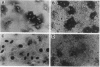
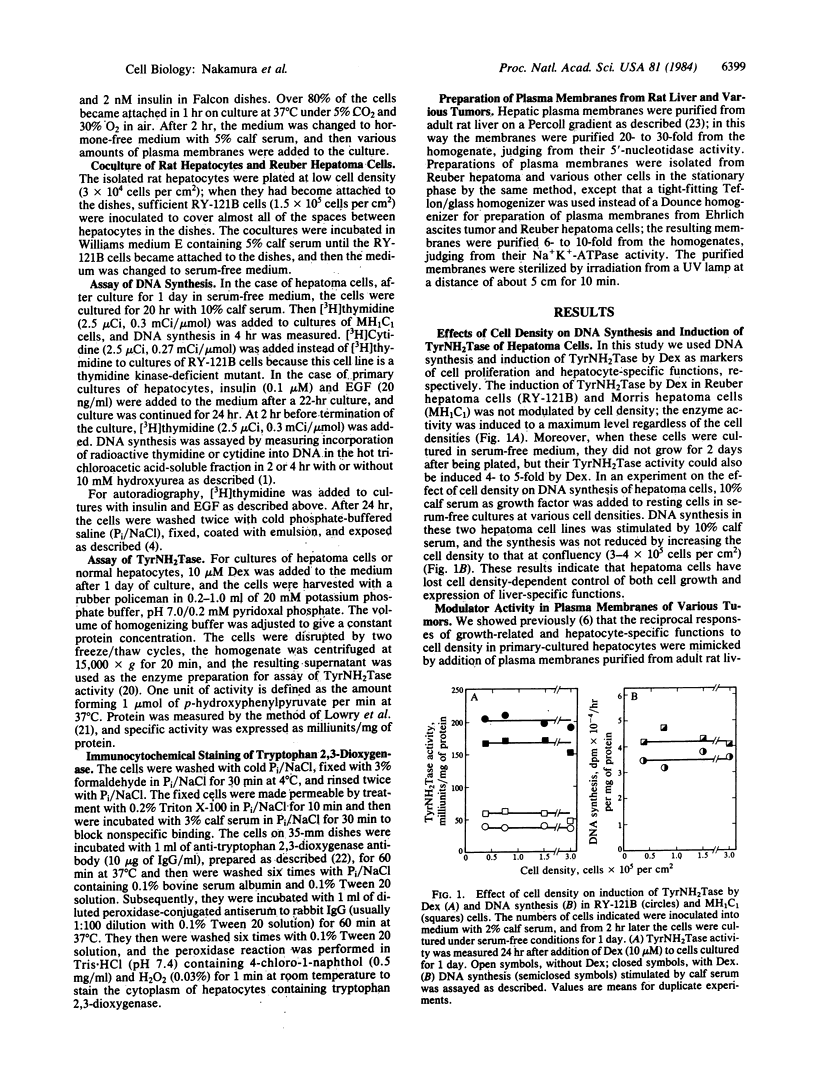
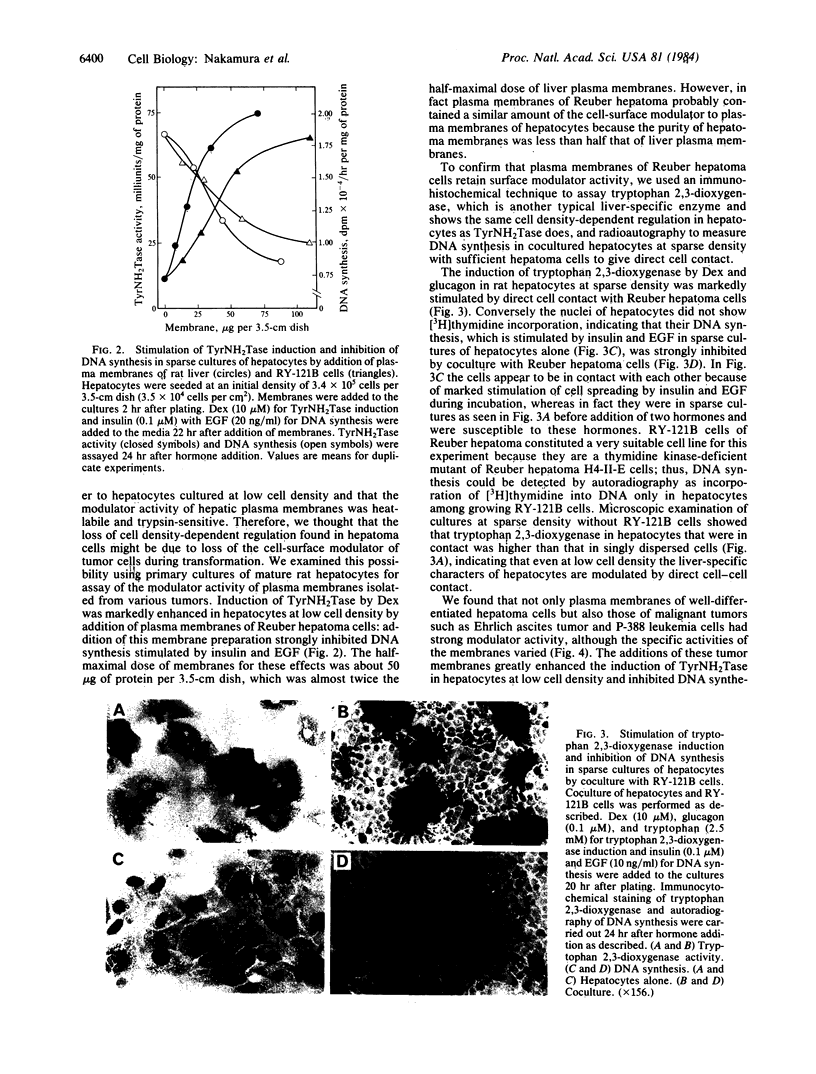
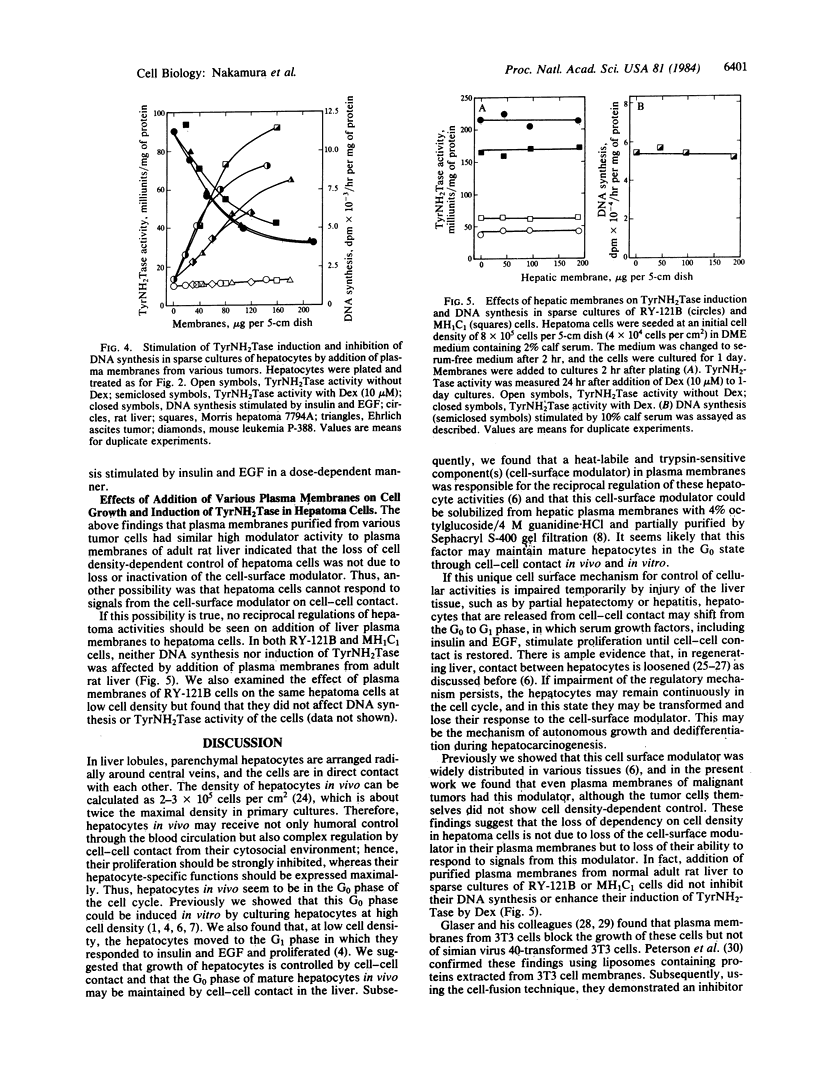
Abstract
In mature rat hepatocytes in primary culture, many metabolic functions and cell growth are controlled reciprocally by cell density, and this reciprocal regulation is mediated by a cell-surface modulator through cell-cell contact. Cultured RY-121B cells from Reuber hepatoma and MH1C1 cells from Morris hepatoma, which retain some liver-specific functions, did not show any cell-density dependency of either cell growth or hepatocyte-specific functions, such as induction of tyrosine aminotransferase by dexamethasone. However, when RY-121B cells were cocultured with a low density of rat hepatocytes as monolayers in direct contact, they exerted contact-dependent control of DNA synthesis and of differentiated function of the hepatocytes. Furthermore, plasma membranes from various tumor cells including these hepatoma cells had strong modulator activity on primary cultures of normal rat hepatocytes, and their activity mimicked the reciprocal effects of cell density on DNA synthesis and induction of tyrosine aminotransferase. On the contrary, addition of plasma membranes from normal adult rat liver to sparse cultures of RY-121B or MH1C1 cells did not cause any inhibition of active DNA synthesis or enhancement of induction of tyrosine aminotransferase in these cells. These results suggest that hepatoma cells have lost cell density-dependent regulations of many cellular activities and cell growth because they have lost the ability to respond to the cell surface modulator, although they retain modulator activity on their plasma membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M. Contact inhibition and malignancy. Nature. 1979 Sep 27;281(5729):259–262. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1705–1711. doi: 10.1073/pnas.56.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge R., Glaser L., Lieberman M., Raben D., Salzer J., Whittenberger B., Woolsey T. Growth control by cell to cell contact. J Supramol Struct. 1979;11(2):175–187. doi: 10.1002/jss.400110207. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Corsaro C. M., Migeon B. R. Comparison of contact-mediated communication in normal and transformed human cells in culture. