Abstract
The signaling context has been found to change the meaning of the silent bared-teeth display (SBT) in pigtail macaques (Macaca nemestrina) such that the SBT in apparently peaceful contexts communicates subordination, a long-term pattern of behavior, whereas in conflict contexts it communicates immediate submission (PNAS, 104: 1581–1586). However, the context dependent nature of the SBT has not yet been explored in other species. We investigated SBT usage with respect to grooming, severe aggression, and signaler-receiver sex, rank difference, and body size in seven captive groups of rhesus macaques. Peaceful SBTs were given most often to male receivers by male and female signalers whereas conflict SBTs were given to both male and female receivers primarily by female signalers. Male signalers rarely gave SBTs (peaceful or conflict) to female receivers. Unlike pigtail macaques, peaceful SBTs in rhesus were often accompanied by withdrawal behavior (referred to as peaceful SBT-leave), which influenced grooming, but not aggression, at the dyadic level. Severe aggression was less frequent among dyads using peaceful SBTs (regardless of withdrawal behavior) than those using conflict SBTs. In contrast, grooming was more frequent among dyads using peaceful SBT-stay signals than those using peaceful SBT-leave signals or conflict SBTs. In total, our results indicate that peaceful SBTs are a functionally different signal from conflict SBTs in rhesus macaques.
Keywords: animal communication, signal, subordination, macaques
INTRODUCTION
The silent bared-teeth display (SBT) is a widely studied signal in primatology, and research continues to reveal new aspects of its usage. Research in pigtail macaques (Macaca nemestrina) shows that the SBT, when given in peaceful contexts (i.e., in response to approach, not aggression), communicates subordination, the long-term state of the dominance relationship, whereas SBTs given in conflict contexts communicate submission for the current agonistic interaction [Flack and de Waal, 2007]. Here, we investigate whether conflict versus peaceful context changes the meaning of the SBT in a closely related species: rhesus macaques (Macaca mulatta).
In most macaques, the SBT is unidirectional and associated with dominance [de Waal and Luttrell, 1985; Preuschoft and van Schaik, 2000], although it is bidirectional in some tolerant species [Thierry, 2000]. Unidirectional signals are reliable indicators of dominance (e.g., more so than direction of aggression or withdrawal behaviors) because the direction is always the same regardless of the presence of allies, third parties, or social leverage [de Waal and Luttrell, 1985; Preuschoft and van Schaik, 2000]. The predictability of unidirectional signals therefore allows communication about the long-term state of the dominance relationship (e.g., subordination), rather than simply immediate submission under the current social circumstances. For example, de Waal’s [1986] concept of a double-layered hierarchy posits that primates distinguish between current interactions and long-term relationships— the SBT being the signal used to communicate about long-term relationships due to its unidirectional pattern.
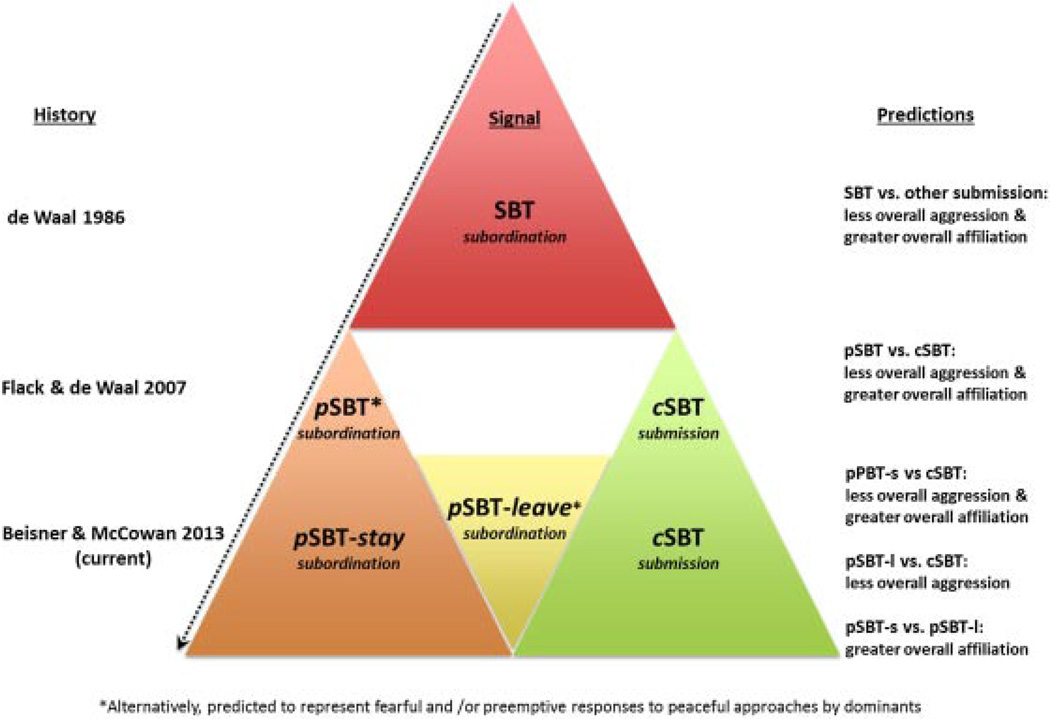
If SBTs given in a peaceful context (hereafter pSBT) truly communicate long-term commitment to subordinate role, then dominants should have less need to reinforce dominance through aggression. Flack and co-workers show that fighting was less frequent (and grooming more frequent) among pigtail dyads that use pSBTs than those that use only conflict SBTs (hereafter cSBT) [Flack and de Waal, 2007]. They reasoned that giving a unidirectional signal, like an SBT, in a new (peaceful) context reduces the dominant’s uncertainty that the subordinate is communicating about subordination rather than immediate submission. If the dominant is more certain that the state of its dominance relationship is settled, there is less need to reinforce dominance (i.e., fight) with this subordinate, which can allow an affiliative relationship to develop. Thus, both the directional