Abstract
In malaria-endemic regions any febrile case is likely to be classified as malaria based on presumptive diagnosis largely caused by a lack of diagnostic resources. A district-wide prevalence study assessing etiologies of fever in 659 patients recruited in rural and semi-urban areas of Bandarban district in southeastern Bangladesh revealed high proportions of seropositivity for selected infectious diseases (leptospirosis, typhoid fever) potentially being misdiagnosed as malaria because of similarities in the clinical presentation. In an area with point prevalences of more than 40% for malaria among fever cases, even higher seroprevalence rates of leptospirosis and typhoid fever provide evidence of a major persistent reservoir of these pathogens.
Introduction
The Chittagong Hill Tracts of Bangladesh (CHTs) are situated toward the border to India and Myanmar and constitute a geographically secluded, forested region with a total population of more than 1.5 million distributed over 14 different ethnical communities.1 Malaria is considered a major health threat in this area and most of the 55,000 laboratory-confirmed malaria cases reported in Bangladesh in 2010 originate from this endemic region.2 The total number including unreported and clinically diagnosed malaria cases in the entire country is estimated around 1.2 million cases per year with Plasmodium falciparum as the predominant species (70% of all cases).2 Because of high incidence rates of malaria, non-malarial febrile illnesses such as leptospirosis, typhoid fever, or dengue are often misdiagnosed as malaria. Epidemiological data on the etiology of febrile diseases in Bangladesh are scarce. Misdiagnosis is therefore considered a major obstacle for the adequate management of malaria and other diseases within its differential diagnosis.
Recent studies in other parts of Bangladesh and Thailand indicate high incidence rates of leptospirosis in the region.3–6 Comparable proportions of typhoid fever have been reported earlier from urban slums in Bangladesh with the highest rates among children < 5 years of age.7,8 Throughout the last century sporadic cases of dengue fever have been reported from major cities of Bangladesh, but since the year 2000 several dengue outbreaks with up to 6,000 clinical cases and a case-fatality rate around 1.6% occurred.9–11 Rickettsial diseases have been reported as being an emerging problem in the subcontinent but the epidemiology remain poorly understood as a result of challenges associated with diagnosis of this complex group of organisms.12
In light of these findings, we investigated the seroprevalence of leptospirosis, typhoid fever, and dengue and their rate of co-infections with malaria among fever cases recruited in malaria-endemic rural and semi-urban areas of the CHTs in Bangladesh.
Materials and Methods
We assessed seroprevalence rates of leptospirosis, typhoid fever, and dengue among febrile patients in a community and a hospital survey between 2007 and 2010. The community survey was performed in the framework of a large-scale cross-sectional, household survey to assess malaria prevalence in Bandarban district, CHTs, in the Southeast of Bangladesh. Details of the cross-sectional study will be reported elsewhere. In brief, during the rainy season of 2007 all seven sub-districts of Bandarban district were surveyed. Through probability proportional to size sampling a total of three villages/communities per sub-district were randomly selected resulting in a total of 21 villages. Nine villages in three of the seven sub-districts were revisited in the following dry season from December 2007 to February 2008. Community sensitization in selected villages was performed by a local field worker 24–48 hours before arrival of the study team. Free diagnosis and treatment was provided to all participants and patients and severe conditions were referred to the closest medical facility. Inhabitants of the visited villages suffering from an episode of fever were encouraged to participate in the ongoing survey. Males and females of any age with fever defined as axillary temperature > 37.5°C, or history of fever within the past 72 hours were eligible.
The hospital survey was conducted throughout the year at the Bandarban Sadar hospital/MARIB (Malaria Research Initiative Bandarban) outpatient department between 2008 and 2010. Patients were self- referred and inclusion criteria were the same as previously mentioned. A questionnaire capturing demographic data, previous malaria infections, recent treatment of the disease, current signs and symptoms, and travel history was completed for all subjects and a physical examination was performed. From each participant one heel or finger prick sample was obtained to perform instant malaria diagnosis by rapid diagnostic test (RDT) and preparation of a microscopic slide. Two drops of blood (2 × 100 mL) were collected on filter paper (903; Schleicher & Schuell BioScience GmbH, Dassel, Germany) for a later confirmation of the diagnosis by polymerase chain reaction (PCR). Venous blood samples were drawn from all individuals 8 years of age or older for serologic assays. Serum was immediately separated by centrifugation and stored at −20°C until further processing. At the central laboratory in Bandarban (MARIB), samples were tested for the presence of Immunoglobulin M (IgM) antibodies (Abs) to assess recent exposure for leptospirosis, dengue, and typhoid fever.
