Abstract
Sarcocystis nesbitti is an intracellular protozoan parasite found as sarcocysts within muscle fibers of intermediate hosts (monkey and baboon). The definitive host is suspected to be the snake. We report two cases from a larger cohort of 89 patients who had fever, headache, and generalized myalgia after a trip to Pangkor Island, Malaysia. Sarcocysts were detected in skeletal muscle biopsy specimens by light and electron microscopy from these two patients. DNA sequencing based on the 18S ribosomal DNA region identified the Sarcocystis species as S. nesbitti. We also identified S. nesbitti sequences in the stools of a snake (Naja naja). Phylogenetic analysis showed that these sequences form a cluster with most of the other known Sarcocystis species for which the snake is a definitive host. We believe these two patients were likely to have symptomatic acute muscular sarcocystosis after S. nesbitti infection that may have originated from snakes.
Sarcocystis species are an intracellular protozoan with a life cycle based on a prey–predator relationship in which various animal species serve as either intermediate or definitive hosts, respectively. Asexual development occurs in the intermediate host after ingestion of sporocysts produced by the definitive host and excreted in the feces. Ingestion of sarcocysts found in the muscles of the intermediate host by the predator definitive host completes the life cycle.1 Humans serve as the definitive hosts for Sarcocystis hominis and S. suihominis after eating improperly cooked, sarcocyst-infected meat from intermediate hosts (cattle and pigs, respectively). Furthermore, humans can also serve as accidental intermediate hosts for some Sarcocystis species of hitherto unknown origin. In these cases, it is assumed that humans became infected through food or drinks contaminated by sporocysts, resulting in skeletal muscle sarcocystosis. Human sarcocystosis is reported to be widely distributed.1
A better understanding of the natural life cycle, including identity of the definitive hosts, is crucial to prevent zoonotic parasitic infections such as human muscular sarcocystosis. Previous light microscopic and ultrastructural studies of the human sarcocyst have suggested that there is some similarity to sarcocysts found in Macaca fascicularis (Malaysian long-tailed macaque).2–4 More recently, nucleotide sequences of the 18S ribosomal DNA (18S rDNA) have been widely used for Sarcocystis species identification in cattle, bison, and water buffalo.5,6 Using this method, Tian and others showed that S. nesbitti found in M. fascicularis shares the closest genetic identity with Sarcocystis species in snakes, suggesting that the snake may be a definitive host for S. nesbitti.7
