Abstract
Although ivermectin treatment can induce serious adverse events (SAEs) in individuals harboring high Loa loa microfilaremia (mf), not all patients with high mf levels develop such reactions, suggesting that cofactors may be involved. A study was conducted in Cameroon to investigate the possible role of Plasmodium coinfection at the time of ivermectin treatment in the development of SAEs. Before their first ivermectin treatment, thick smears were obtained from 4,175 individuals to determine the burden of Plasmodium sp., L. loa, and Mansonella perstans. After treatment, 18 (4.3 per 1,000) patients developed a non-neurologic SAE. Logistic regression analysis, adjusting for age, sex, P. falciparum infection, and M. perstans infection intensities, confirmed that L. loa mf was the main risk factor for SAEs. We found no evidence that coinfection with P. falciparum at the time of ivermectin treatment was associated with the occurrence of Loa-related SAEs in this population.
Ivermectin, the drug used for onchocerciasis control as Mectizan® (Merck & Co., Inc., Whitehouse Station, NJ), can induce serious adverse events (SAEs) in individuals heavily infected with Loa loa. Although rare, these potentially fatal SAEs continue to impede the progress of the African Program for Onchocerciasis Control and the Global Program to Eliminate Lymphatic Filariasis in areas where loiasis is endemic.1 The major risk factor for the development of post-ivermectin SAEs is a high L. loa microfilarial (mf) density.2 It has been estimated that the relative risk of developing non-neurologic marked adverse events or SAEs, as defined previously,3 is significantly increased when the Loa density exceeds 8,100 or 30,000 mf/mL blood, respectively.3 However, most individuals potentially at risk because of their high mf density do not develop an SAE after ivermectin treatment. Therefore, cofactors related to the host or the parasite are likely involved in the development of SAEs.
In a previous study, we examined the possibility that a simian L. loa strain (with nocturnally periodic microfilaremia) could be associated with the occurrence of SAEs in humans.4 For this purpose, the periodicity of L. loa mf was compared in individuals who did and did not develop an SAE. Diurnal periodicity was observed in all subjects, which is inconsistent with the hypothesis that an infection with a simian L. loa was responsible for the SAE cases.4 The role of a genetic mutation in a P-glycoprotein gene called mdr-1 (ABCB1) that enhances the permeability of the blood–brain barrier to ivermectin was also assessed in individuals who had experienced an SAE.5 No association between this mutation and the occurrence of SAEs was shown.
Involvement of coinfections in post-ivermectin and post-diethylcarbamazine SAEs has also been suggested.2 In this respect, it is interesting to note that Loa-related SAEs and severe malaria share many clinical features, including fever, impaired consciousness, and white-centered retinal hemorrhages.6 In addition, Plasmodium infection has been observed in a number of individuals who developed post-ivermectin SAEs.7 Thus, Plasmodium infection has been considered as a potential cofactor facilitating the development of SAEs.2
In the present study, the presence of P. falciparum infection at the time of ivermectin treatment was examined as a potential risk factor for Loa-related SAEs. The following protocol was approved by the National Ethics Committee of Cameroon.
Parasitological surveys were conducted in 2005 in 74 communities of the East Region of Cameroon (Lom and Djerem and Upper Nyong Divisions) at the same time as the first Community Directed Treatment with Ivermectin campaign targeting onchocerciasis in this area. Before the surveys, the Ministry of Health informed the local authorities and populations that a mass drug administration of Mectizan would soon take place in their village. On this occasion, they were also informed of the objective and protocol of the study. All healthy individuals 13 years of age or older who had been living in the village for more than 6 months were offered a parasitological examination for assessment of Plasmodium spp., L. loa, and Mansonella perstans infections. Refusal to participate did not exempt the eligible individuals from the Mectizan treatment.
