Abstract
Uganda is the only African country whose onchocerciasis elimination program uses a two-pronged approach of vector control and mass drug distribution. The Ugandan program relies heavily upon the use of serosurveys of children to monitor progress toward elimination. The program has tested over 39,000 individuals from 11 foci for Onchocerca volvulus exposure, using the Ov16 ELISA test. The data show that the Ov16 ELISA is a useful operational tool to monitor onchocerciasis transmission interruption in Africa at the World Health Organization (WHO) recommended threshold of < 0.1% in children. The Ugandan experience has also resulted in a re-examination of the statistical methods used to estimate the boundary of the upper 95% confidence interval for the WHO prevalence threshold when all samples tested are negative. This has resulted in the development of Bayesian and hypergeometric statistical methods that reduce the number of individuals who must be tested to meet the WHO criterion.
Introduction
Onchocerciasis has historically been one of the most important causes of infectious blindness.1,2 The disease is caused by the filarial nematode parasite Onchocerca volvulus. It is estimated that 120 million individuals worldwide are at risk of O. volvulus infection, with most residing in rural Africa.3 Onchocerca volvulus is transmitted by black flies of the genus Simulium, insects that breed in fast flowing water. Thus, the infection is most intense in areas located along rivers, leading to the common name of “river blindness” for the disease. Unfortunately, the areas bordering the river basins contain much of the fertile land found in sub-Saharan African savanna ecosystems. By preventing the agricultural use of the most fertile lands, onchocerciasis has had a significant negative impact on the economic growth of many of the poorest countries of Africa.
The devastating impact that onchocerciasis has historically had upon some of the poorest people on the planet has attracted the attention of the international community, which has supported several programs to control or eliminate the disease. Strategies originally focused on vector control, but this approach has been largely supplanted with the discovery that ivermectin was a safe and effective treatment of human onchocerciasis, having a potent effect on the microfilarial stage of O. volvulus.4 The offer of Merck & Co, Inc. to donate ivermectin free of charge for the treatment of onchocerciasis for as long as needed resulted in the establishment of two major regional programs, the African Program for Onchocerciasis Control, (APOC) and the Onchocerciasis Elimination Program of the Americas (OEPA). The strategies of these programs are to use population-based chemotherapy (mass drug administration) with ivermectin to control morbidity from onchocerciasis in Africa (APOC) or to completely eliminate the parasite from the Americas (OEPA). It was initially believed that ivermectin distribution alone could not successfully eliminate onchocerciasis in Africa, as a result of the widespread distribution of the infection and the intensity of transmission.5 However, recent data have suggested that this is not the case, and that long-term community wide distribution of ivermectin may be capable of eliminating onchocerciasis in at least some foci in Africa.6–10 This discovery has resulted in a refocusing of the international community from an emphasis on control of onchocerciasis in Africa toward an emphasis upon possible elimination.11,12
Monitoring and evaluation activities are especially necessary in elimination efforts to document the effectiveness of program operations and eventually in showing that transmission had been interrupted. The latter task requires that assays with high negative predictive values be used to test large numbers of samples to verify that transmission has been interrupted. To this end, in 2001 the World Health Organization (WHO) adopted two key criteria for transmission interruption: 1) An absence or near absence of infective stage larvae (L3) in the vector population and; 2) Infection rates of < 0.1% in children residing in the endemic area.13 Infection rates in children have operationally been measured by detecting the presence of IgG4 antibodies to a parasite-specific 16 kDa antigen (Ov16) using an enzyme-linked immunosorbent assay (ELISA) format. Using conventional statistical methods,14 the WHO 2001 guidelines noted that it would be necessary to test 3,000 individuals to conclude that the upper bound of the 95% confidence interval (CI) of the prevalence estimate was < 0.1%.
In 2007, Uganda declared a goal of national elimination of onchocerciasis by 2020, becoming one of the first countries in Africa to do so.15 Uganda contains 18 distinct onchocerciasis transmission zones (foci). With the exception of the Victoria and Mount Elgon foci, all of the foci are found in the western and northern regions of the country (Figure 1). The vector in the western foci is Simulium neavei, whereas S. neavei and Simulium damnosum sensu lato serve as vectors in the northern foci.16,17 Onchocerciasis was eliminated by DDT river treatments in the Victoria focus in the 1960s.18,19 The Ugandan Onchocerciasis Elimination Program (UOEP) is unique in that it is currently the only program that incorporates both mass ivermectin distribution and vector control or local elimination into its strategic plan.15 This combination of approaches has resulted in the rapid interruption of transmission of O. volvulus in at least two foci in Uganda.9,20–22 However, the incorporation of vector control and focal elimination into the UEOP's strategic plan has often made it difficult or impossible to collect the number of vector black flies necessary to meet (with 95% confidence) the first WHO criterion of < 0.05% infective stage larvae in the vector population.22 For this reason, the UEOP has relied heavily upon the second WHO criterion (< 0.1% infection in children). To accomplish this, the Ov16 ELISA assay is used as the infection assessment tool for interruption of onchocerciasis transmission. Here, we present an overview of the operational experience of the UEOP with the Ov16 ELISA, which has included testing serum samples from over 39,000 children from 11 of the 17 active foci in the county. We also propose some alternative statistical approaches that suggest it may not be necessary to test 3,000 individual serum samples to determine, with 95% certainty, that the OV16 antibody prevalence is < 0.1%.