predictability of the signal and its shift to a new (peaceful) context were necessary to reduce the receiver’s uncertainty that the signal was communicating subordination rather than immediate submission. Flack and de Waal [2007] modified de Waal’s [1986] double-layered hierarchy concept, showing that only SBTs in peaceful contexts are status signals which communicate the long-term dominance asymmetry of the dyadic relationship (the long-term relationship “layer”), thereby allowing actual expression of the aggressive relationship (the present interaction “layer”) to vary [Flack and de Waal, 2004]. In essence, only pSBTs represent a formal communication of the state of the relationship, not all SBTs, as originally hypothesized [de Waal, 1986], at least in pigtail macaques.
Further testing is needed to determine whether Flack and co-worker’s conclusions are applicable to other species. Rhesus macaques are ideal for testing context dependent signal meaning, as SBTs are unidirectional and given in conflict and peaceful contexts (see Fig. 1 for picture of peaceful SBT) [de Waal and Luttrell, 1985]. To test cross-species applicability, we compare this context modulation hypothesis with our modified version of this hypothesis and two alternative hypotheses as described below.
Fig. 1.
Photo of a peaceful SBT from a rhesus group at Yerkes National Primate Research Center (photo credit: Lisa A. Parr).
Context Modulation Hypothesis
Currently, there is debate over whether animal communication conveys information to perceivers [Seyfarth et al., 2010] or simply induces a response in perceivers [Rendall et al., 2009]. If pSBTs communicate about the long-term state of the relationship, then the signal likely conveys information rather than induces a nervous-system response because it seems unlikely that a long-term response of not fighting could be induced by a single SBT interaction, especially since aggression is relatively frequent whereas pSBTs are relatively rare. In other words, if a subordinate induces a dominant to not fight with him by giving a pSBT, it seems unreasonable to expect that this induced reduction in fighting will persist in the long term, unless pSBTs were given as frequently as the occurrence of aggression. Therefore, in our context modulation hypothesis, we view pSBTs as conveying information to the perceiver about the nature of their relationship.
We hypothesize that in rhesus macaques, like pigtails, pSBTs communicate long-term commitment to a subordinate role whereas cSBTs communicate submission in the current agonistic interaction. Under this context modulation hypothesis, we expect dyads using pSBTs will fight less and groom more than dyads using only cSBTs or none. Alternatively, if de Waal’s original formal signal hypothesis is correct that all SBTs communicate long-term commitment to a subordinate role, then dyads using any type of SBT are expected to fight less and groom more than dyads that do not use SBTs (top triangle of Fig. 2).
Fig. 2.
Diagram showing the historical change in the hypothesized definition of the SBT as a subordination signal. *Alternatively, predicted to represent fearful and/or preemptive responses to peaceful approaches by dominants.
However, accompanying withdrawal behavior has the potential to affect these predictions. In pigtail macaques, pSBTs are almost never accompanied by withdrawal behaviors such as runaway [Flack and de Waal, 2007] whereas half of all pSBTs in rhesus macaques are accompanied by withdrawal behavior [de Waal and Luttrell, 1985].
Peaceful SBTs accompanied by withdrawal behavior (referred to here as pSBT-leave) may be given by individuals with such subservient temperament that overt aggression is not required to elicit the signal. Under this modification of the context modulation hypothesis, only pSBT-stay signals (i.e., pSBTs in which the signaler remains in proximity and does not show withdrawal behavior) should be associated with more grooming than cSBTs. However, both pSBT-stay and -leave signals are still expected to be associated with less fighting (bottom layer of triangles in Fig. 2).