Leptospirosis.
The IgM-Abs against leptospirosis in patients sera were detected using commercial enzyme-linked immunosorbent assay (ELISA) kits (E-LEP01M, Panbio Limited, Queensland, Australia). Three control sera included in the kit were treated in a similar way as the test samples, to determine the cut off concentration. As recommended by the manufacturer, all readings were divided by the cut-off value and then classified as seronegative if the value was > 0.9, considered positive if < 1.1, and retested if a value between these two values was calculated.
Typhoid fever.
Presence of anti-Salmonella O9 IgM in collected sera was detected by a semi-quantitative rapid in vitro assay (TUBEX, IDL Biotech AB, Bomma, Sweden). In the presence of anti-Salmonella O9 IgM, a reaction between antigen-coated and Ab-coated magnetic microglobuli is inhibited in a suspension of microglobuli, serum, and solution. Applying a magnetic field results in a color change of the suspension, this is then compared with a color scale ranging from 1 to 10. Based on previously published data the cut-off value was set to > 5 to improve specificity.13,14
Dengue.
Seropositivity for dengue was tested with a capture-ELISA (E-DEN01M, Panbio Limited, Queensland, Australia) detecting the presence of IgM-Ab. Similar calculations as for the leptospirosis-ELISA were performed.
Malaria.
All participants were instantly screened for the presence of P. falciparum (HRP2) and Plasmodium vivax antigens (pLDH) by RDT (Falcivax, Zephyr Biomedicals, Goa, India) and later confirmed microscopically in a Giemsa (Merk KGaA, Darmstadt, Germany) stained thick and thin film. If results between RDT and microscopy were discordant, an additional reading obtained by a second expert microscopist was considered final. For the statistical analysis only results from microscopy were considered. Filter paper samples were analyzed at the laboratories of the Medical University of Vienna for PCR confirmation of the malaria diagnosis and results were reported previously.15,16
Statistical analysis.
Data were entered into a customized MS-Excel (Microsoft Corp., Reston, VA) database and analyzed using the OpenEpi statistical calculator (http://www.openepi.com). Proportions of test positivity for malaria, typhoid, and the remaining samples (test positive for leptospirosis, dengue, or fever not specified) and clinical categorical variables were compared using Yates corrected Pearson's χ2 (Chi-square) and Fisher's Exact test. Comparisons of continuous data were made using Student's t test at a significance level of P < 0.05.
Ethics.
The study was approved by the ethics committee of the ICDDR,B (International Center of Diarrheal Disease Research, Bangladesh) and written informed consent was obtained from all participants or their legal representatives prior to sampling.
Results
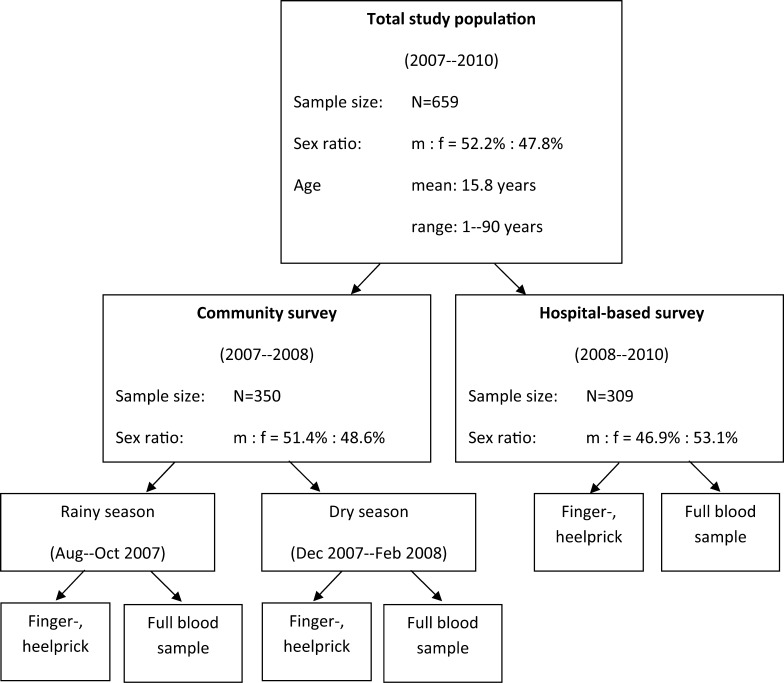
A total of 659 febrile individuals were enrolled and 506 (76.8%) blood and serum samples for serological analysis were collected from participants 8 years of age or older. From 105 patients < 8 years of age only finger/heel prick samples were collected. Approximately half of the patients were enrolled in the hospital survey (N = 309) and the other half in the community survey (N = 350), whereby 280 patients were enrolled in the first part of the community survey during the rainy season (7 sub-districts visited) and 70 in the second part (3 sub-districts visited) in the dry season. Figure 1 shows the flow chart for the enrollments during community (rainy and dry season) and hospital-based survey.