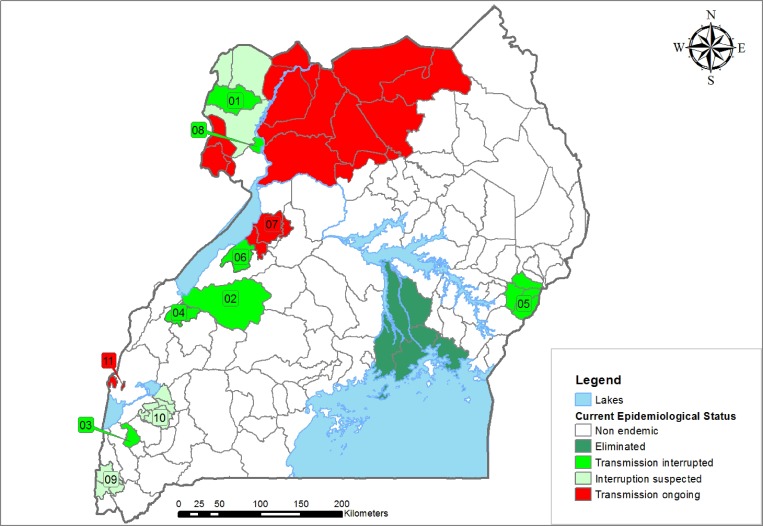
Figure 1.
Map of onchocerciasis foci in Uganda included in this study: The foci included in this study and their current epidemiological status are shown by different color codes. The names of the foci are as follows: 1 = Maracha Terengo; 2 = Mpamba-Nkusi; 3 = Imaramagambo; 4 = Itwara; 5 = Mt Elgon; 6 = Wambabya-Rwamarongo; 7 = Budongo; 8 = Wadelai; 9 = Bwindi; 10 = Kashoya; 11 = Nyamugasani.
Materials and Methods
Sample collection and ethical clearance.
Samples were collected by staff of the Uganda Ministry of Health as part of the monitoring activities for onchocerciasis elimination efforts in the country. The study was determined not to meet the definition of human subjects research by the Uganda Ministry of Health and the Institutional Review Boards for Human Subjects Research of Emory University and the University of South Florida.
Blood samples were collected by finger prick from children resident in 11 Ugandan foci. Blood was placed on Whatman #2 filter paper squares and the blood spots permitted to dry. The samples were stored in a sealed plastic bag with a silica dessicant at −20°C until analyzed.
Children for survey were selected by a multistage stratified sampling scheme applied at the parish administrative unit level. In small foci, all parishes were sampled, although in large foci parishes were chosen for sampling using a simple random sampling scheme. In every selected parish, all children < 10 years of age had equal chance of being selected. The minimum number of children sampled from each parish was calculated based upon the percentage of overall population of the focus that resided in each parish. For example, if a parish contained 20% of the total estimated population of the focus, attempts were made to collect samples from a minimum of 600 children from that parish (20% of the 3,000 needed from the focus as a whole). Where the focus had < 3000 children, all available and consenting children were enrolled in the study.
Ov16 ELISA assay.
The presence of IgG4 antibodies recognizing the Ov16 antigen in the blood spots was determined by ELISA, essentially as previously described.23,24 A detailed protocol describing this procedure is found in Supplemental File S1. In brief, serum samples eluted from dried blood spots were exposed to plates coated with purified recombinant Ov16–glutathione S-transferase (GST) antigen. Bound antibodies were detected by exposure to biotin conjugated goat anti-human IgG4 and streptavidin conjugated with alkaline phosphatase. The plates were developed with paranitrophenol phosphate (PNPP) substrate (Sigma Chemical, St. Louis, MO). Putatively positive samples were retested with plates coated with Ov16–GST and with control GST. Samples that gave a positive reading in both Ov16 assays and were negative for GST alone were scored as confirmed positive.
Statistical methods.
Calculation of 95% CIs when positive samples were found.
The 95% CIs for collections in which positive serum samples were identified were calculated as previously described,14 using the formula,
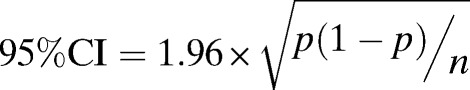 |
where p = the proportion of positive samples and n = the total number of samples tested.
Calculation of CIs from a sample collected from a large population when no samples are positive.
The conventional method of calculating 95% CIs from discrete (count) data14 requires that at least one positive sample be included in the analysis. Thus, this method cannot be used to calculate 95% CIs from data sets in which all samples are negative; at least a single positive sample must be assumed to exist. However, this problem may be addressed using Bayesian methods. The Bayesian approach to inference is often used with an objective (non-informative) prior distribution on the parameter of interest. One of the most commonly chosen is the Jeffreys' prior, which has the important property that the inferences made are invariant under reparameterization of the model. In the case of the Binomial sampling model, this prior also turns out to be a conjugate prior, which is a desirable property. In the Bayesian paradigm, it is common to develop Highest Posterior Distribution (HPD) credible intervals rather than equal-tailed intervals. When no positive pools are observed, then the HPD interval does not exist. In this case, a one-sided credible interval is calculated in which the upper tail area of the posterior distribution is excluded from the interval. The program Poolscreen 2.025 uses this strategy for calculating frequentist CIs when no positive sample pools are observed, and was thus adapted to calculate CIs for the Ov16 data, where individual samples (i.e., pool size = 1) are screened and no positive samples are found.