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4476–4480. doi: 10.1073/pnas.74.10.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Characterization of a tyrosine-specific kinase activity in human fibroblast membranes stimulated by platelet-derived growth factor. J Biol Chem. 1982 Sep 10;257(17):10486–10492. [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Watanabe K., Koga M. Induction of mitosis in primary cultures of adult rat hepatocytes under serum-free conditions. Biochem Biophys Res Commun. 1982 Jan 15;104(1):259–265. doi: 10.1016/0006-291x(82)91968-4. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Böhlen P., Fava R., Baldwin J. H., Kleeman G., Armour R. Purification of kidney epithelial cell growth inhibitors. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5989–5992. doi: 10.1073/pnas.77.10.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. M., Barry J. M., Wang J. L. Growth control in cultured 3T3 fibroblasts: neutralization and identification of a growth-inhibitory factor by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2107–2111. doi: 10.1073/pnas.81.7.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Growth control of differentiated adult rat hepatocytes in primary culture. Ann N Y Acad Sci. 1980;349:111–127. doi: 10.1111/j.1749-6632.1980.tb29520.x. [DOI] [PubMed] [Google Scholar]
- LEVINE E. M., BECKER Y., BOONE C. W., EAGLE H. CONTACT INHIBITION, MACROMOLECULAR SYNTHESIS, AND POLYRIBOSOMES IN CULTURED HUMAN DIPLOID FIBROBLASTS. Proc Natl Acad Sci U S A. 1965 Feb;53:350–356. doi: 10.1073/pnas.53.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J. B., Farrelly J. G., Iype P. T. Purification and properties of a rat liver protein that specifically inhibits the proliferation of nonmalignant epithelial cells from rat liver. Proc Natl Acad Sci U S A. 1982 Jan;79(2):456–460. doi: 10.1073/pnas.79.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Yancey S. B., Revel J. P. Intercellular communication in normal and regenerating rat liver: a quantitative analysis. J Cell Biol. 1981 Nov;91(2 Pt 1):505–523. doi: 10.1083/jcb.91.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., Cianciulli H. D., Novotny A. R., Kligerman A. D., Strom S. C., Jirtle R. L. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982 Nov;42(11):4673–4682. [PubMed] [Google Scholar]