Figure 1.
Flow chart for enrollment and sampling including demographic characteristics for community and hospital survey.
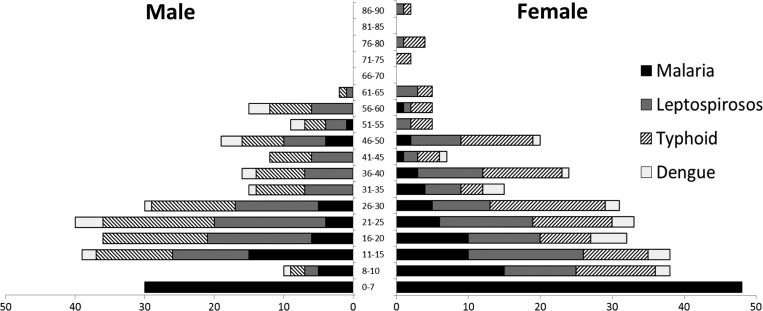
The study population was well balanced regarding gender and age with a high number of fever patients at a young age, as shown in Figure 2. All patients were screened for malaria and among the participants 8 years of age or older 500 patients (98.8%) could be tested for antibodies against dengue, 488 (96.4%) for typhoid fever, and 455 (89.9%) for leptospirosis.
Figure 2.
Test positivity of malaria, leptospirosis, typhoid fever, and dengue by age group and gender. In the age group 0–7 years only malaria diagnosis was performed.
We found 37.3%, 69.8%, and 39.9% of the samples positive for anti-Salmonella O9 IgM in the community survey during the rainy and dry season and in the hospital survey, respectively. Similarly, we found 42.0%, 40.0%, and 44.4% positive for IgM against leptospirosis. The IgM against dengue was found in the community survey only during the rainy season in 1.2% and in the hospital survey in 13.4% of the patients' sera (Table 1). In total 171 (25.9%) patients of all age groups were positive for malaria of which 126 (73.7%) were diagnosed as P. falciparum, 34 (19.9%) were P. vivax, 6 (3.5%) were mixed infection of P. falciparum and P. vivax, and 2 (1.2%) individuals were positive for Plasmodium malariae (Table 1).
Table 1.
Test positivity for malaria, typhoid fever, leptospirosis, and dengue in the rainy and dry season and among individuals enrolled in the hospital-based fever survey
| Community survey | P value* | Hospital survey | ||
|---|---|---|---|---|
| Rainy season† | Dry season‡ | |||
| Fever cases | 280 | 70 | 309 | |
| Test positive (%) | ||||
| P. falciparum | 95 (33.9%) | 12 (17.1%) | 0.01 | 19 (6.2%) |
| P. vivax | 15 (5.4%) | 2 (2.9%) | 0.58 | 18 (5.8%) |
| P. falciparum + P. vivax | 4 (1.4%) | 1 (1.4%) | 0.67 | 1 (0.3%) |
| P. malariae | 0 (0%) | 0 (0%) | NA | 2 (0.7%) |
| TUBEX | 63 (37.3%) | 30 (69.8%) | 0.00 | 110 (39.9%) |
| Leptospirosis IgM | 68 (42.0%) | 18 (40%) | 0.88 | 126 (44.4%) |
| Dengue IgM | 2 (1.2%) | 0 (0%) | NA | 38 (13.4%) |
Significance of difference between rainy and dry season using χ2 or Fisher's exact test as appropriate; level of significance > 0.05.
Seven sub-districts (21 villages) surveyed.
