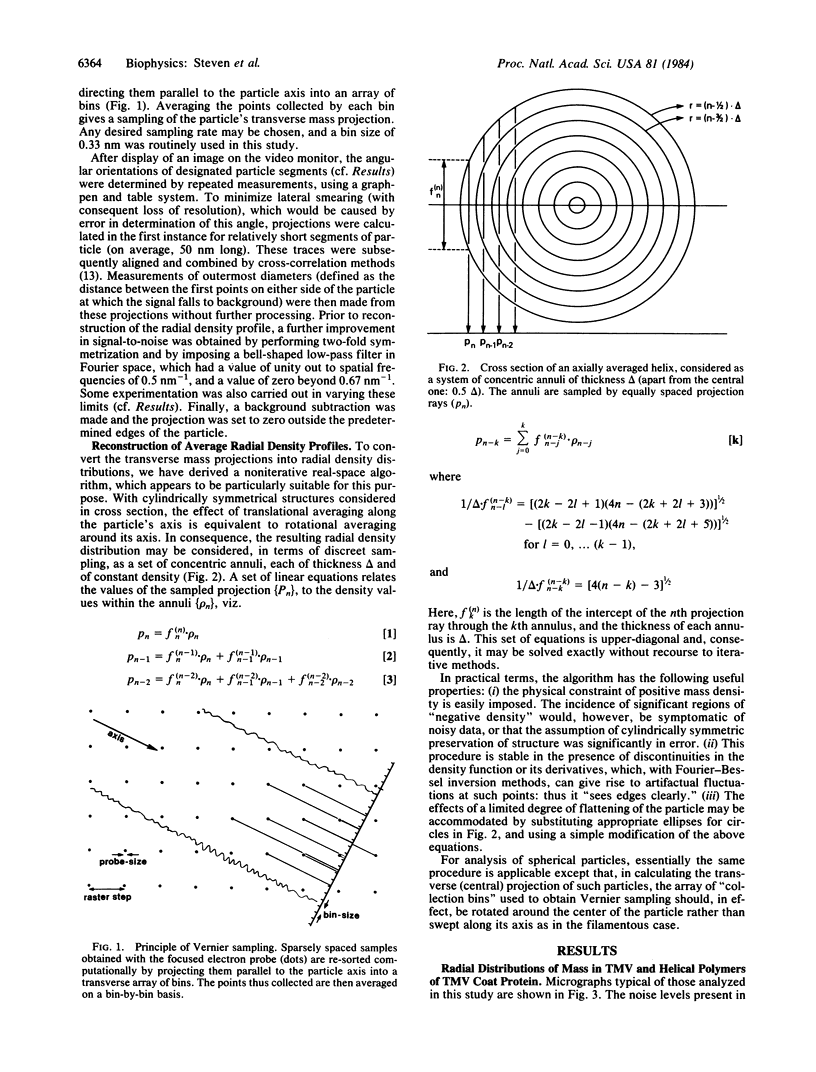
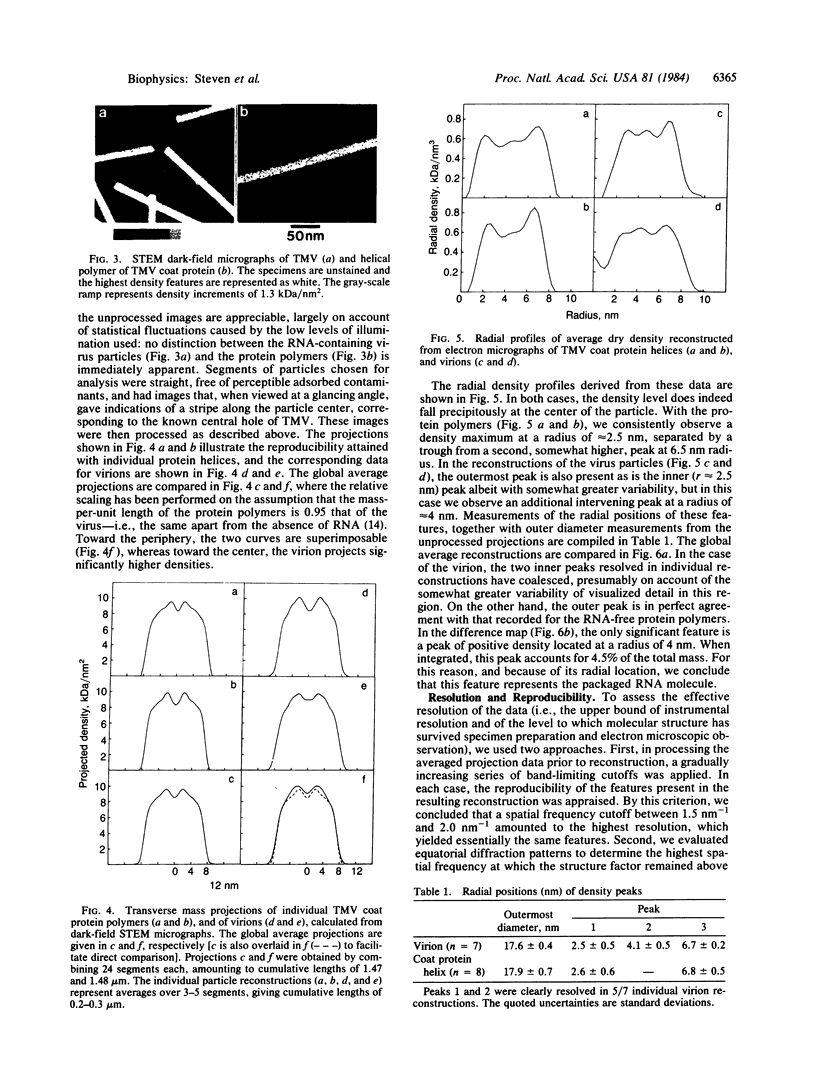
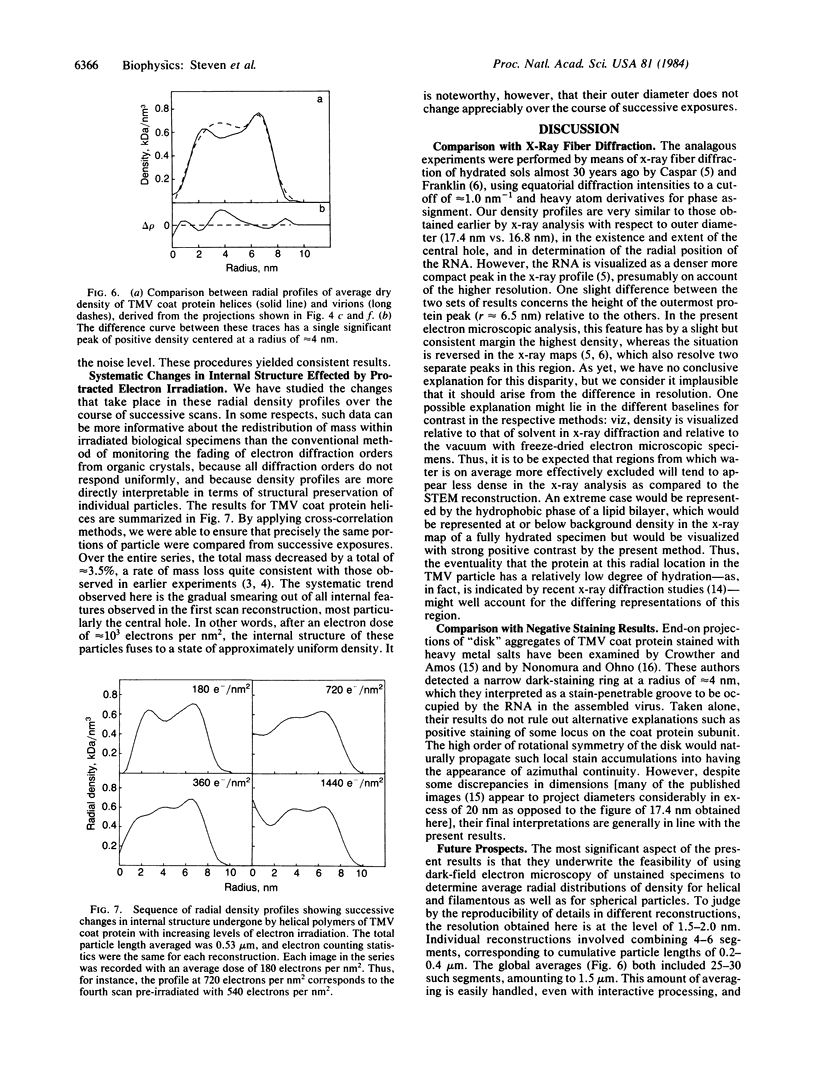
Abstract
A procedure has been developed for direct determination of radial distributions of density in filamentous and spheroidal particles by analyzing dark-field scanning transmission electron micrographs of unstained freeze-dried specimens. Unlike electron microscopic methods based on staining or shadowing with heavy atoms, this approach can be used to probe the internal structure of macromolecular complexes. As an experimental proving ground, we have applied the procedure to tobacco mosaic virus (TMV) and to RNA-free helical polymers of TMV coat protein. Both structures are found to project outermost diameters of 17.6 +/- 0.4 nm, to have empty axial holes approximately equal to 3.5 nm in diameter, and to have density peaks at radii of 2.5 +/- 0.5 and 6.7 +/- 0.3 nm. Thus visualized, the only significant difference between them is the presence in the virion of an additional density peak at 4.1 +/- 0.5 nm contributed by its internalized RNA molecule. We have also used the procedure to monitor the structural expression