To date, there are only limited studies on human Sarcocystis spp. infection in Malaysia. A study found antibodies against Sarcocystis species in 20% of Malaysians.8 Titers were highest among the Orang Asli ethnic group (aboriginals), followed by the Malay, Indian, and Chinese ethnic groups, possibly reflecting food habits and environmental sanitation levels. A histopathologic study of tongue skeletal muscle found 21 of a series of 100 autopsy cases from a local urban population to be positive for sarcocysts.9 In 1999, an outbreak of eosinophilic myositis attributed to skeletal muscle Sarcocystis spp. infection was reported and involved 7 of 15 U.S. military personnel who had operated in rural Malaysia.10 More recently, outbreaks of symptomatic acute muscular sarcocystosis in foreign travelers to Tioman Island, off the east coast of peninsular Malaysia were reported.11,12
We report two symptomatic cases of acute muscular sarcocytosis in which S. nesbitti has been identified. These two patients were from a larger cohort of 89 adults who went for a holiday in Pangkor Island, off the west coast of peninsular Malaysia, and showed development of fever, headache, and generalized myalgia approximately 10 days after the trip. Fever and myalgia were also reported in recent cases of suspected acute muscular sarcocystosis from Tioman Island, Malaysia.12
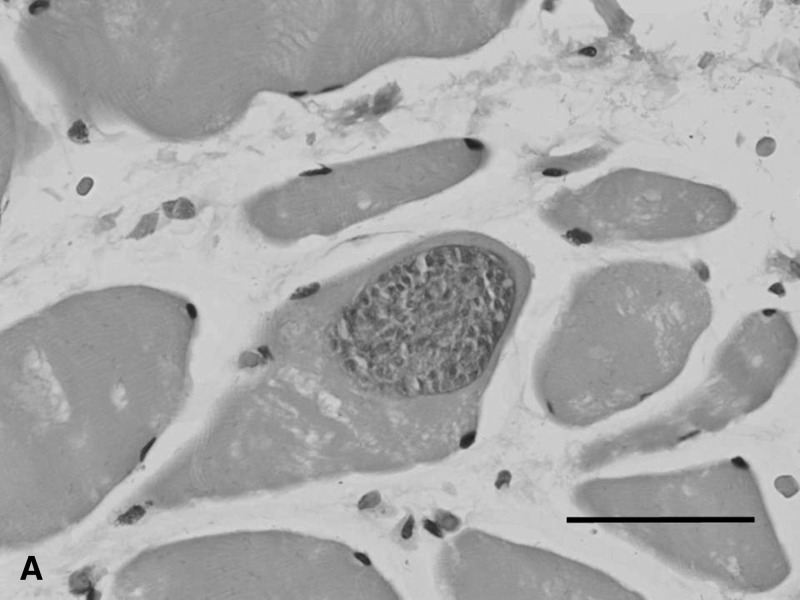
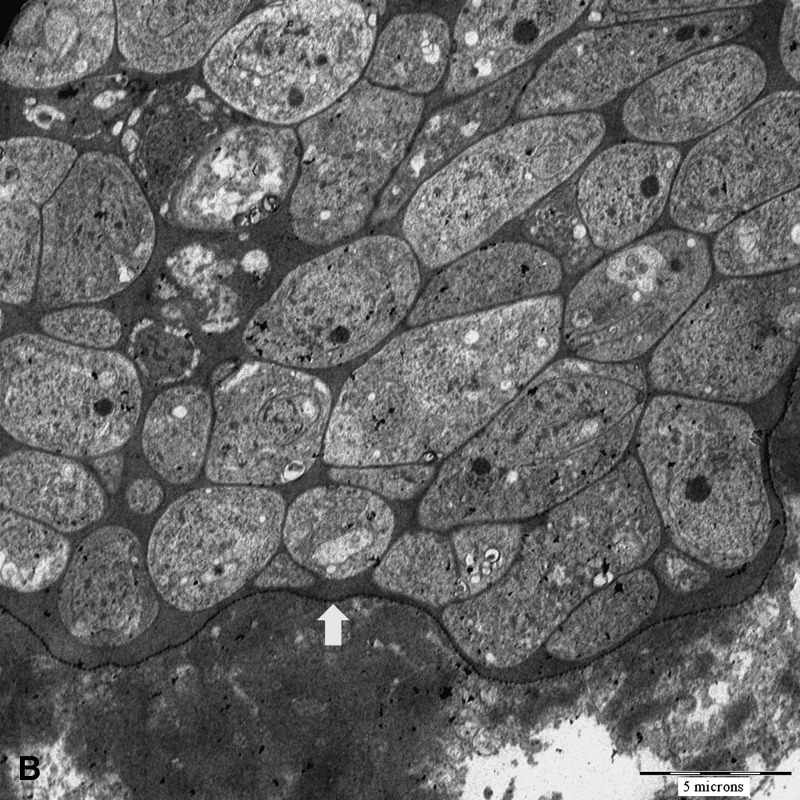
Blood investigations for the first patient (MAL-1) approximately two weeks after symptoms onset showed a transient increase in liver enzyme levels, but the eosinophil count and creatine kinase level were normal. For the second patient (MAL-3), blood investigations after approximately two weeks of symptoms showed normal liver enzyme levels, eosinophil count, and creatine kinase levels. The creatine kinase level was increased (1,391 IU/L, reference range = 22–198 IU/L) at approximately week 10 of symptoms. Subsequently, in the first patient, a muscle biopsy of a swollen jaw muscles was performed at week four of symptoms. In the second patient, a leg muscle biopsy was performed at week 12. Magnetic resonance imaging findings for both types of muscle showed changes consistent with myositis. Both biopsies showed intramuscular sarcocysts (Figure 1A), and mild-to-moderate inflammation. Electron microscopy of formalin-fixed tissues confirmed that the sarcocyst consisted of mainly closely-packed metrocytes surrounded by a thin cyst wall (Figure 1B).
