by using a minimum least squares estimate separately for the ducklings and chicks. The standard equation that was optimized was PFUTRANS = α.AGEβ.f(t,μ,σ), where α and β are scale values to accommodate the magnitude of viremia and the extent to which that was influenced by the age of the bird at infection. β = 0 implies no difference in infection rates as a function of age; β < 0 implies that infection rate decreases as the age at infection increases; t is the time in days post-infection; and μ and σ are the parameters of the lognormal distribution given as f(t, μ, σ) = exp[−(ln(t/μ))2/(2σ2)]/[tσ(2π)½]. The quality of fit of the equation was assessed by determining the coefficient of variation (R-square) as the variance in the dependent variable explained by the equation divided by its total variance.
Results
Morbidity and mortality after infection with JEV.
None of the chicks or ducklings died before being euthanized on day 10 post-infection. None of the chicks showed overt signs of morbidity during the 10-day infection period, although lack of appetite was difficult to observe because the chick's natural behavior involved scratching and spreading their feed across the pen floor. In contrast, a fraction of the ducklings infected with both viruses showed clinical signs of disease as described below; there were no differences in severity or type of signs between ducklings infected with JE-VN or JE-P3 viruses. All ducklings infected on day two or four post-hatch showed clear signs of disease starting one or two days after infection, which consisted of piloerection of their down, reduced activity, and less aversion to investigators; in comparison to uninfected ducklings of the same age, they preferred to remain curled up under the heat lamp. There was an apparent reduced feed intake. However, this was not measured but based on observation and the refilling of the bowls.
None of the ducklings showed neurologic signs during the infection period, although apparent weakness was observed in one duckling infected on day 4. Ducklings infected on day 2 post-hatch regained their appetite and activity level after nine days, and those infected on day 4 had a faster recovery and showed no signs of reduced activity after six days of infection. Individual ducklings in the groups infected on days 7 and 10 after hatching showed slight signs of distress (fluffing and reduced activity for one or two days), but no clear clinical signs encompassing whole groups were observed. Ducklings infected beyond 10 after hatching did not show obvious clinical signs of disease.
Chicks and ducklings infected between 2 and 21 days post-hatch were weighed on day 10 post-infection and compared with the control group of the same age weighed on the same day of age. Chicks and ducklings in all groups showed evidence of stunted growth at 10 days after infection in comparison to the control animals supporting the observation of reduced appetite in the ducklings (Table 1). In spite of the fact that the average weights of the infected bird samples were always less than the average weights of the corresponding control birds, the differences were only significant in six cases. Statistics in Table 1 show that chicks infected 2 days and 10 days post-hatch showed significant evidence of stunted growth (P < 0.05) as did ducklings infected 2, 5, 7, and 10 days post-hatch (P < 0.01). No difference in weight was measured between JE-VN- and JE-P3-infected animals.
Table 1.
Weight comparisons 10 days post-infection as a function of age in chicks and ducklings infected with Japanese encephalitis virus*
| Bird | Variable | 2 Days | 5 Days | 7 Days | 10 Days | 14 Days | 21 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected | Control | Infected | Control | Infected | Control | Infected | Control | Infected | Control | Infected | Control | ||
| Chicks | Mean weight (grams) | 71.7 | 95.8 | 92.5 | 112.5 | 108.3 | 139.2 | 98.3 | 173.3 | 210.8 | 245.0 | 295.0 | 335.0 |
| SD | 15.6 | 22.0 | 37.8 | 13.3 | 24.0 | 61.9 | 33.5 | 75.3 | 40.2 | 57.1 | 127.1 | 65.4 | |
| P† | 0.046 | 0.176 | 0.193 | 0.046 | 0.184 | 0.303 | |||||||
| Ducklings | Mean weight (grams) | 114.2 | 229.2 | 140.0 | 261.7 | 203.3 | 413.3 | 334.2 | 453.3 | 471.7 | 498.3 | 650.0 | 666.0 |
| SD | 7.5 | 12.8 | 6.1 | 24.8 | 9.3 | 26.2 | 17.5 | 29.4 | 43.2 | 34.5 | 40.6 | 48.9 | |
| P | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.189 | 0.316 | |||||||
All comparisons involved six observations with the exception of chicks on day 21, where five observations were made. Days indicate days of age at which birds were inoculated with virus.