- Nakamura T., Nakayama Y., Ichihara A. Reciprocal modulation of growth and liver functions of mature rat hepatocytes in primary culture by an extract of hepatic plasma membranes. J Biol Chem. 1984 Jul 10;259(13):8056–8058. [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Ichihara A. Density-dependent growth control of adult rat hepatocytes in primary culture. J Biochem. 1983 Oct;94(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a134444. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomomura A., Noda C., Shimoji M., Ichihara A. Acquisition of a beta-adrenergic response by adult rat hepatocytes during primary culture. J Biol Chem. 1983 Aug 10;258(15):9283–9289. [PubMed] [Google Scholar]
- Nakamura T., Yoshimoto K., Nakayama Y., Tomita Y., Ichihara A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell--cell contact and cell membranes. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7229–7233. doi: 10.1073/pnas.80.23.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi S., Nakamura T., Nawa K., Ichihara A. Hormonal regulation of translatable mRNA of tryptophan 2,3-dioxygenase in primary cultures of adult rat hepatocytes. J Biochem. 1983 Nov;94(5):1697–1706. [PubMed] [Google Scholar]
- Niwa A., Yamamoto K., Sorimachi K., Yasumura Y. Continuous culture of Reuber hepatoma cells in serum-free arginine-, glutamine- and tyrosine-deprived chemically defined medium. In Vitro. 1980 Nov;16(11):987–993. doi: 10.1007/BF02619337. [DOI] [PubMed] [Google Scholar]
- Noda C., Nakamura T., Ichihara A. alpha-Adrenergic regulation of enzymes of amino acid metabolism in primary cultures of adult rat hepatocytes. J Biol Chem. 1983 Feb 10;258(3):1520–1525. [PubMed] [Google Scholar]
- Peterson S. W., Lerch V. Inhibition of DNA synthesis in SV3T3 cultures by isolated 3T3 plasma membranes. J Cell Biol. 1983 Jul;97(1):276–279. doi: 10.1083/jcb.97.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. W., Lerch V., Moynahan M. E., Carson M. P., Vale R. Partial characterization of a growth-inhibiting protein in 3T3 cell plasma membranes. Exp Cell Res. 1982 Dec;142(2):447–451. doi: 10.1016/0014-4827(82)90386-x. [DOI] [PubMed] [Google Scholar]