Just before ivermectin treatment (standard dose of 150 μg/kg), blood smears were obtained from all consenting subjects. A calibrated blood thick smear (50 μL) from a finger prick was performed between 10:00 am and 4:00 pm for the quantitative diagnosis of L. loa (diurnally periodic microfilaremia) and M. perstans (aperiodic). An additional slide, with a thick smear and a thin smear, was prepared for the diagnosis of Plasmodium infection. All blood smears were stained with Giemsa stain within 2 days of preparation and examined by two experienced laboratory technicians using optical microscopy. The slides from subjects who developed an SAE were examined immediately because of the impact of the results on therapeutic management. The remaining slides were examined after a delay of several weeks by the same two laboratory technicians. All parasites present on the slide were identified and counted. An active surveillance procedure for adverse events was established in all communities for 7 days starting at the time of ivermectin distribution, with health teams actively seeking side effects. This procedure was then complemented by passive surveillance up to 30 days after treatment. Patients who did not show any disorders of consciousness or objective neurologic signs3,8 but developed a functional impairment with severity that was such that it would have probably required at least 1 week of full-time assistance at home by their family to undertake normal activities and hospitalization was, thus, considered necessary were considered as having a non-neurologic SAE. All individuals presenting with a non-SAE were closely monitored and benefitted from free management until complete recovery. Our definitions of SAEs/non-SAEs correspond to the standard definition for gradation of adverse events linked to ivermectin in mass treatment, which has been adapted by Merck & Co. Inc. from the definitions of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use and agreed on by the Mectizan Expert Committee under the auspices of the Special Programme for Research and Training in Tropical Diseases (TDR)/World Health Organization.8
A total of 4,175 individuals underwent parasitological examination before receiving ivermectin. Overall prevalence of L. loa, M. perstans, and Plasmodium sp. was 26.3%, 29.6%, and 23.2%, respectively (Table 1). Coinfection with L. loa and M. perstans was more frequent than under the assumption of independence (χ2 = 345.28, P < 0.0001), indicating a statistical association between the filarial infections. No such association was found between Plasmodium infection and L. loa (χ2 = 1.46, P = 0.23) or M. perstans (χ2 = 0.48, P = 0.49).
Table 1.
Prevalence and intensity of infection with L. loa, P. falciparum, and M. perstans in 18 individuals presenting post-ivermectin SAEs and the rest of the population (N = 4,157)
| L. loa | P. falciparum | M. perstans | ||||
|---|---|---|---|---|---|---|
| SAE cases | Non-SAE cases | SAE cases | Non-SAE cases | SAE cases | Non-SAE cases | |
| Prevalence (%) | 100 | 25.9 | 16.7 | 23.7 | 44.4 | 29.5 |
| Arithmetic mean* (95% CI) | 63,948.5 (43,346.8–84,550.3) | 1,779 (1,495.4–2,062.6) | 17.8 (0–51.4) | 502.8 (363–642.7) | 1,970 (0–5,717.2) | 129 (105.4–152.6) |
| Williams' geometric mean* (95% CI) | 50,524.3 (34,181.5–74,681.5) | 4.7 (4.2–6.3) | 0.9 (0–3.1) | 2.4 (2.2–2.7) | 14.7 (1.9–85) | 3.1 (2.9–3.4) |
Arithmetic and Williams' geometric means have been calculated including zero counts and are expressed in mf per milliliter for L. loa and M. perstans and trophozoites per milliliter for P. falciparum.
Plasmodium infections were mainly caused by P. falciparum alone (N = 969). P. malariae alone was observed in 47 individuals, and mixed infections (P. falciparum and P. malariae) were observed in 27 individuals. The mean P. falciparum trophozoite density is shown in Table 1.
Post-ivermectin SAEs occurred in 18 individuals (4.3 per 1,000), none of whom presented with neurologic signs. The most commonly presented clinical signs and symptoms were low back pain (88.9%), headache (83.3%), fever (61.1%), and asthenia (44.4%). The group of SAE cases was composed of 14 men and 4 women, with a mean age of 41.8 years (range = 22–70 years). All of the subjects were hospitalized and discharged after an average of 3.7 days (range = 3–6 days) when their condition had improved to such an extent that full-time assistance was not required. L. loa mf were present on thick smears from all 18 subjects who experienced SAEs, and 17 of the subjects (94.4%) had more than 8,000 L. loa mf/mL (the lowest count being 5,620 mf/mL). Incidence rate of SAEs as a function of L. loa mf density is shown in Table 2. P. falciparum infection was less frequent in the SAE cases (16.7%) than the rest of the population (23.7%). Among those individuals presenting with P. falciparum infection, trophozoite density tended to be lower in 3 SAE cases than 966 non-SAE cases (geometric mean = 42.7 versus 196.7 trophozoites/mL, respectively; Kolmogorov–Smirnov test: P = 0.358). M. perstans infection was more frequent in the SAE cases (44.4%) than the rest of the population (29.5%) (Table 1). Among those individuals presenting with M. perstans infection, mf density tended to be higher in 8 SAE cases than 1,226 non-SAE cases (geometric mean = 490.8 versus 121.6 mf/mL, respectively; Kolmogorov–Smirnov test: P = 0.076).