Fearful Temperament Hypothesis
An alternative hypothesis is that all pSBTs, regardless of accompanying withdrawal behavior, are given out of fearful or subservient temperament. The potential role of fear in SBTs can be assessed by temperament scoring. Four temperament dimensions have been identified in infant rhesus macaques that are predictive of juvenile and adult social behavior: vigilant, gentle, confident, and nervous [Golub et al., 2009]. Under this fearful temperament hypothesis, pSBTs should be given more frequently by individuals scoring highly on the nervous temperament scale than those scoring lower on the scale.
Preempt Hypothesis
A final alternative hypothesis is that pSBTs are given to an approaching dominant to prevent a fight. Under this preempt hypothesis, we expect no association between pSBTs and long-term grooming behavior. Furthermore, pSBTs should be given in response to most, if not all, approaches.
Why Signal Long-Term Subordination? The Role of Individual Attributes
Another key component of Flack and co-workers research on pSBTs in pigtail macaques is their use in determining the power structure of the society. It is argued that animals are willing to signal subordination using pSBTs because they acknowledge the dominant’s ability to use force against them [Flack and de Waal, 2007; Flack and Krakauer, 2006]. The ability to use force in polyadic social situations to stop a fight is called power [Bierstedt, 1950; Flack and Krakauer, 2006]. Given that power is likely a determinant of intervention success, Flack and coworkers conclude that pSBTs communicate acknowledgment of the dominant’s ability to use force because dominants who receive pSBTs from a wide variety of subordinates also perform impartial interventions most successfully [Flack et al., 2005a].
If the context modulation hypothesis is true, we further hypothesize that the underlying reason for signaling subordination is acknowledgment of the receiver’s power. Under this attribute hypothesis, we expect animals with greater ability to use force will receive pSBTs more frequently than those with lesser power. Below, we describe three potential measures of an animal’s ability to use force: (1) body size or weaponry, (2) rank disparity, or (3) number of potential allies. In sexually dimorphic species such as macaques, both sex and age are correlates of body size and weaponry. Males are larger in size and have larger canines than females, making males more physically capable of using force against others [Smith and Jungers, 1997]. In addition, a dominant that is much higher ranking than a subordinate may be more capable of using force against that subordinate than if their ranks were very close. Finally, several monkeys allied together can dominate a single individual that is physically larger. In rhesus, kin alliances are the most important in defining one’s rank and social competitive ability [Datta, 1986], suggesting that individuals from larger kin groups may be more powerful than those from smaller kin groups.
METHODS
Data Collection
The subjects of this study were seven groups of rhesus macaques at the California National Primate Research Center. All research reported in this manuscript adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates as well as all laws of the US Government. This research was approved by the University of California, Davis Institutional Animal Care and Use Committee, protocol no. 11843. The seven groups (one per half-acre cage) were studied between June 2008 and December 2009 for a total of 1,500 hr. Of the 19,241 possible dyads across these seven groups, we focused on only non-kin dyads (N=16,274) to avoid the potential influence of kinship.
Each group was observed for 6 hr on 4 days per week for 1 week of each month during each group’s study period (Table I). Observers used an event sampling design to record aggressive, submissive, and status interactions among group members. Both aggressive (Levels 1–8) and submissive (Levels 1–11) behaviors were categorized in increasing levels of severity. Aggression included threat, vocal threat or threat and follow, lunge or mild slap, chase <3m, chase >3m or grapple, bite <5 sec, chase and bite <5 sec, and bite >5 sec. Submission included SBT, turn away, turn away with SBT, move away, move away with SBT, run away <3m, run away <3m with SBT, run away >3m, run away >3m with SBT, prolonged scream, and crouch (animal stops resisting aggression). Severe aggression included any aggression with biting. We also define the following types of SBT:
Peaceful SBT-stay: an SBT given spontaneously or in response to the approach of a dominant, and not accompanied by any additional submissive or withdrawal behaviors. Peaceful context was conservatively assessed—SBTs were recorded as peaceful if the dominant’s face was visible, and there was no indication of overt or subtle threat, such as hard stare or body posture suggestive of threatening behavior.
Peaceful SBT-leave: an SBT given spontaneously or in response to a dominant approach that is accompanied by withdrawal behaviors, such as move away or crouch.
Conflict SBT: an SBT given in response to aggression, regardless of other submission.