Three sub-districts (9 villages) surveyed.
A total of 201 (30.5%) patients were negative for malaria parasites and seronegative for dengue, typhoid, and leptospirosis and were summarized as fever not specified.
A significant reduction (P = 0.002) of malaria cases was observed between the rainy season (114 positive cases, 40.7%) and dry season (15 positive cases, 21.4%). The proportion of leptospirosis was stable between the rainy and dry season, whereas typhoid fever showed a significant increase (P < 0.001) in the dry season compared with the monsoon months.
Most fever cases were single test positive. However, 108 (16.4%) participants 8 years of age or older were found to have two positive test results and 21(3.2%) were triple positive (Table 2).
Table 2.
Double and triple positive cases with corresponding axillary temperature
| Test positivity | Positive cases* N (%) | Temperature °C mean (range) |
|---|---|---|
| Double positive (108) | ||
| malaria + leptospirosis | 16 (3.2%) | 37.1 (35.8–42.0) |
| malaria + typhoid fever | 17 (3.4%) | 37.1 (35.9–39.6) |
| malaria + dengue | 5 (1.0%) | 37.4 (36.5–38.5) |
| leptospirosis + typhoid fever | 56 (11.1%) | 36.9 (35.0–38.9) |
| leptospirosis + dengue | 3 (0.6%) | 37.4 (36.4–39.5) |
| dengue + typhoid fever | 11 (2.2%) | 37.2 (34.8–39.2) |
| Triple positive (21) | ||
| malaria + leptospirosis + dengue | 13 (2.6%) | 36.4 (36.1–39.5) |
| malaria + leptospirosis + typhoid fever | 2 (0.4%) | 37.7 (36.7–39.5) |
| malaria +typhoid fever + dengue | 1 (0.2%) | 38.8 |
| leptospirosis + typhoid fever + dengue | 5 (1.0%) | 37.9 (36.2–39.3) |
Individuals < 8 years of age are not included in this analysis.
Clinical signs and symptoms.
Patients with any of the described seroprevalences showed rather unspecific clinical signs. Main symptoms included fatigue, fever, dizziness, and headache. Patients' positive for anti-Salmonella O9 IgM were significantly older and presented with lower body temperature than the rest of the cohort (P < 0.05). In general, malaria-positive children < 8 years of age had more severe symptoms (headache, myalgia, and dizziness) and a greater probability of vomiting than older malaria patients. The only significant clinical predictor for malaria was spleen enlargement (Table 3). No significant difference in the clinical presentation between single, double, and triple (sero-) positive cases was found.
Table 3.
Demographics and clinical signs and symptoms by mutual exclusive test positivity groups for malaria (children versus adults), typhoid fever and “others” (test positive for leptospirosis, dengue, or fever not specified)
| Group 1 malaria | Group 2 malaria | Group 3 typhoid | Group 4 others* | P value* | ||||
|---|---|---|---|---|---|---|---|---|
| < 8 years (N = 78) | ≥ 8 years (N = 39) | ≥ 8 years (N = 100) | ≥ 8 years (N = 313) | Group 1 vs. 2 | Group 2 vs. 3 | Group 2 vs. 4 | Group 3 vs. 4 | |
| Mean age (years) | 3.2 | 18.9 | 30.3 | 12.2 | NA | 0.00 | 0.03 | 0.00 |
| Male (%) | 48 (61.5) | 25 (64.1) | 49 (49.0) | 158 (50.5) | 0.95 | 0.16 | 0.15 | 0.89 |
| Chills (%) | 30 (38.5) | 21 (53.8) | 47 (47.0) | 190 (60.7) | 0.17 | 0.69 | 0.52 | 0.02 |
| Headache (%) | 37 (47.4) | 33 (84.6) | 84 (84.0) | 243 (77.6) | 0.00 | 0.87 | 0.43 | 0.22 |
| Myalgia (%) | 25 (32.1) | 24 (61.5) | 73 (73.0) | 184 (58.8) | 0.00 | 0.27 | 0.88 | 0.01 |
| Arthralgia (%) | 11 (14.1) | 11 (28.2) | 49 (49.0) | 109 (34.8) | 0.11 | 0.04 | 0.52 | 0.02 |
| Dizziness (%) | 35 (44.9) | 27 (69.2) | 73 (73.0) | 222 (70.9) | 0.02 | 0.81 | 0.97 | 0.79 |
| Fatigue (%) | 72 (92.3) | 38 (97.4) | 99 (99.0) | 297 (94.9) | 0.49 | 0.92 | 0.76 | 0.13 |
| Skin rash (%) | 1 (1.3) | 1 (2.6) | 1 (1.0) | 13 (4.2) | 0.80 | 0.92 | 0.96 | 0.23 |
| Abd. pain (%) | 36 (46.2) | 20 (51.3) | 50 (50.0) | 140 (44.7) | 0.74 | 0.96 | 0.55 | 0.42 |
| Nausea (%) | 31 (39.7) | 11 (28.2) | 41 (41.0) | 121 (38.7) | 0.31 | 0.23 | 0.27 | 0.76 |
| Vomiting (%) | 25 (32.1) | 5 (12.8) | 17 (17.0) | 61 (19.5) | 0.04 | 0.73 | 0.43 | 0.68 |
| Diarrhea (%) | 8 (10.3) | 5 (12.8) | 9 (9.0) | 22 (7.0) | 0.91 | 0.71 | 0.34 | 0.66 |