Comparisons of Means tests were undertaken by using the approximate t-statistic with independent variance estimates and Satterthwaite's (1946) approximation of degrees of freedom. The t-statistics and probability values (P) are interpreted in the standard manner.
Viremia as a function of age at infection.
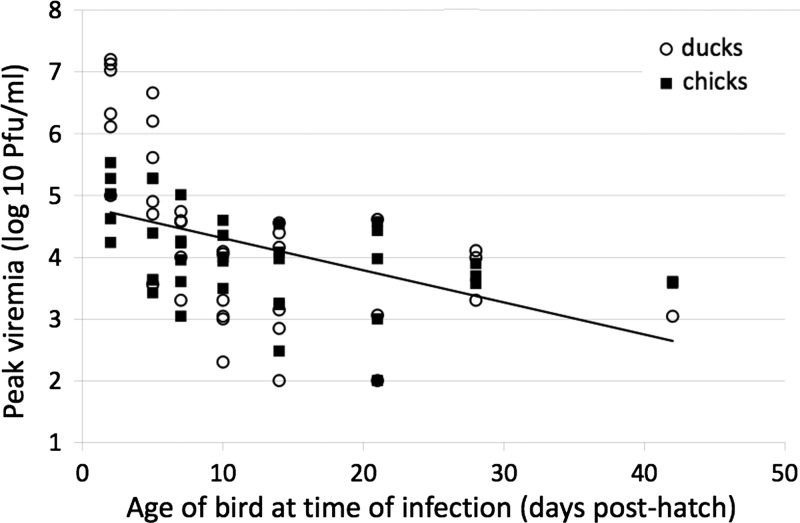
All ducklings and chicks infected within two weeks post-hatch developed detectable viremia, but the fraction of birds that became viremic decreased with age of inoculation, reaching 36% in animals six weeks of age (Table 2). There was no difference in magnitude of viremia based on infecting strain of virus (P > 0.1), and data from birds infected with both strains were pooled. There was no significant difference between chicks and ducklings in the rate of decrease in the proportion of birds that developed viremia (P = 0.34), although the overall decrease in detectable viremia as a function of age at infection was significant; the slope (β) coefficients for both equations were negative and significant (P < 0.01). Peak viremia was observed two days post-inoculation in the animals that developed a detectable viremia (Figure 1 ), and there was a reduction in time to peak viremia post-infection as a function of age at infection (P < 0.01). The mean peak viremia for ducklings and chicks infected two days post-hatch were 7.3 × 106 PFU/mL and 1.3 × 105 PFU/mL, respectively. The mean peak viremia decreased to 1.8 × 102 PFU/mL and 2.0 × 103 PFU/mL for ducklings and chicks infected 42 days post-hatch.
Table 2.
Relationship between the age at infection and development of viremia in chicks and ducklings infected with Japanese encephalitis virus
| Bird | Variable | Days of age when inoculated with virus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 7 | 10 | 14 | 21 | 28 | 42 | ||
| Chicks | No. tested | 6 | 6 | 6 | 6 | 6 | 5 | 5 | 5 |
| No. viremic | 6 | 6 | 5 | 6 | 6 | 3 | 3 | 1 | |
| Proportion viremic | 1.00 | 1.00 | 0.83 | 1.00 | 1.00 | 0.60 | 0.60 | 0.20 | |
| Ducklings | No. tested | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| No. viremic | 6 | 6 | 6 | 5 | 6 | 5 | 3 | 3 | |
| Proportion viremic | 1.00 | 1.00 | 1.00 | 0.83 | 1.00 | 0.83 | 0.50 | 0.50 | |
Figure 1.