Calculation of the proportion of a finite population that must be screened to ensure an upper bound of a 95% CI of a prevalence of < 0.1%.
Sampling small populations represents a special case in which sampling without replacement is carried out on a finite population. Approximate CIs are often found by means of the application of asymptotic theory based upon the Classical Central Limit Theorem (CLT). However, when sampling is done without replacement (such as in a standard serosurvey), the sample observations are not independent and the CLT does not apply. Fortunately, there is a special version of the CLT that applies in this case.26,27 Agresti and Coull28 provide a survey of methods for constructing CIs for binomial proportions, a number of which are based on the use of asymptotic theory. In particular, the interval suggested by Wilson29 leads to an interval that can be applied when no positive individuals are found. The derivation of the algorithms for calculating CIs when a large proportion of a finite population is samples and where all samples are found to be negative may be found in the Supplemental Material S2.
Results
Serum from 39,444 children (residents of 11 of the 17 active Ugandan transmission zones) were tested for the presence of Ov16 antibodies during a period from 2008 to 2012 (Table 1). The samples were run in 1,892 plates. Of these, 27 (1.4%) did not meet the quality assurance standards described in the detailed protocol provided in the Supplemental Material. The most common reason for plate rejection was a finding that the positive controls did not fall within the acceptable optical density range of values.
Table 1.
Summary of Ov16 ELISA serosurveys in Uganda
| Focus no.* | Focus name | Year surveyed | Age group | No. tested | No. positive | % positive | 95% CI (%) |
|---|---|---|---|---|---|---|---|
| 1 | Maracha Terengo | 2011 | 1–4 | 3,171 | 0 | 0 | 0–0.06 |
| 5–9 | 3,450 | 0 | 0 | 0–0.06 | |||
| 10–15 | 13 | 0 | 0 | 0–3.5 | |||
| TOTAL | 6,634 | 0 | 0 | 0–0.03 | |||
| 2 | Mpamba-Nkusi | 2009 | 1–4 | 1,378 | 2 | 0.14 | 0–0.35 |
| 5–9 | 1,011 | 4 | 0.40 | 0–0.78 | |||
| 10–15 | 962 | 13 | 1.35 | 0.62–2.08 | |||
| TOTAL | 3,351 | 19 | 0.57 | 0.30–0.80 | |||
| 2012 | 1–4 | 1,942 | 0 | 0 | 0–0.1 | ||
| 5–9 | 1,465 | 1 | 0.07 | 0–0.20 | |||
| TOTAL | 3,407 | 1 | 0.03 | 0–0.09 | |||
| 3 | Imaramagambo | 2009 | 1–4 | 1,392 | 2 | 0.14 | 0–0.34 |
| 5–9 | 968 | 4 | 0.41 | 0.01–0.82 | |||
| 10–15 | 972 | 7 | 0.72 | 0.19–1.25 | |||
| TOTAL | 3,332 | 13 | 0.39 | 0.18–0.60 | |||
| 4 | Itwara | 2010 | 1–4 | 1,898 | 1 | 0.05 | 0–0.16 |
| 5–9 | 1,409 | 1 | 0.07 | 0–0.21 | |||
| 10–15 | 7 | 0 | 0 | 0–23.2 | |||
| TOTAL | 3,307 | 2 | 0.05 | 0–0.14 | |||
| 5 | Mt Elgon | 2008 | 1–4 | 1,123 | 0 | 0 | 0–0.17 |
| 5–9 | 1,032 | 0 | 0 | 0–0.19 | |||
| 10–15 | 907 | 1 | 0.11 | 0–0.33 | |||
| TOTAL | 3,062 | 1 | 0.03 | 0–0.1 | |||
| 6 | Wambabya-Rwamarongo | 2009 | 1–10 | 3,005 | 49 | 1.63 | 1.17–2.08 |
| TOTAL | 3,005 | 49 | 1.63 | 1.17–2.08 | |||
| 7 | Budongo | 2008 | 1–4 | 1,339 | 64 | 4.78 | 3.64–5.92 |
| 5–9 | 967 | 104 | 10.75 | 8.8–12.71 | |||
| 10–15 | 853 | 131 | 15.36 | 12.94–17.78 | |||
| TOTAL | 3,159 | 299 | 9.46 | 8.44–10.48 | |||
| 8 | Wadelai | 2008 | 1–4 | 1,078 | 0 | 0 | 0–0.18 |
| 5–9 | 1,058 | 2 | 0.19 | 0–0.45 | |||
| 10–15 | 873 | 1 | 0.11 | 0–0.34 | |||
| TOTAL | 3,009 | 3 | 0.10 | 0–0.21 | |||
| 9 | Bwindi | 2010 | 1–4 | 2,012 | 0 | 0 | 0–0.095 |
| 5–9 | 1,484 | 0 | 0 | 0–0.13 | |||
| 10–15 | 876 | 0 | 0 | 0–0.22 | |||
| TOTAL | 4,372 | 0 | 0 | 0–0.04 | |||
| 10 | Kashoya | 2010 | 1–4 | 709 | 1 | 0.14 | 0–0.42 |
| 5–9 | 653 | 10 | 1.53 | 0.59–2.47 | |||
| TOTAL | 1,362 | 11 | 0.81 | 0.33–1.28 | |||
| 11 | Nyamugasani | 2011 | 1–4 | 505 | 0 | 0 | 0–0.38 |
| 5–9 | 900 | 0 | 0 | 0–0.21 | |||
| 10–15 | 32 | 0 | 0 | 0–5.78 | |||
| TOTAL | 1,437 | 0 | 0 | 0–0.13 |
Numbers correspond to the focus numbers depicted in Figure 1.