| Resp. sympt. (%) | 46 (59.0) | 18 (46.2) | 64 (64.0) | 228 (72.8) | 0.26 | 0.08 | 0.00 | 0.1 |
| Mean Temp† | 38.0 | 37.5 | 36.8 | 37.2 | 0.06 | 0.00 | 0.12 | 0.00 |
| Days of fever | 4.5 | 4.2 | 5.6 | 4.8 | 0.82 | 0.62 | 0.42 | 0.66 |
| Spleen‡ (%) | 40 (51.3) | 6 (15.4) | 2 (2.0) | 17 (5.4) | 0.00 | 0.01 | 0.04 | 0.25 |
| Liver§ (%) | 6 (7.7) | 1 (2.6) | 1 (1.0) | 7 (2.2) | 0.49 | 0.92 | 0.90 | 0.72 |
Note: Abd. pain = Abdominal pain; Resp. sympt. = Respiratory symptoms (including cough, running nose, and sore throat); Temp. = Temperature in degree Celsius. Double and triple test-positive cases (108 cases and 21 cases, respectively) were excluded from this analysis.
Using χ2 or student's t test (continuous data).
Axillary measurement in degree Celsius.
Palpable spleen enlargement.
Palpable liver enlargement.
Discussion
Bandarban is known to be one of the 13 malaria-endemic districts in Bangladesh, but there is a lack of data on the prevalence and importance of other communicable diseases with similar clinical presentation. The aim of this study was to provide greater knowledge of the distribution of selected pathogens in the surveyed area to improve awareness of diseases within the differential diagnosis of malaria. The well-matched ratio of women to men within the study population and among the test-positive fractions suggests similar relative risk for infection with any of the investigated pathogens, regardless of gender and possibly occupation. The high number of fever cases among children may be attributed to a generally increased susceptibility and vulnerability at this age, but also to the population structure in the CHTs featuring a high proportion of young individuals, as it is typical for developing countries.17 Malaria prevalence rates in this study were similar to previous reports from this area but even higher seroprevalence rates were found for leptospirosis and typhoid fever, providing evidence of a major persistent reservoir of these pathogens in a region, where malaria is considered to be the main health threat.18 In contrast to recent reports from hospital-based fever surveys indicating similar prevalence rates for dengue in urban and rural populations in different districts of Bangladesh, only a few seropositive cases of dengue were identified in the course of this study.19 An explanation for these findings might be that dengue is a largely urban problem in Bangladesh, whereas the rural population of Bandarban district is rather stable with very little migration background from other parts of the county or from abroad. Recent data on occurrence and frequency of mosquitoes as vectors in the CHT remain scarce and are limited to Anopheles species and malaria transmission20; because migration and urbanization is likely to increase over the next decades, patterns of transmission might change, potentially leading to rising numbers of dengue cases in this area in the future.21–23
High seroprevalence rates for the pathogens tested in this study have been reported from other parts of South and Southeast Asia. Similar high seroprevalence rates have been reported from western Thailand and Vietnam and diseases like leptospirosis are likely to be major contributors to the spectrum of febrile illnesses in the region.24–26 In neighboring India they contribute to the vast challenge of infectious diseases and are likely to be underreported.27 Typhoid fever remains an important public health problem and a major cause of morbidity and mortality not just in Asia but throughout the developing world.28
The seropositivity for leptospirosis was stable throughout the year, whereas malaria and typhoid fever showed a distinct seasonality. Malaria had a peak in the rainy season, when conditions for the vector seem to be favorable. Poor quality and shortage of water, on the other hand, may contribute to increased numbers of individuals' seropositivity for typhoid fever during dry season.7 Data from the community and hospital survey were not used for comparison because of essential differences in the surveyed populations (encouraged to participate versus self-referred).