Peak viremia as a function of age of ducklings and chicks when infected with Japanese encephalitis virus. The graph plots the peak viremia observed in each of the chicks and ducklings against the age of the bird at infection. The vertical axis is on a logarithmic scale so that the fitted model is presented as a straight line. The underlying equation is PFUPEAK = α×βAGE, which transforms to log10(PFUPEAK) = log10(α)+log10(β)×AGE, where AGE is the age of the bird at infection. PFU = plaque-forming units.
Peak viremia data were analyzed by using a natural logarithmic regression model. The model showed a relationship between age and reduction in viremia and a coefficient of determination (R-square) of 0.40, ln(β) = −0.15, and P < 0.01. Each of the regressions was significant (P < 0.01), and coefficients that were consistent with peak viremia levels were inversely related to the age of the bird at infection. The coefficients of determination of the regressions of 0.36 and 0.45 for chicks and ducklings, respectively, indicate that a significant proportion of the peak viremia found in infected birds is a function of their age at infection. Younger birds develop significantly higher viremia levels than older birds.
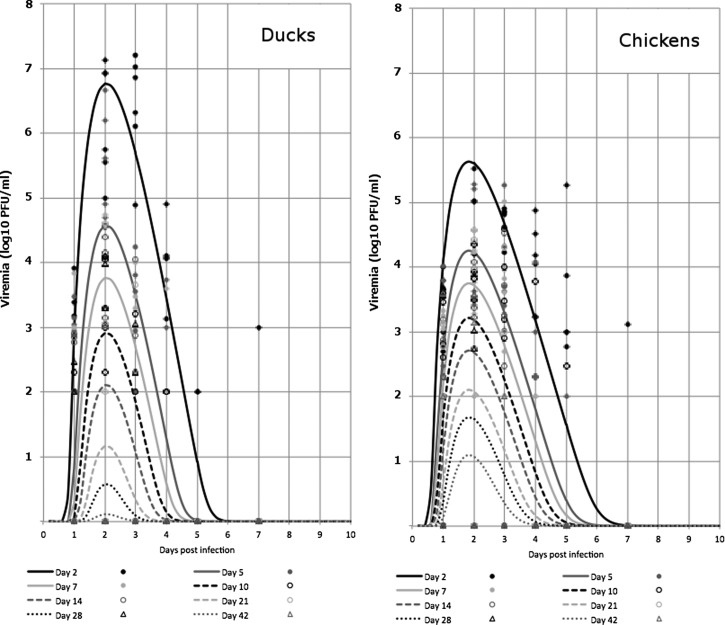
Values for peak viremia measured over the five days after virus inoculation are shown for all birds in Table 3. Fully fitted models that relate the path of viremia development in infected chicks and ducklings as a function of their age at infection are shown in Figure 2 . These complex curves clearly show that peak viremia is higher in younger birds, that ducklings develop higher magnitude viremia than chicks, and the magnitude of viremia is highest two days post-infection, and then decreases over the next eight days. The model accounts for 57% of the observed deviations for chicks and 67% of the observed deviations for ducklings.
Table 3.