A total of 473 serum samples were scored as positive in the initial screening assays. Of these, 420 (89%) were scored positive in the confirmatory assay. Just four individuals were found to produce antibodies that reacted with the GST fusion partner of the Ov16 recombinant antigen.
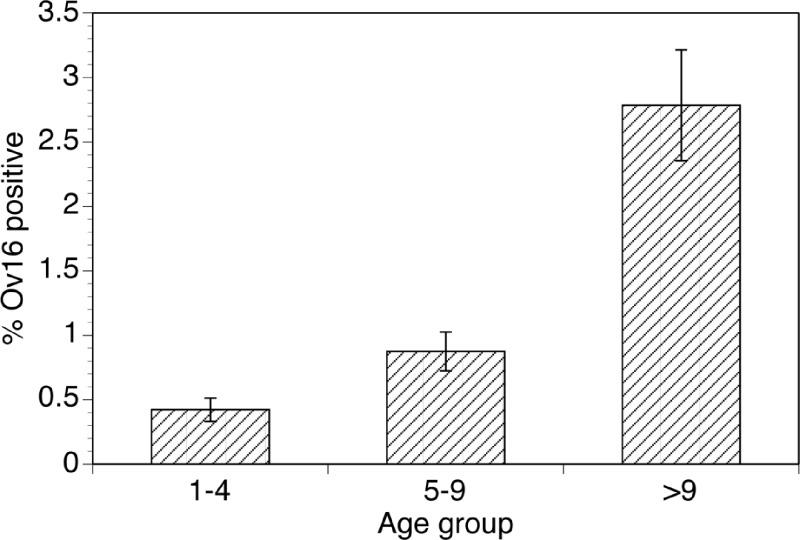
Ov16 antibody prevalence varied from a low of 0% (95% CI 0–0.03%) in Maracha-Terango to a high of 9.5% (95% CI 8.4–10.5%) in Budongo (Table 1). The age profile of antibody positivity among all foci is shown in Figure 2. As expected for an endemic infection, the prevalence of antibodies to Ov16 (and presumably exposure to the parasite) increased with age, reaching a high of 33.5/1,000 in children 10 years of age and older.
Figure 2.
Prevalence of IgG4 antibodies recognizing Ov16 in children in Ugandan foci of onchocerciasis by age group: Error bars indicate 95% confidence intervals (CIs) for the prevalence estimates.
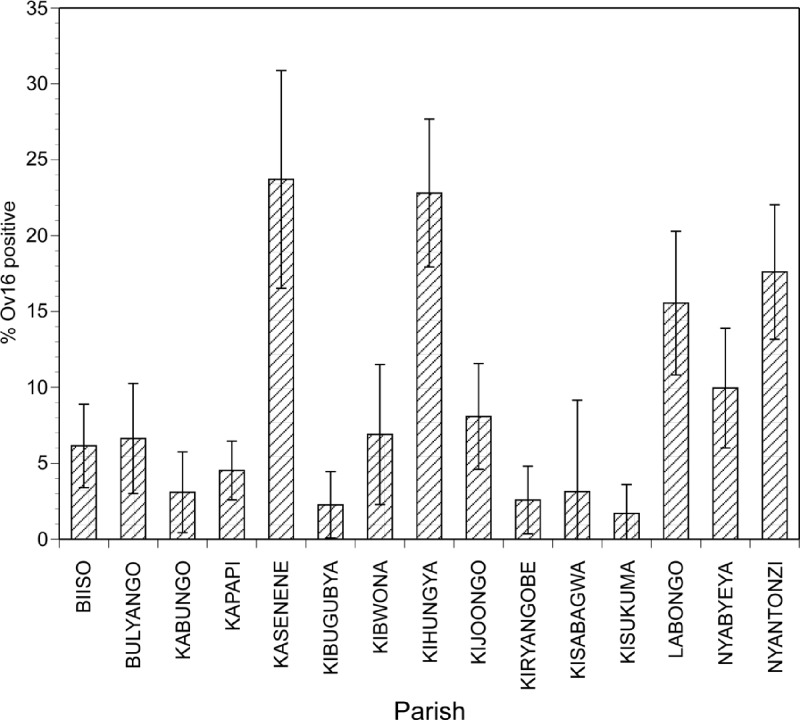
Within foci exhibiting evidence of ongoing exposure to O. volvulus, the prevalence of antibodies to Ov16 varied widely by parish, indicating a varying force of transmission within a given focus. For example, in the Budongo focus, the prevalence of antibody positive children ranged from a low of 1.7% in Kisukuma parish to a high of 23.7% in Kasenene parish (Figure 3).