- Potter V. R. The present status of the blocked ontogeny hypothesis of neoplasia: the thalassemia connection. Oncodev Biol Med. 1981;2(4):243–266. [PubMed] [Google Scholar]
- Richardson U. I., Tashjian A. H., Jr, Levine L. Establishment of a clonal strain of hepatoma cells which secrete albumin. J Cell Biol. 1969 Jan;40(1):236–247. doi: 10.1083/jcb.40.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Steck P. A., Blenis J., Voss P. G., Wang J. L. Growth control in cultured 3T3 fibroblasts II. Molecular properties of a fraction enriched in growth inhibitory activity. J Cell Biol. 1982 Feb;92(2):523–530. doi: 10.1083/jcb.92.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Sato M., Tomita Y., Ichihara A. Biochemical studies on liver functions in primary cultured hepatocytes of adult rats. I. Hormonal effects on cell viability and protein synthesis. J Biochem. 1978 Oct;84(4):937–946. doi: 10.1093/oxfordjournals.jbchem.a132207. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Nakamura T., Ichihara A. Control of DNA synthesis and ornithine decarboxylase activity by hormones and amino acids in primary cultures of adult rat hepatocytes. Exp Cell Res. 1981 Oct;135(2):363–371. doi: 10.1016/0014-4827(81)90172-5. [DOI] [PubMed] [Google Scholar]
- Traub O., Drüge P. M., Willecke K. Degradation and resynthesis of gap junction protein in plasma membranes of regenerating liver after partial hepatectomy or cholestasis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):755–759. doi: 10.1073/pnas.80.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenberger B., Glaser L. Inhibition of DNA synthesis in cultures of 3T3 cells by isolated surface membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2251–2255. doi: 10.1073/pnas.74.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. G., Revel J. P. Loss and reappearance of gap junctions in regenerating liver. J Cell Biol. 1978 Aug;78(2):554–564. doi: 10.1083/jcb.78.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K., Nakamura T., Ichihara A. Reciprocal effects of epidermal growth factor on key lipogenic enzymes in primary cultures of adult rat hepatocytes. Induction of glucose-6-phosphate dehydrogenase and suppression of malic enzyme and lipogenesis. J Biol Chem. 1983 Oct 25;258(20):12355–12360. [PubMed] [Google Scholar]