Peak viremia (log10 plaque-forming units/mL) as a function of day infected with Japanese encephalitis virus for chicks and ducklings infected between 2 and 42 days post-hatch*
| Bird | Day post-inoculation | Day of infection post-hatch | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 7 | 10 | 14 | 21 | 28 | 42 | ||
| Chicks | 1 | 3.5 | 2.7 | 3.3 | 2.0 | 1.5 | 2.1 | < 1.0 | < 1.0 |
| 2 | 5.0 | 4.3 | 3.8 | 2.7 | 3.0 | 2.3 | 1.3 | 1.4 | |
| 3 | 4.7 | 4.0 | 3.7 | 3.2 | 3.0 | 0.5 | < 1.0 | < 1.0 | |
| 4 | 4.1 | 1.8 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | |
| 5 | 3.2 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | |
| Ducklings | 1 | 2.6 | 2.4 | 1.3 | < 1.0 | 1.5 | < 1.0 | < 1.0 | < 1.0 |
| 2 | 6.3 | 5.2 | 3.2 | 3.4 | 2.9 | 1.9 | 2.3 | < 1.0 | |
| 3 | 6.5 | 2.9 | 1.6 | < 1.0 | 1.1 | < 1.0 | < 1.0 | < 1.0 | |
| 4 | 3.6 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | |
| 5 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | < 1.0 | |
Values are the highest daily virus titer measured in samples collected 1–5 days after virus inoculation.
Figure 2.
Dynamics of viremia in ducklings and chicks in the days post-infection as a function of age when infected with Japanese encephalitis virus. The eight lines in each graph give the fitted model for the trend in viremia as a function of time post-infection by using the equation PFU = (α×AGEβ×f(t,μ,σ))6.67, where f is the lognormal distribution with parameters α and σ. The individual points are the actual data observations of viremia count for all the infected animals on days 1, 2, 3, 4, 5 and 7 post-infection. PFU = plaque-forming units.
Discussion
Japanese encephalitis virus induces severe encephalitic disease in humans and horses and is currently endemic to large areas of Asia. There has been continued expansion in the geographic range of this pathogen over the past 20 years, and there is concern about its continued spread into Europe and the New World.6,25,26 The sylvatic transmission cycle involves ardeid birds and mosquitoes, which explains why the virus is found primarily in wetter agricultural regions such as rice paddy fields in Asia. However, JEV also infects domestic pigs and amplifies to titers sufficient for transmission to mosquitoes,27 With the expansion of pig production in Asia, the virus has expanded to regions with lower densities of ardeid birds.10 It is possible that other birds may play a significant role in virus transmission. Previous studies have shown that chickens develop high viremia at a young age and that antibodies have been detected in ducklings and chickens in JEV-endemic regions.15,19,28 These domestic birds are high in abundance throughout Asia, often farmed and traded year round, and live in large numbers in near humans. In this study, we investigated the hypothesis that domestic poultry produce high enough viremia for an extended period of time to serve as a possible alternative source of JEV infection and transmission to humans.
Chicks and ducklings were infected with JEV via subcutaneous needle inoculation at various ages from 2 to 42 days post-hatch. In contrast to previous studies using young birds, no disease-related mortality was observed in any of the birds, although noticeable morbidity was observed in young ducklings. Interestingly, many of the clinical signs observed in JEV-infected ducklings are similar to those reported for infection with avian influenza virus, which is endemic to the same locales as JEV.29 It is possible that some outbreaks of JEV in poultry are mistakenly assumed to be caused by influenza virus or any number of other pathogens. Documenting JEV infection in young poultry in the field would clearly aid in interpreting the significance of the current experimental studies.
Analysis of viremia profiles in ducklings and chicks showed a clear relationship between age and magnitude of JEV viremia, and reduction of viremic response with age was best described by a negative logarithmic relationship that can be predicted with R-square. The viremia found specifically in the young birds were high, and similar to those reported.15,19 Previous research indicated that a viremia of ≥ 104 PFU/mL in 1–3-day old chicks resulted in 100% transmission to Cx. tritaeniorhynchus mosquitoes, and that infection of approximately 10% of mosquitoes occurred from feeding on birds having a viremia of only approximately 102 PFU/mL.19 A similar study using Cx. pipiens (molestus) reported 51% transmission of JEV from chicks with viremia titers of 104.5–105.4 PFU/mL.21
Considering these estimates for vector competence, the results suggest that efficient transmission of JEV to mosquitoes likely occurs from young viremic chicks and ducklings. Considering the vast numbers of such free-ranging hosts in disease-endemic regions, they may play an epidemiologically significant role in JEV transmission. Field studies to elucidate the force of infection in these hosts during JEV transmission events are necessary to further validate their role in JEV transmission dynamics.