of radiation damage in the low electron dose regime prior to the onset of significant mass loss. Changes in the radial density profiles are detected at average doses as low as approximately equal to 400 electrons per nm2: the trend is for the internal structure of these particles to fuse toward a state of uniform density, although the values of their outermost diameters remain unaffected.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crewe A. V. The current state of high resolution scanning electron microscopy. Q Rev Biophys. 1970 Feb;3(1):137–175. doi: 10.1017/s0033583500004431. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Holmes K. C. Protein-RNA interactions during TMV assembly. J Supramol Struct. 1979;12(3):305–320. doi: 10.1002/jss.400120304. [DOI] [PubMed] [Google Scholar]
- Knott G. D. Mlab--a mathematical modeling tool. Comput Programs Biomed. 1979 Dec;10(3):271–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- Lamvik M. K. Muscle thick filament mass measured by electron scattering. J Mol Biol. 1978 Jun 15;122(1):55–68. doi: 10.1016/0022-2836(78)90108-0. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Hainfeld J., Wall J., Haschemeyer R. H. Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol. 1981 Dec 15;153(3):695–718. doi: 10.1016/0022-2836(81)90414-9. [DOI] [PubMed] [Google Scholar]
- Nonomura Y., Ono T. Direct visualization of the RNA location in cucumber green mottle mosaic virus and tobacco mosaic virus protein disk. J Mol Biol. 1974 Dec 15;90(3):523–527. doi: 10.1016/0022-2836(74)90232-0. [DOI] [PubMed] [Google Scholar]
- Paglini S., Lauffer M. A. Polymerization-depolymerization of tobacco mosaic virus protein. XI. Osmotic pressure studies of solutions in water and in deuterium. Biochemistry. 1968 May;7(5):1827–1835. doi: 10.1021/bi00845a030. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Schuster T. M. Letter: Hysteresis of proton binding to tobacco mosaic virus protein associated with metastable polymerization. J Mol Biol. 1975 May 25;94(3):519–525. doi: 10.1016/0022-2836(75)90218-1. [DOI] [PubMed] [Google Scholar]
- Smith P. R. An integrated set of computer programs for processing electron micrographs of biological structures. Ultramicroscopy. 1978;3(2):153–160. doi: 10.1016/s0304-3991(78)80021-7. [DOI] [PubMed] [Google Scholar]