Figure 1.
A, Light microscopy of an intramuscular sarcocyst found in the temporalis muscle (hematoxylin and eosin stained, magnification ×40 objective, scale bar = 50 μm). B, Electron micrograph of a sarcocyst showing numerous metrocytes within a thin, electron dense cyst wall with no prominent protrusions (arrow) (magnification ×513, scale bar = 5 μm).
We analyzed 18S rDNA from sarcocysts found in biopsy specimens and confirmed their identity as S. nesbitti in both cases. DNA was extracted by using the DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA). The 18S rDNA gene was amplified by nested polymerase chain reaction (PCR) and primer 1L (5′-CCATGCATGTCTAAGTATAAGC-3′) and primer 1H (5′-TATCCCCATCACGATGCATAC-3′) in the primary reaction, followed by primer 3L (5′-CTAGTGATTGGAATGATGGG-3′) and primer 2H (5′-ACCTGTTATTGCCTCAAACTTC-3′) in the secondary reaction.5 Four microliters of DNA template was used in a 25-μL PCR sample and the following reaction conditions: 35 mM Tris-HCl, pH 9.0, 25 mM KCl, 3.5 mM MgCl2, 5 pmoles of each primer, 1 mM dNTPs, and 1 unit of polymerase. The PCR was performed as follows: 95°C for 2 minutes; followed by 35 cycles at 94°C for 40 seconds, 50°C for 30 seconds, and 72°C for 1.5 minutes; followed by 72°C for 6 minutes.
The product was separated by agarose gel electrophoresis, and the nucleotide sequence of the amplified fragment was analyzed and compared with 18S rDNA sequences of Sarcocystis species available in GenBank. Multiple sequence alignment was conducted by using ClustalW (www.clustal.org/), and phylogenetic analysis was conducted by using the maximum-parsimony method in MEGA4.13 Amplified 18S rDNA gene was approximately 1,000 basepairs, and aligned sequences of this fragment were 962 basepairs (GenBank accession no. 1554238). The S. nesbitti 18S rDNA found in these 2 patients (denoted as S. nesbitti MAL-1 and MAL-3) showed a variation of 1%, which is likely caused by intraspecific variation, an apparently common feature within the Sarcocystis species.5 A BLAST result indicated that these sequences share 99% homology with S. nesbitti found in the muscle of M. fascicularis from Yunnan Province, China.7
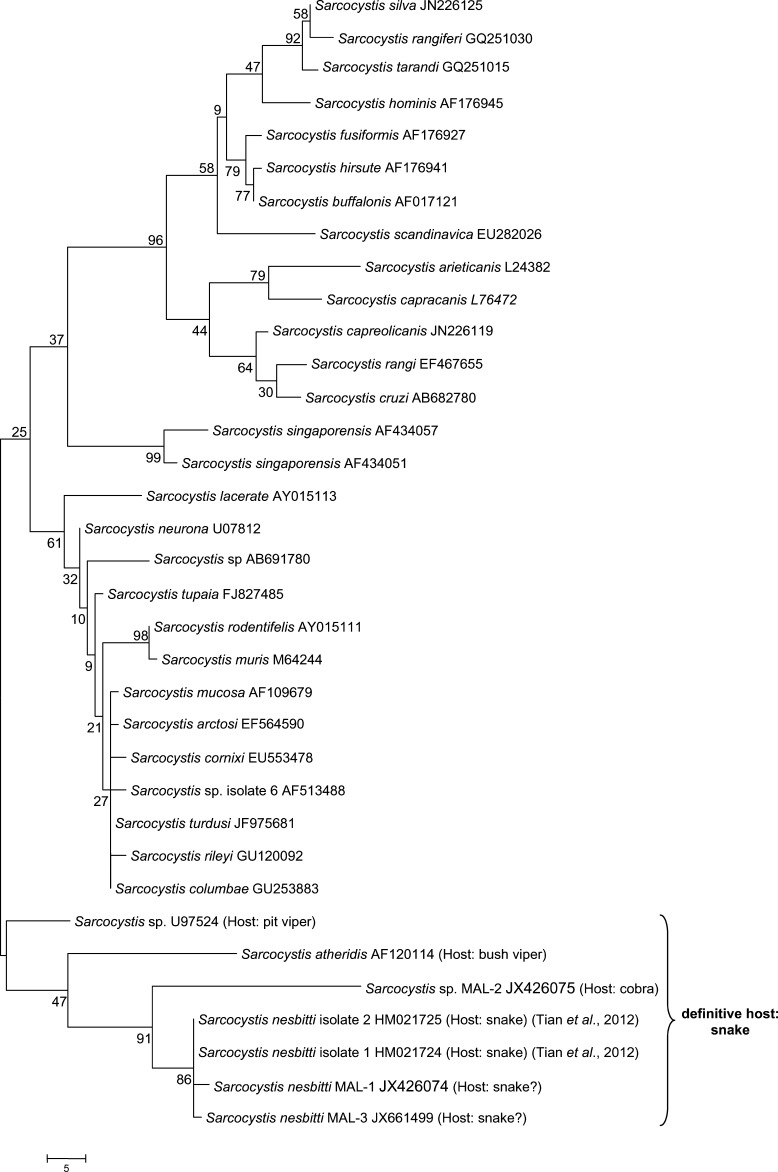