ACKNOWLEDGMENTS
We are grateful to Barry Miller and Alan Barrett for providing JEV strains and to Paul Gordy for technical support.
Footnotes
Financial support: This study was supported by a VSB Fonds fellowship to Natalie B. Cleton and by subcontract N01-AI25489 from the National Institute of Allergy and Infectious Diseases.
Authors' addresses: Natalie B. Cleton, National Institute for Public Health and the Environment, Centre for Infectious Disease Control, Bilthoven, The Netherlands, E-mail: natalie.cleton@rivm.nl. Angela Bosco-Lauth and Richard A. Bowen, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO, E-mails: mopargal@colostate.edu and rbowen@colostate.edu. Michael J. Page, Office of the Provost, Bentley University, Waltham, MA, E-mail: mpage@bentley.edu.
References
- 1.Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 2.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–774. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Japanese Encephalitis. 2011. www.who.int/nuvi/je/en/ Available at. Accessed July 22, 2012.
- 4.Halstead SB, Jacobson J. Japanese encephalitis. Adv Virus Res. 2003;61:103–138. doi: 10.1016/s0065-3527(03)61003-1. [DOI] [PubMed] [Google Scholar]
- 5.Schneider RJ, Firestone MH, Edelman R, Chieowanich P, Pronpibul R. Clinical sequelae after Japanese encephalitis: a one year follow-up study in Thailand. Southeast Asian J Trop Med Public Health. 1974;5:560–568. [PubMed] [Google Scholar]
- 6.Nett RJ, Campbell GL, Reisen WK. Potential for the emergence of Japanese encephalitis virus in California. Vector Borne Zoonotic Dis. 2008;9:511–517. doi: 10.1089/vbz.2008.0052. [DOI] [PubMed] [Google Scholar]
- 7.Van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 9.Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol. 2002;267:11–48. doi: 10.1007/978-3-642-59403-8_2. [DOI] [PubMed] [Google Scholar]
- 10.Rosen L. The natural history of Japanese encephalitis virus. Annu Rev Microbiol. 1986;40:395–414. doi: 10.1146/annurev.mi.40.100186.002143. [DOI] [PubMed] [Google Scholar]
- 11.Buescher EL, Scherer WF, Rosenberg MZ, Gresser I, Hardy JL, Bullock HR. Ecologic studies of Japanese encephalitis virus in Japan. II. Mosquito infection. Am J Trop Med Hyg. 1959;8:651–654. doi: 10.4269/ajtmh.1959.8.651. [DOI] [PubMed] [Google Scholar]
- 12.Scherer WF, Buescher EL, Fleming MB, Noguchi A, Scanlon J. Ecologic studies of Japanese encephalitis virus in Japan. III. Mosquito factors. Zootropism and vertical flight of Culex tritaeniorhynchus with observations on variations in collections from animal-baited traps in different habitats. Am J Trop Med Hyg. 1959;8:665–677. [PubMed] [Google Scholar]
- 13.Scherer WF, Buescher EL, McClure HE. Ecologic studies of Japanese encephalitis virus in Japan. V. Avian factors. Am J Trop Med Hyg. 1959;8:689–697. doi: 10.4269/ajtmh.1959.8.689. [DOI] [PubMed] [Google Scholar]
- 14.Scherer WF, Moyer JT, Izumi T, Gresser I, McCown J. Ecologic studies of Japanese encephalitis virus in Japan. VI. Swine infection. Am J Trop Med Hyg. 1959;8:698–706. doi: 10.4269/ajtmh.1959.8.698. [DOI] [PubMed] [Google Scholar]
- 15.Soman RS, Rodrigues FM, Guttikar SN, Guru PY. Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in ardeid birds. Indian J Med Res. 1977;65:709–718. [PubMed] [Google Scholar]