Table 2.
Incidence rate of SAEs as a function of L. loa mf density
| L. loa mf density (mf/mL) | No. of individuals | No. SAE cases | Incidence rate (%) | 95% CI* |
|---|---|---|---|---|
| 0–4,999 | 3,844 | 0 | 0 | 0–0.1 |
| 5,000– 9,999 | 123 | 1 | 0.81 | 0.02–4.45 |
| 10,000–19,999 | 95 | 0 | 0 | 0–3.8 |
| 20,000–39,999 | 65 | 7 | 10.77 | 4.44–20.94 |
| 40,000–59,999 | 27 | 3 | 11.11 | 2.35–29.2 |
| 60,000–79,999 | 5 | 0 | 0 | 0–52.2 |
| 80,000–99,999 | 7 | 4 | 57.14 | 18.4–90.1 |
| ≥ 100,000 | 9 | 3 | 33.33 | 7.49–70.1 |
| Total | 4,175 | 18 | 0.43 | 0.26–0.68 |
Exact 97.5% CI is given when incidence rate is zero.
Using multivariate logistic regression, we assessed the associations between the occurrence of an SAE (variable of interest) and five potential risk factors, namely P. falciparum infection (absence/presence), L. loa mf density [log10(x + 1) transformed], age (13–29 [reference category], 30–44, 45–59, and ≥ 60 years), and sex (reference category: female); following the trend previously observed, according to which M. perstans infection was positively associated with occurrence of SAE,3 M. perstans mf density [log10(x + 1) transformed] was also included in the model as a potential risk factor.
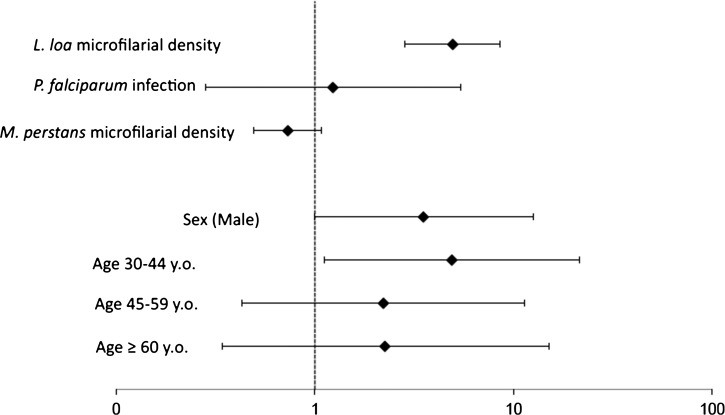
Infection with P. falciparum was not associated with the occurrence of an SAE (odds ratio [OR] = 1.23, 95% confidence interval [95% CI] = 0.28–5.40, P = 0.788) (Figure 1). In contrast, as expected, L. loa mf density was significantly associated with the occurrence of an SAE, with an OR of 4.94 (95% CI = 2.84–8.58, P < 0.001), indicating a nearly fivefold increase in the risk of an SAE for every unit increase in the mf density on a log10 scale (10/100/1,000/10,000).
Figure 1.
OR for risk factors of post-ivermectin SAEs estimated by multivariate logistic regression. y.o. = years old.
M. perstans mf density was not significantly associated with the risk for an SAE (OR = 0.73, 95% CI = 0.49–1.08, P = 0.12). Males tended to have an increased risk for an SAE (OR = 3.51, 95% CI = 0.99–12.53, P = 0.053) compared with females. Subjects 30–44 years old exhibited a significantly increased risk for an SAE (OR = 4.89, 95% CI, 1.11–21.44, P = 0.035) compared with younger subjects.