To investigate the possibility that snakes may be a definitive host for S. nesbitti,7 snake feces were collected from several locations in Malaysia, including Pangkor Island. Exhaustive efforts were made to look for oocysts and/or sporocysts in these feces, including using the fecal concentration method, but none were found. The PCR evidence of S. nesbitti (denoted as Sarcocystis sp. MAL-2) was found in feces of a cobra (Naja naja) collected in Kelantan, a state on the east coast of peninsular Malaysia. A phylogenetic tree based on 18S rDNA sequences distinctly shows that S. nesbitti MAL-1 and MAL-3 form a cluster with Sarcocystis sp. MAL-2, S. nesbitti isolates 1 and 2 found in monkey,7 and other Sarcocystis species such as S. atheridis and Sarcocystis sp. U97524 for which snakes are definitive hosts (Figure 2). Not surprisingly, S. nesbitti MAL-1and MAL-3 are only distantly related to S. hominis because Sarcocystis species with different definitive hosts probably have evolutionarily diverged from each other.
Figure 2.
Phylogenetic tree based on 18S ribosomal DNA sequences of Sarcocystis species. Tree was constructed by using the maximum-parsimony method. The percentage of replicate trees in which the associated isolates cluster together in the bootstrap test (1,000 replicates) are shown next to the branches. Phylogenetic analysis was conducted using MEGA4.13 GenBank accession numbers are given after the species name. Sale bar indicates nucleotide substitutions per site.
The two patients with acute muscular sarcocystosis reported are assumed to be accidental intermediate hosts of S. nesbitti because humans have no known predators. We believe that the acute symptoms were likely caused by recent S. nesbitti infection because there were no other causes identified. Nevertheless, the sarcocysts could have originated from a previous infection. Like M. fascicularis, our patients may have been infected after consuming sporocysts excreted from a predator such a snake,14,15 possibly via contaminated water or food. Further investigations are needed to confirm this possibility.
Footnotes
Financial support: This study was supported by UM High Impact Research Grant UM-MOHE (UM.C/HIR/MOHE/MED/18) from the Ministry of Higher Education Malaysia, UMRG (grant no. RP011-2012) from University of Malaya and UM High Impact Research Grant UM.C/625/1/HIR/MOHE/MED-06.