- 16.Gresser I, Hardy JL, Hu SMK, Scherer WF. Factors influencing transmission of Japanese B encephalitis virus by a colonized strain of Culex tritaeniorhynchus giles, from infected pigs and chicks to susceptible pigs and birds. Am J Trop Med Hyg. 1958;7:365–373. doi: 10.4269/ajtmh.1958.7.365. [DOI] [PubMed] [Google Scholar]
- 17.Sazawa H. Japanese encephalitis in domestic animals. Bull Office International des el Pizooties. 1968;70:627–633. [PubMed] [Google Scholar]
- 18.Hammon WM, Reeves WC, Sather GE. Japanese B encephalitis virus in the blood of experimentally inoculated birds; epidemiological implications. Am J Epidemiol. 1951;53:249–261. doi: 10.1093/oxfordjournals.aje.a119452. [DOI] [PubMed] [Google Scholar]
- 19.Dhanda V, Banergee K, Deshmukh PK, Ilkal MA. Experimental viraemia and transmission of Japanese encephalitis virus by mosquitoes in domestic ducks. Indian J Med Res. 1977;66:881–888. [PubMed] [Google Scholar]
- 20.Banerjee K, Deshmukh PK. Transmission of Japanese encephalitis virus to chicks by individual Culex tritaeniorhyncus mosquitoes. Indian J Med Res. 1987;86:726–727. [PubMed] [Google Scholar]
- 21.Turell MJ, Mores CN, Dohm DJ, Komilov N, Paragas J, Lee JS, Shermuhemedova D, Endy TP, Kodirov A, Khodjaev S. Laboratory transmission of Japanese encephalitis and West Nile viruses by molestus form of Culex pipiens (Diptera: Culicidae) collected in Uzbekistan in 2004. J Med Entomol. 2006;43:296–300. doi: 10.1603/0022-2585(2006)043[0296:ltojea]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Ni H, Barrett ADT. Nucleotide and deduced amino acid sequence of the structural protein genes of Japanese encephalitis viruses from different geographical locations. J Gen Virol. 1995;76:401–407. doi: 10.1099/0022-1317-76-2-401. [DOI] [PubMed] [Google Scholar]
- 23.Westaway EG. Assessment and application of a cell line from pig kidney for plaque assay and neutralization tests with twelve group B arboviruses. Am J Epidemiol. 1966;84:439–456. doi: 10.1093/oxfordjournals.aje.a120657. [DOI] [PubMed] [Google Scholar]
- 24.Handelsman DJ. Optimal power transformations for analysis of sperm concentration and other semen variables. J Androl. 2002;23:629–634. [PubMed] [Google Scholar]
- 25.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platonov AE, Rossi G, Karan LS, Mironov KO, Busani L, Rezza G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Euro Surveill. 2012;17:20241. doi: 10.2807/ese.17.32.20241-en. [DOI] [PubMed] [Google Scholar]
- 27.Ilkal MA, Prasanna Y, Jacob G, Geevarghese G, Banerjee K. Experimental studies on the susceptibility of domestic pigs to West Nile virus followed by Japanese encephalitis virus infection and vice versa. Acta Virol. 1994;38:157–161. [PubMed] [Google Scholar]
- 28.Ogata M, Nagao Y, Jitsunari F, Kitamura N, Okazaki T. Infection of herons and domestic fowls with Japanese encephalitis virus with specific reference to maternal antibody of hen (epidemiological study on Japanese encephalitis 26) Acta Med Okayama. 1970;24:175–184. [PubMed] [Google Scholar]
- 29.Swayne DE, Pantin-Jackwood M. Pathogenicity of avian influenza viruses in poultry. Dev Biol (Basel) 2006;124:61–67. [PubMed] [Google Scholar]