The diseases investigated in this study were very difficult to distinguish by their clinical presentation. Splenomegaly remains the only significant predictor for malaria, especially among children, who generally present with more severe symptoms than adults with malaria. In a household surveillance conducted in the same rural communities of Bandarban in parallel with this fever study, high proportions of oligo- and asymptomatic malaria-positive adults were identified, indicating a greater probability for semi-immunity by increasing age.
Another finding of this study is the high proportion of participants testing positive for more than one of the pathogens similar to previous reports from the Thai-Myanmar border24; considering a potential overlap more elaborate diagnostic measures for causes of fever other than malaria may therefore be indicated, especially for severe cases and patients with persistent symptoms under antimalarial treatment.
The diagnostic tools used in this study were selected by their field applicability. For malaria, microscopy provides high sensitivity and specificity and remains the gold standard whenever working in the field. Serological assays, however, have major limitations regarding sensitivity and specificity.29 Molecular techniques or blood culture could potentially provide more accurate diagnosis, but these techniques are relatively expensive and time-consuming, making them less suitable for the use in field studies and for local clinicians. The setup in this study did not allow for follow-up samples to be collected. However, the IgM seropositivity can provide an indication of recent infections as IgM can be detected as early as 1 week after the emergence of symptoms. A single positive result for leptospirosis by ELISA will provide evidence of seroconversion within the preceding months to 1 year, as IgM antibodies against Leptospira remain detectable in the blood for up to 300 days or even longer30,31; this fact might contribute to the non-existing seasonality for leptospirosis found in this study.
False positive results for dengue can be caused by cross-reactivity of the ELISA with other arboviruses thereby decreasing the specificity of the dengue test used.32 Positive ELISA results for leptospirosis and dengue were not felt to be convincing evidence of an acute infection and therefore not separately considered in the calculations of clinical predictors. According to the TUBEX manual, all cases exhibiting a score of > 3 shall be considered positive. We found this value to be too low for the region and considered all patients with a score of > 5 to suffer or have undergone a recent typhoid infection. This assumption is supported by studies recently conducted in the capital Dhaka.13
With this study, we intended to bring attention to the diagnostic challenges and the effects of misdiagnosis and underreporting of febrile diseases in malaria-endemic regions. Our findings confirm the existence of a major reservoir of other causes of fever than malaria with possible dual and triple infections and underline the need to improve strategies for the diagnosis and management of febrile conditions in resource-limited settings.
ACKNOWLEDGMENTS
We thank all study participants for their time and cooperation and the staff of the Sadar Hospital Bandarban for the kind assistance in conducting the study. We also thank all MARIB staff for their effort and support during the community surveys and Oliver Graf, Anja Siedl, and Verena Hofecker for their assistance during the hospital survey. We express our gratitude to IDL Biotech for the providing of TUBEX tests for the diagnosis of typhoid fever.
Footnotes
Financial support: This study was supported by the MARIB.
Authors' addresses: Paul Swoboda, Benedikt Ley, Peter Starzengruber, Kamala Ley-Thriemer, Mariella Jung, Julia Matt, Markus A. Fally, Milena K. S. Mueller, Johannes A. B. Reismann, and Harald Noedl, Institute of Specific Prophylaxis and Tropical Medicine, Medical University of Vienna, Kinderspitalgasse 15, Vienna, Austria, E-mails: paul.swoboda1@gmail.com, ley.benedikt@gmail.com, peter.starzengruber@meduniwien.ac.at, k.leythriemer@gmail.com, mariella.jung@gmx.de, julia.matt@meduniwien.ac.at, markus.fally@gmx.at, milenamueller@yahoo.de, Johannes.Reismann@gmx.de, and harald.noedl@meduniwien.ac.at. Hans-Peter Fuehrer, Department of Pathobiology, Institute of Parasitology, University of Veterinary Medicine Vienna, Veterinärplatz 1, Vienna, Austria, E-mail: hans-peter.fuehrer@vetmeduni.ac.at. Rashidul Haque and Wasif A. Khan, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mails: rhaque@icddrb.org and wakhan@icddrb.org.