Figure 3.
Prevalence of IgG4 antibodies recognizing Ov16 in children in different parishes of the Budongo focus: Error bars indicate 95% confidence intervals for the prevalence estimates.
Surveys were conducted in Mpamba-Nkusi in 2009 and again in 2012. In 2009, 6 of 2,389 children < 10 years of age were identified with Ov16 antibodies (Table 1). In 2012, a significant decline in the rate of seropositive children was seen, with just 1 of 3,047 children < 10 years of age found to be seropositive (Table 1; P = 0.017; χ2 test).
The 2001 WHO guidelines require an infection prevalence of < 0.1%, and conclude that a minimum of 3,000 children must be tested to reach this threshold.13 This critical number is derived from classical statistical methods for calculating 95% CIs.14 These can be used to show that when 3,000 samples are tested and a single sample is positive, the upper bound of the 95% CI for the prevalence of exposure in the community from which the samples were drawn will be 0.098%, whereas if two samples are positive, the upper bound of the prevalence of infection will be 0.15%. However, the classical method of calculating 95% CIs cannot be applied to data in which all samples are negative. The problem of the calculation of 95% CIs when all samples are negative has been addressed previously in situations involving screening pools of samples of insects for the presence of infectious agents.25,30,31 As described in the Materials and Methods, Bayesian methods employing a non-informative prior distribution can be used for this purpose. Taking the serosurvey data as a special case of pool screening where the pool size consisted of one sample (N = 1), it was possible to explore the use of Bayesian statistical methods to predict the upper bound of the 95% creditability interval in the population prevalence when all samples tested were negative. The results of this analysis are summarized in Table 2; when all samples are negative, the Bayesian upper bound of the creditability interval remains below 0.1% when 2,000 samples are tested.
Table 2.
Bayesian creditability intervals for the upper limit of the 95% confidence interval (CI) of prevalence for different sample sizes when all samples are negative
| Number screened | Upper limit 95% CI |
|---|---|
| 2,250 | 0.085% |
| 2,000 | 0.096% |
| 1,750 | 0.11% |
| 1,500 | 0.128% |
| 1,250 | 0.153% |
| 1,000 | 0.191% |
Because onchocerciasis is a disease of rural populations, in many foci the total population of resident children in a focus may be less than the 3,000. In such situations, the 2001 WHO guidelines call for testing the entire population of eligible children residing in that focus. It is quite difficult to obtain the 100% compliance necessary to meet this alternative criterion. However, when one is attempting to sample every individual in the target population, a significant proportion of the eligible individuals will be included. This represents a special case in which conventional statistical methods, which assume that one draws a random sample from an essentially infinite population, do not apply. However, the problem of determining an upper bound of the prevalence of infection in situations where a substantial proportion of the eligible population is included in the study can be addressed using a hypergeometric distribution, as described in the Materials and Methods. Based upon this, it is possible to calculate the proportion of a population that must be tested and found to be negative to ensure that a test based on the Hypergeometric model would reject the null hypothesis H0 : θ ≥ 0.1% in favor of the alternative that Ha : θ < 0.1%. For populations of < 2,000 individuals, a difficulty arises in that an upper bound for a 0.1% prevalence often cannot be explicitly calculated, because the number of positive individuals allowed must be expressed in whole numbers, although fractions are required to generate a prevalence estimate of 0.1%. For example, in a population of 1,750 individuals, a prevalence of 0.1% would correspond to 1.75 individuals. Because it is not possible to have a fraction (0.75) of an individual, the test must be revised to exclude the possibility that more than one individual in the entire population tested would be positive when populations of < 2,000 individuals are tested. Table 3 summarizes the results of these calculations, providing sample sizes that must be tested to ensure that the overall prevalence in the target population of children < 10 years of age is ≤ 0.1%, given the ordinal nature of the prevalence calculations.
Table 3.
Proportion of a finite target population that needs to be tested to conclude that the prevalence in the entire target population is ≤ 0.1% when none of the samples tested are positive
| Total population size (children < 10) | Maximum no. positives allowed in total population of children < 10 | Actual allowed upper bound of prevalence | No. to test |
|---|---|---|---|
| 1,750 | 1 | 0.057% | 1,663 |
| 1,500 | 1 | 0.067% | 1,425 |
| 1,250 | 1 | 0.08% | 1,188 |
| 1,100 | 1 | 0.09% | 1,045 |
Discussion
The previous results represent the largest operational experience of the Ov16 ELISA assay in programmatic activities in Africa. The data show that the Ov16 ELISA assay can be effectively used in an operational setting to monitor the success of onchocerciasis elimination activities in Africa. The Uganda laboratory has used the Ov16 ELISA without encountering any major technical obstacles, and has produced data that have met established quality control criteria for over 98% of the plates analyzed. Collection and shipping of blood spots to the central laboratory in Kampala for analysis has also been carried out without any major difficulties. The only minor operational difficulty encountered has been the need to maintain a cold chain during the shipment of the temperature-sensitive reagents (the antigens and antibody conjugates). This has required close cooperation between the shippers and the recipients in Kampala to ensure timely delivery of these reagents. However, to date this has been accomplished successfully without incident.