Authors' addresses: Yee Ling Lau, Phooi Yee Chang, Chong Tin Tan, Mun Yik Fong, Rohela Mahmud, and Kum Thong Wong, Department of Parasitology, Department of Medicine, and Department of Pathology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: lauyeeling@um.edu.my, phooiyee@gmail.com, chongtin.tan@gmail.com, fongmy@um.edu.my, rohela@um.edu.my, and wongkt@ummc.edu.my.
References
- 1.Fayer R. Sarcocystis spp. in human infections. Clin Microbiol Rev. 2004;17:894–902. doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kan SP, Prathap K, Dissanaike AS. Light and electron microstructure of a Sarcocystis sp. from the Malaysian long-tailed monkey, Macaca fascicularis. Am J Trop Med Hyg. 1979;28:634–642. [PubMed] [Google Scholar]
- 3.Wong K, Clarke G, Pathmanathan R, Hamilton P. Light microscopic and three-dimensional morphology of the human muscular sarcocyst. Parasitol Res. 1994;80:138–140. doi: 10.1007/BF00933781. [DOI] [PubMed] [Google Scholar]
- 4.Wong KT, Pathmanathan R. Ultrastructure of the human skeletal muscle sarcocyst. J Parasitol. 1994;80:327–330. [PubMed] [Google Scholar]
- 5.Fischer S, Odening K. Characterization of bovine Sarcocystis species by analysis of their 18S ribosomal DNA sequences. J Parasitol. 1998;84:50–54. [PubMed] [Google Scholar]
- 6.Yang ZQ, Zuo YX, Ding B, Chen XW, Luo J, Zhang YP. Identification of Sarcocystis hominis-like (Protozoa: Sarcocystidae) cyst in water buffalo (Bubalus bubalis) based on 18S rRNA gene sequences. J Parasitol. 2001;87:934–937. doi: 10.1645/0022-3395(2001)087[0934:IOSHLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Tian M, Chen Y, Wu L, Rosenthal BM, Liu X, He Y, Dunams DB, Cui L, Yang Z. Phylogenetic analysis of Sarcocystis nesbitti (Coccidia: Sarcocystidae) suggests a snake as its probable definitive host. Vet Parasitol. 2012;183:373–376. doi: 10.1016/j.vetpar.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 8.Thomas V, Dissanaike AS. Antibodies to Sarcocystis in Malaysians. Trans R Soc Trop Med Hyg. 1978;72:303–306. doi: 10.1016/0035-9203(78)90211-0. [DOI] [PubMed] [Google Scholar]
- 9.Wong KT, Pathmanathan R. High prevalence of human skeletal muscle sarcocystosis in south-east Asia. Trans R Soc Trop Med Hyg. 1992;86:631–632. doi: 10.1016/0035-9203(92)90161-5. [DOI] [PubMed] [Google Scholar]
- 10.Arness MK, Brown JD, Dubey JP, Neafie RC, Granstrom DE. An outbreak of acute eosinophilic myositis attributed to human Sarcocystis parasitism. Am J Trop Med Hyg. 1999;61:548–553. doi: 10.4269/ajtmh.1999.61.548. [DOI] [PubMed] [Google Scholar]
- 11.Husna Maizura AM, Khebir V, Chong CK, Azman Shah AM, Azri A, Lokman Hakim S. Surveillance for sarcocystosis in Tioman Island, Malaysia. Malaysian J of Public Health Med. 2012;12:39–44. [Google Scholar]
- 12.Centers for Disease Control and Prevention . Notes from the Field: Acute Muscular Sarcocystosis Among Returning Travelers—Tioman Island, Malaysia, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2012. [PubMed] [Google Scholar]
- 13.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 14.Pathmanathan R, Kan S. Human Sarcocystis infection in Malaysia. Southeast Asian J Public Health Trop Med. 1981;12:247–250. [Google Scholar]
- 15.Beaver PC, Gadgil K, Morera P. Sarcocystis in man: a review and report of five cases. Am J Trop Med Hyg. 1979;28:819–844. [PubMed] [Google Scholar]