References
- 1.Bangladesh Bureau of Statistics Population & Housing Census 2011: Preliminary Results. 2011. http://www.bbs.gov.bd/WebTestApplication/userfiles/Image/BBS/PHC2011Preliminary%20Result.pdf Available at. Accessed August 8, 2013.
- 2.World Health Organization World Malaria Report 2011. 2011. http://www.who.int/malaria/world_malaria_report_2011 Available at. Accessed May 10, 2012.
- 3.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 4.Myint KS, Gibbons RV, Murray CK, Rungsimanphaiboon K, Supornpun W, Sithiprasasna R, Gray MR, Pimgate C, Mammen MP, Jr, Hospenthal DR. Leptospirosis in Kamphaeng Phet, Thailand. Am J Trop Med Hyg. 2007;76:135–138. [PubMed] [Google Scholar]
- 5.Kendall EA, LaRocque RC, Bui DM, Galloway R, Ari MD, Goswami D, Breiman RF, Luby S, Brooks WA. Leptospirosis as a cause of fever in urban Bangladesh. Am J Trop Med Hyg. 2010;82:1127–1130. doi: 10.4269/ajtmh.2010.09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morshed MG, Konishi H, Terada Y, Arimitsu Y, Nakazawa T. Seroprevalence of leptospirosis in a rural flood prone district of Bangladesh. Epidemiol Infect. 1994;112:527–531. doi: 10.1017/s0950268800051220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ram PK, Naheed A, Brooks WA, Hossain MA, Mintz ED, Breiman RF, Luby SP. Risk factors for typhoid fever in a slum in Dhaka, Bangladesh. Epidemiol Infect. 2007;135:458–465. doi: 10.1017/S0950268806007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks WA, Hossain A, Goswami D, Nahar K, Alam K, Ahmed N, Naheed A, Nair GB, Luby S, Breiman RF. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis. 2005;11:326–329. doi: 10.3201/eid1102.040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman M, Rahman K, Siddque AK, Shoma S, Kamal AH, Ali KS, Nisaluk A, Breiman RF. First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis. 2002;8:738–740. doi: 10.3201/eid0807.010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podder G, Breiman RF, Azim T, Thu HM, Velathanthiri N, Mai le Q, Lowry K, Aaskov JG. Origin of dengue type 3 viruses associated with the dengue outbreak in Dhaka, Bangladesh, in 2000 and 2001. Am J Trop Med Hyg. 2006;74:263–265. [PubMed] [Google Scholar]
- 11.Raheel U, Faheem M, Riaz MN, Kanwal N, Javed F, Zaidi NS, Qadri I. Dengue fever in the Indian subcontinent: an overview. J Infect Dev Ctries. 2011;26:239–247. doi: 10.3855/jidc.1017. [DOI] [PubMed] [Google Scholar]
- 12.Miah MT, Rahman S, Sarker CN, Khan GK, Barman TK. Study on 40 cases of Rickettsia. Mymensingh Med J. 2007;16:85–88. doi: 10.3329/mmj.v16i1.259. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Siddique AK, Tam FC, Sharmin S, Rashid H, Iqbal A, Ahmed S, Nair GB, Chaignat CL, Lim PL. Rapid detection of early typhoid fever in endemic community children by the TUBEX O9-antibody test. Diagn Microbiol Infect Dis. 2007;58:275–281. doi: 10.1016/j.diagmicrobio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Ley B, Thriemer K, Ame SM, Mtove GM, von Seidlein L, Amos B, Hendriksen IC, Mwambuli A, Shoo A, Kim DR, Ochiai LR, Favorov M, Clemens JD, Wilfing H, Deen JL, Ali SM. Assessment and comparative analysis of a rapid diagnostic test (Tubex) for the diagnosis of typhoid fever among hospitalized children in rural Tanzania. BMC Infect Dis. 2011;11:147. doi: 10.1186/1471-2334-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuehrer HP, Starzengruber P, Swoboda P, Khan WA, Matt J, Ley B, Triemer K, Haque R, Yunus EB, Hossain SM, Walochnik J, Noedl H. Indigenous Plasmodium ovale malaria in Bangladesh. Am J Trop Med Hyg. 2011;83:75–78. doi: 10.4269/ajtmh.2010.09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuehrer HP, Fally MA, Habler VE, Starzengruber P, Swoboda P, Noedl H. Novel nested direct PCR technique for malaria diagnosis using filter paper samples. J Clin Microbiol. 2011;49:1628–1630. doi: 10.1128/JCM.01792-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyder AA, Morrow RH. In: Measures of health and disease in populations. International Public Health, Diseases, Programs, Systems, and Policies. Second edition. Merson MH, Black RE, Mills AJ, editors. Sudbury, MA: Jones and Bartlett; 2005. pp. 2–53. [Google Scholar]