The results present a rapidly changing serological prevalence pattern of onchocerciasis in childhood that is in line with expected patterns of a reduction in ongoing transmission. In foci where transmission has been interrupted or eliminated, the Ov16 ELISA also showed its ability to serve as a metric to assess if the 2001 WHO transmission breakpoint threshold indicator of < 0.1% infection rate among children was met. Together, these data support the hypothesis that the Ov16 ELISA assay is a reliable tool for onchocerciasis transmission monitoring in the African elimination paradigm. Our experience also suggests that the use of finger stick blood spots are more acceptable to communities (and children) than the traditional skin snips for measuring the prevalence of microfilardermia.6
The data also show heterogeneous force of transmission within a given focus, with some parishes having a high prevalence of exposure, whereas others located in the same focus have little or no evidence of exposure. This suggests the intensity of exposure to O. volvulus varies dramatically from parish to parish. This difference is probably attributable to differences in the local ecology of the parishes (e.g., distance from a breeding site, or activities conducted by community members that expose them to black fly bites). Similar focal distributions in infection intensity have been documented using traditional parasitological methods in foci in Mali and Senegal6 and in entomological surveys in Mexico.32 Although such heterogeneity may complicate sampling designs to accurately measure prevalence within a focus while transmission is ongoing, this complication disappears once transmission has been eliminated and all children in the affected communities are expected to be negative for exposure.
The UOEP is currently the only onchocerciasis control program that is using a combined approach of mass ivermectin distribution and vector control for elimination. As a result, vector flies are difficult to collect in the numbers necessary to verify transmission elimination, leading the UOEP to rely on Ov16 serological testing (together with ongoing entomological surveys to verify the lack of vectors in areas subject to vector control) as the primary metrics in certifying transmission elimination. Given the difficulty of capturing flies in other areas of Africa, other programs may also need to rely on this tool.
It is currently not known how long the IgG4 antibody response to the Ov16 antigen persists in exposed individuals. Thus, although the Ov16 ELISA is a useful indicator of exposure, it cannot differentiate recent from historical exposure. However, the data presented from the two serosurveys conducted in Mpamba-Nkusi in 2009 and 2012 indicated a significant decline in the number of Ov16-positive children < 10 years of age in this focus. There are two possible explanations for this decline. First, it is possible that most of the seropositive children detected in the serosurvey in 2009 were not included in the 2012 survey, either because they were not present in 2012 or because they had aged out of the under 10 cohort. Alternatively, it is possible that many of the seropositive children converted to seronegativity in the 3 years between the two studies. Because individual sample identifiers were not available to us in this study, the current data cannot be used to differentiate between these possibilities. Longitudinal studies in which patient identifiers are collected, conducted in foci in Uganda where other evidence suggests that transmission has been interrupted, would be useful to address this question.
The 2001 WHO guidelines requiring a minimum of 3,000 serum samples from children be tested to exclude the < 0.1% infection prevalence threshold13 were derived from classical statistical methods for calculating 95% CIs that require that at least one sample be presumed positive. This makes this criterion quite conservative. The application of Bayesian methods that do permit the calculation of a 95% creditability interval when all samples are negative suggest that a minimum of 2,000 samples are sufficient to conclude that the prevalence is < 0.1%, if all samples are negative. Reducing the number of samples necessary to confirm an infection prevalence of < 0.1% will make it operationally easier and less expensive to meet this criterion of the 2001 WHO guidelines.
The 2001 WHO guidelines require that the entire population of eligible children must be tested in situations where the total target population is small. However, the analyses presented previously suggest that it is possible to conclude with a high degree of certainty that the prevalence of exposure is < 0.1% if one samples a relatively large proportion of eligible individuals, even when the total population is < 2,000 individuals. However, when the number of individuals in the population drops below 2,000, it is often not possible to set the upper bound of the cutoff prevalence at exactly 0.1%, caused by the ordinal nature of the sampling data in small populations. Thus, one is forced to except a cutoff that is more conservative than 0.1%. In some cases, it may be advisable to consider relaxing the < 0.1% criterion slightly to take this into account. For example, if the total population of eligible children is 1,750, it is necessary to sample 1,663 individuals and confirm that all samples are negative to conclude that there is at most one positive individual in the entire population. However, it is necessary to sample only 1,359 individuals to conclude that there are two or fewer positive individuals. Accepting an upper bound that permits two or fewer individuals to be positive would result in an upper bound of 0.11%, which is only slightly above 0.1%. However, allowing only one positive individual results in a prevalence of 0.057%, which is substantially more conservative than the < 0.1% cutoff. Finally, it should be noted that if the population is 1,000, it is no longer possible to use statistical methods to conclude that the prevalence is < 0.1%. In this case, the entire population must be sampled if one is to conclude that transmission has been interrupted.