In summary, the results concerning the influence of sex, age, and L. loa and M. perstans infections on the development of a post-ivermectin SAE confirm the results already documented.3,8 We found no evidence that coinfection with P. falciparum at the time of ivermectin treatment was associated with the occurrence of Loa-related SAEs in this population. An unavoidable limitation of our study is that subjects with mild and moderate adverse effects were treated immediately for ethical reasons. If symptomatic treatments reduced the risk of progressing to SAE, associations between SAE and the potential cofactors may have been biased to the null hypothesis. However, when extending the group of adverse event cases to those patients who presented any kind of adverse events and using the same model and covariates, no association was found between P. falciparum infection and occurrence of the latter (OR = 0.85, 95% CI = 0.64–1.14, P = 0.27).
The absence of neurologic SAEs in the study population after ivermectin treatment precludes any conclusions regarding a possible association between P. falciparum infection and neurologic SAEs. Animal models allowing the development of both Loa and Plasmodium infections may be particularly useful to test the possible association between L. loa, Plasmodium, and neurologic post-ivermectin SAEs.
ACKNOWLEDGMENTS
The authors thank the Mectizan Donation Program for financial support.
Footnotes
Authors' addresses: Joël Fokom-Domgue, Patrick Nguipdop-Djomo, and Joseph Kamgno, Center for Research on Filariasis and other Tropical Diseases, Yaounde, Cameroon, E-mails: joeldomgue@yahoo.fr, patrick.nguipdop-djomo@lshtm.ac.uk, and kamgno@crfilmt.org. Raceline Gounoue, Faculty of Sciences, University of Yaounde, Yaounde, Cameroon, E-mail: gounoue@crfilmt.org. Julie Akame, Helen Keller International, Yaounde, Cameroon, E-mail: jakame@hki.org. Nana A. Y. Twum-Danso and Björn Thylefors, Mectizan Donation Program, Decatur, GA, E-mails: ntwumdanso@yahoo.com and bthylefors@gmail.com. Sébastien D. Pion and Michel Boussinesq, UMI233, Institut de Recherche pour le Développement, Montpellier, France, E-mails: sebastien.pion@ird.fr and michel.boussinesq@ird.fr.
References
- 1.Zouré HGM, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, Remme JHF. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the rapid assessment procedure for loiasis (RAPLOA) PLoS Negl Trop Dis. 2011;5:e1210. doi: 10.1371/journal.pntd.0001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boussinesq M, Gardon J, Gardon-Wendel N, Chippaux J-P. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003;2((Suppl 1)):S4. doi: 10.1186/1475-2883-2-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardon J, Gardon-Wendel N, Demanga-Ngangue, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- 4.Kamgno J, Pion SD, Mackenzie CD, Thylefors B, Boussinesq M. Loa loa microfilarial periodicity in ivermectin-treated patients: comparison between those developing and those free of serious adverse events. Am J Trop Med Hyg. 2009;81:1056–1061. doi: 10.4269/ajtmh.2009.09-0356. [DOI] [PubMed] [Google Scholar]
- 5.Bourguinat C, Kamgno J, Boussinesq M, Mackenzie CD, Prichard RK, Geary TG. Analysis of the mdr-1 gene in patients co-infected with Onchocerca volvulus and Loa loa who experienced a post-ivermectin serious adverse event. Am J Trop Med Hyg. 2010;83:28–32. doi: 10.4269/ajtmh.2010.09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White V, Lewallen S, Beare N, Molyneux M, Taylor T. Retinal pathology of pediatric cerebral malaria in Malawi. PLoS One. 2009;4:e4317. doi: 10.1371/journal.pone.0004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamgno J, Boussinesq M, Labrousse F, Nkegoum B, Thylefors BI, Mackenzie CD. Encephalopathy after ivermectin treatment in a patient infected with Loa loa and Plasmodium spp. Am J Trop Med Hyg. 2008;78:546–551. [PubMed] [Google Scholar]
- 8.Twum-Danso NAY. Serious adverse events following treatment with ivermectin for onchocerciasis control: a review of reported cases. Filaria J. 2003;2((Suppl 1)):S3. doi: 10.1186/1475-2883-2-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]