- 18.Haque U, Ahmed SM, Hossain S, Huda M, Hossain A, Alam MS, Mondal D, Khan WA, Khalequzzaman M, Haque R. Malaria prevalence in endemic districts of Bangladesh. PLoS One. 2009;4:e6737. doi: 10.1371/journal.pone.0006737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faruque LI, Zaman RU, Alamgir AS, Gurley ES, Haque R, Rahman M, Luby SP. Hospital-based prevalence of malaria and dengue in febrile patients in Bangladesh. Am J Trop Med Hyg. 2012;86:58–64. doi: 10.4269/ajtmh.2012.11-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam MS, Chakma S, Khan WA, Glass GE, Mohon AN, Elahi R, Norris LC, Podder MP, Ahmed S, Haque R, Sack DA, Sullivan DJ, Jr, Norris DE. Diversity of anopheline species and their Plasmodium infection status in rural Bandarban, Bangladesh. Parasit Vectors. 2012;5:150. doi: 10.1186/1756-3305-5-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Githeko AK, Lindsay SW, Confalonieri UE, Patz JA. Climate change and vector-borne diseases: a regional analysis. Bull World Health Organ. 2000;78:1136–1147. [PMC free article] [PubMed] [Google Scholar]
- 23.Ali M, Wagatsuma Y, Emch M, Breiman RF. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in an urban outbreak. Am J Trop Med Hyg. 2003;69:634–640. [PubMed] [Google Scholar]
- 24.Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, Gray MR, Uthaimongkol N, Buathong N, Sriwichai S, Phasuk R, Yingyuen K, Mathavarat C, Miller RS. Causes of fever in adults on the Thai-Myanmar border. Am J Trop Med Hyg. 2006;74:108–113. [PubMed] [Google Scholar]
- 25.Laras K, Cao BV, Bounlu K, Nguyen TK, Olson JG, Thongchanh S, Tran NV, Hoang KL, Punjabi N, Ha BK, Ung SA, Insisiengmay S, Watts DM, Beecham HJ, Corwin AL. The importance of leptospirosis in Southeast Asia. Am J Trop Med Hyg. 2002;67:278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 26.Van CT, Thuy NT, San NH, Hien TT, Baranton G, Perolat P. Human leptospirosis in the Mekong delta, Viet Nam. Trans R Soc Trop Med Hyg. 1998;92:625–628. doi: 10.1016/s0035-9203(98)90787-8. [DOI] [PubMed] [Google Scholar]
- 27.John TJ, Dandona L, Sharma VP, Kakkar M. Continuing challenge of infectious diseases in India. Lancet. 2011;377:252–269. doi: 10.1016/S0140-6736(10)61265-2. [DOI] [PubMed] [Google Scholar]
- 28.Buckle GC, Walker CL, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health. 2012;2:10401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad SN, Shah S, Ahmad FM. Laboratory diagnosis of leptospirosis. J Postgrad Med. 2005;51:195–200. [PubMed] [Google Scholar]
- 30.Adler B, Murphy AM, Locarnini SA, Faine S. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J Clin Microbiol. 1980;11:452–457. doi: 10.1128/jcm.11.5.452-457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupidi R, Cinco M, Balanzin D, Delprete E, Varaldo PE. Serological follow-up of patients involved in a localized outbreak of leptospirosis. J Clin Microbiol. 1991;29:805–809. doi: 10.1128/jcm.29.4.805-809.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzmán MG, Kourí G. Dengue diagnosis, advances and challenges. Int J Infect Dis. 2004;8:69–80. doi: 10.1016/j.ijid.2003.03.003. [DOI] [PubMed] [Google Scholar]