In summary, the Ugandan experience has shown that the Ov16 ELISA is a useful operational tool in monitoring and verifying onchocerciasis elimination. Furthermore, the Ugandan operational experience has resulted in a re-examination of the statistical methods underpinning the 2001 WHO criterion of confidently showing an infection prevalence of < 0.1% in children, and this has resulted in lower estimates for the number of individuals who must be tested in a focus. These findings have been given due consideration in an upcoming revision of the WHO guidelines for onchocerciasis elimination and should be useful elsewhere in Africa as the continent moves from an emphasis on control to an era of onchocerciasis elimination.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Uganda Ministry of Health, the national and district onchocerciasis coordinators, the affected communities (especially the children), The Carter Center, the African Programme for Onchocerciasis Control, Lions Clubs International Foundation together with the Lions Clubs of Uganda, and the Carter Center Uganda staff. The late Nancy Cruz Ortiz, who supervised the OEPA onchocerciasis elimination reference laboratory in Guatemala, assisted in establishing the OV16 laboratory that tested the blood specimens reported in this paper.
Footnotes
Financial support: John Moores provided critical financial support for establishing the Uganda onchocerciasis molecular laboratory and launching the 2007 elimination field activities.
Authors' addresses: David Oguttu, Vector Control Division, Ministry of Health, Kampala, Uganda, E-mail: dguttu@gmail.com. Edson Byamukama and Peace Habomugisha, The Carter Center Uganda, Kampala, Uganda, E-mails: edson.navs@gmail.com and provia5@hotmail.com. Charles R. Katholi, Department of Biostatistics, School of Public Health, University of Alabama at Birmingham, Birmingham, AL, E-mail: ckatholi@uab.edu. Christine Nahabwe, Monica Ngabirano, and Thomson Lakwo, Vector Control Division, Ministry of Health, Kampala, Uganda, E-mails: ch.nahabwe@gmail.com, ngabiranomonica@ymail.com, and tlakwo@gmail.com. Hassan K. Hassan and Thomas R. Unnasch, Global Health Infectious Disease Research, Department of Global Health, University of South Florida, Tampa, FL, E-mails: hhassan@health.usf.edu and tunnasch@health.usf.edu. Moses Katabarwa and Frank O. Richards, Emory University and The Carter Center, Atlanta, GA, E-mails: mkataba@emory.edu and frich01@emory.edu.
References
- 1.Thylefors B. Ocular Onchocerciasis. Bull World Health Organ. 1978;56:63–72. [PMC free article] [PubMed] [Google Scholar]
- 2.African Programme for Onchocerciasis Control . Ouagadougou, Burkina Faso: APOC; 2005. Final communiqué of the 11th session of the Joint Action Forum (JAF) of APOC, Paris, France 6–9 December 2005. [Google Scholar]
- 3.Winthrop KL, Furtado JM, Silva JC, Resnikoff S, Lansingh VC. River blindness: an old disease on the brink of elimination and control. J Glob Infect Dis. 2011;3:151–155. doi: 10.4103/0974-777X.81692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White AT, Newland HS, Taylor HR, Erttmann KD, Keyvan-Larijani E, Nara A, Aziz MA, D'Anna SA, Williams PN, Greene BM. Controlled trail and dose finding study of ivermectin for the treatment of onchocerciasis. J Infect Dis. 1987;156:463–470. doi: 10.1093/infdis/156.3.463. [DOI] [PubMed] [Google Scholar]
- 5.Dadzie Y, Neira M, Hopkins D. Final report of the conference on the eradicability of onchocerciasis. Filaria J. 2003;2:2. doi: 10.1186/1475-2883-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diawara L, Traore MO, Badji A, Bissan Y, Doumbia K, Goita SF, Konate L, Mounkoro K, Sarr MD, Seck AF, Toe L, Touree S, Remme JH. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLOS Neg Trop Dis. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tekle AH, Elhassan E, Isiyaku S, Amazigo UV, Bush S, Noma M, Cousens S, Abiose A, Remme JH. Impact of long-term treatment of onchocerciasis with ivermectin in Kaduna State, Nigeria: first evidence of the potential for elimination in the operational area of the African Programme for Onchocerciasis Control. Parasit Vect. 2012;5:28. doi: 10.1186/1756-3305-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traore MO, Sarr MD, Badji A, Bissan Y, Diawara L, Doumbia K, Goita SF, Konate L, Mounkoro K, Seck AF, Toe L, Toure S, Remme JH. Proof-of-principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLOS Neg Trop Dis. 2012;6:e1825. doi: 10.1371/journal.pntd.0001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katabarwa M, Walsh F, Habomugisha P, Lakwo T, Agunyo S, Oguttu D, Unnasch TR, Unoba D, Byamukama E, Tukesiga E, Ndyomugyenyi R, Richards FO. Transmission of onchocerciasis in Wadelai focus of northwestern Uganda has been interrupted and the disease eliminated. J Parasitol Res. 2012;2012(748540) doi: 10.1155/2012/748540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higazi TB, Zarroug IMA, Mohamed HA, ElMubark WA, Deran TC, Aziz N, Katabarwa M, Hassan HK, Unnasch TR, Mackenzie CD, Richards F, Hashim K. Interruption of onchocerciasis transmission in the Abu Hamed Focus, Sudan. Am J Trop Med Hyg. 2013;89:51–57. doi: 10.4269/ajtmh.13-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.African Programme for Onchocerciasis Control . Informal Consultation on elimination of Onchocerciasis Transmission with Current Tools in Africa “Shrinking the Map.”. Ouagadougou, Burkina Faso: African Programme for Onchocerciasis Control; 2009. [Google Scholar]
- 12.Mackenzie CD, Homeida MM, Hopkins AD, Lawrence JC. Elimination of onchocerciasis from Africa: possible? Trends Parasitol. 2012;28:16–22. doi: 10.1016/j.pt.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Criteria for Certification of Interruption of Transmission/Elimination of Human Onchocerciasis. Geneva: World Health Organization; 2001. WHO report WHO/CDS/CPE/CEE/2001.18a. [Google Scholar]
- 14.Steel RGD, Torrie JH, Dickey DA. Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw-Hill; 1997. [Google Scholar]
- 15.Ndyomugyenyi R, Lakwo T, Habomugisha P, Male B. Progress towards the elimination of onchocerciasis as a public-health problem in Uganda: opportunities, challenges and the way forward. Ann Trop Med Parasitol. 2007;101:323–333. doi: 10.1179/136485907X176355. [DOI] [PubMed] [Google Scholar]
- 16.Kruger A, Nurmi V, Yocha J, Kipp W, Rubaale T, Garms R. The Simulium damnosum complex in western Uganda and its role as a vector of Onchocerca volvulus. Trop Med Int Health. 1999;4:819–826. doi: 10.1046/j.1365-3156.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 17.Lakwo TL. Preliminary studies on the biting activity and transmission of Onchocerca volvulus by Simulium neavei (Diptera: Simuliidae) in Kashoya-Kitomi focus. Western Uganda. East Afr. Med. J. 2004;81:244–247. doi: 10.4314/eamj.v81i5.9167. [DOI] [PubMed] [Google Scholar]
- 18.Brown AW. A survey of Simulium control in Africa. Bull World Health Organ. 1962;27:511–527. [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh JF. Review of vector control prior to the OCP. Acta Leiden. 1990;59:61–78. [PubMed] [Google Scholar]
- 20.Ndyomugyenyi R, Tukesiga E, Buttner DW, Garms R. The impact of ivermectin treatment alone and when in parallel with Simulium neavei elimination on onchocerciasis in Uganda. Trop Med Int Health. 2004;9:882–886. doi: 10.1111/j.1365-3156.2004.01283.x. [DOI] [PubMed] [Google Scholar]
- 21.Garms R, Lakwo TL, Ndyomugyenyi R, Kipp W, Rubaale T, Tukesiga E, Katamanywa J, Post RJ, Amazigo UV. The elimination of the vector Simulium neavei from the Itwara onchocerciasis focus in Uganda by ground larviciding. Acta Trop. 2009;111:203–210. doi: 10.1016/j.actatropica.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Lakwo TL, Garms R, Rubaale T, Katabarwa M, Walsh F, Habomugisha P, Oguttu D, Unnasch T, Namanya H, Tukesiga E, Katamanywa J, Bamuhiiga J, Byamukama E, Agunyo S, Richards F. The disappearance of onchocerciasis from the Itwara focus, western Uganda after elimination of the vector Simulium neavei and 19 years of annual ivermectin treatments. Acta Trop. 2013;126:218–221. doi: 10.1016/j.actatropica.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH, Dominguez A, Cruz-Ortiz N, Unnasch TR, Punkosdy GA, Richards J, Sauerbrey M, Castro J, Catu E, Oliva O, Richards FO., Jr Elimination of Onchocerca volvulus transmission in the Santa Rosa focus of Guatemala. Am J Trop Med Hyg. 2007;77:334–341. [PubMed] [Google Scholar]
- 24.Gonzalez RJ, Cruz-Ortiz N, Rizzo N, Richards J, Zea-Flores G, Dominguez A, Sauerbrey M, Catu E, Oliva O, Richards FO, Lindblade KA. Successful interruption of transmission of Onchocerca volvulus in the Escuintla-Guatemala focus, Guatemala. PLoS Negl Trop Dis. 2009;3:e404. doi: 10.1371/journal.pntd.0000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katholi C. Poolscreen v2.0. 2010. http://www.soph.uab.edu/bst/poolscreen Available at. Accessed June 14, 2012.
- 26.Hajek J. Limiting distributions in simple random sampling from a finite population. Publ. Math. Inst. Hung. Acad. 1960;5:361–374. [Google Scholar]
- 27.Lehmann EL. Nonparametrics: Statistical Methods Based on Ranks. Oakland, CA: Holden-Day; 1975. [Google Scholar]
- 28.Agresti A, Coull BA. Approximation is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 29.Wilson EB. Probabilistic inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- 30.Katholi CR, Toe L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis. 1995;172:1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- 31.Gu W, Unnasch TR, Katholi CR, Lampman R, Novak RJ. Fundamental issues in mosquito surveillance for arboviral transmission. Trans R Soc Trop Med Hyg. 2008;102:817–822. doi: 10.1016/j.trstmh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Pérez MA, Lilley BG, Domínguez-Vázquez A, Segura-Arenas R, Lizarazo-Ortega C, Mendoza-Herrera A, Reyes-Villanueva F, Unnasch TR. Polymerase chain reaction monitoring of transmission of Onchocerca volvulus in two endemic states in Mexico. Am J Trop Med Hyg. 2004;70:38–45. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.