SBT style: a categorical variable describing SBT usage for each dyad. There were two possible definitions: (1) dyads categorized by their most frequent SBT type, for example, a dyadwith one pSBT-stay signal and two cSBTs would be categorized as “cSBT,” or (2) dyads categorized on the basis of whether or not pSBTs were used, as in Flack and de Waal [2007], for example, a dyad with at least one peaceful-SBT-stay signal would be categorized as “pSBT-stay” regardless of the frequency of SBT-leave or cSBT. In addition, pSBT-leave signals may communicate the same meaning as pSBT-stay, if the withdrawal behavior does not change the meaning, or may communicate the same meaning as cSBT, if the withdrawal behavior is a preemptive response to potential aggression. We therefore tested three additional category definitions: (1) pSBTs divided into stay versus leave, (2) both types of pSBT lumped into a single pSBT category, and (3) pSBT-leave lumped together with cSBT into a single cSBT category.
TABLE I.
Characteristics of Study Groups
| Group | Mean group size |
Males (>3 years) |
Females (>2 years) |
Non-kin dyads |
|---|---|---|---|---|
| 1B | 177.6 | 21 | 52 | 2,459 |
| 5 | 136.6 | 15 | 61 | 2,621 |
| 8 | 156.9 | 20 | 76 | 3,989 |
| 10B | 164.9 | 7 | 67 | 2,134 |
| 14B | 108.3 | 8 | 37 | 878 |
| 16D | 149.4 | 10 | 55 | 1,721 |
| 18B | 197.2 | 13 | 64 | 2,472 |
Dyads with high interaction frequency have greater opportunity to use SBTs, groom, or fight. To account for the influence of interaction frequency in our analyses, we included the following variables:
Aggression frequency: count of fights per dyad, to account for interaction frequency when analyzing the frequency of SBT usage, especially cSBTs. We divided aggression frequency into mild aggression and severe aggression to allow analysis of severe aggression frequency and use mild aggression frequency as a control for opportunity to fight.
Displacement frequency: count of approach-avoid interactions (with no SBT) per dyad, such that the subordinate moves away or otherwise withdraws from a dominant’s approach. This was used to account for interaction frequency when analyzing frequency of SBT use.
Dominance interaction frequency: the sum of fights per dyad using mild aggression (i.e., threat, lunge, or chase <3m) and displacement (approach–avoid) interactions per dyad, to account for interaction frequency when analyzing severe aggression frequency.
Contact-sitting frequency: count of social contact interactions per dyad, including ventral-ventral, ventral-dorsal, or side-by-side contact. This was used to account for interaction frequency when analyzing groom frequency.
To measure kin group size, we counted the number individuals 3 years and older in each matriline. Males in these groups cannot disperse, so males also have maternal kin present in the group. Both females and natal males in the study groups are known to form alliances with maternal kin [Beisner et al., 2011].
We used a new method for computing dominance relationships called the Beta random field method [Fushing et al., 2011]. First, we constructed a win/loss matrix using agonistic interactions. Then dyadic dominance potentials were calculated using dominance transitivity, whereby multiple indirect dominance pathways in the network (via common third parties) were used to infer missing data in the win/ loss matrix. Dominance ranks were determined from these dyadic dominance potentials by re-ordering the matrix using a simulated annealing algorithm for minimizing the appearance of values greater than 0.5 in the lower triangle [Fushing et al., 2011].
Temperament
Of the 506 study subjects, 215 had previously participated in an infant BioBehavioral Assessment (BBA) program at the CNPRC. As infants (3 months old), subjects were removed from their home field cages, separated from their mothers, and placed in an unfamiliar indoor testing room for 25 hr. During the assessment period, infants were given a series of tests to evaluate behavioral and physiological reactivity, such as behavioral response to separation and relocation, interactions with novel stimuli, and response to human intruder. See Golub et al. [2009] for complete details. Most importantly, at the end of the 25-hr testing period, infants’ temperament was rated by an experienced observer on a 1–7 scale for each of 16 traits (e.g., active, calm, fearful). Four temperament rating scales were determined using exploratory and confirmatory factor analysis of the 16 traits: (1) vigilant scale (vigilant, not depressed, not tense, not timid), (2) gentle scale (gentle, calm, curious, flexible), (3) nervous scale (nervous, fearful, timid, not calm, not confident), and (4) confident scale (confident, active, bold, curious, playful).
Two additional scales were identified using exploratory and confirmatory factor analyses on subsets of data from the assessment: (1) activity (time spent moving, rate of environmental exploration, whether the subject ate or drank) and (2) emotionality (rate of cooing, rate of barking, whether the subject scratched, displayed, or lip-smacked) [Golub et al., 2009]. These scales reflect behavioral response to immediate separation and relocation (Day 1 activity and emotionality)and adaptation to the situation (Day 2 activity and emotionality), and thus represent measuresofanindividual’sacute reactivity to stressful situations and general emotional reactivity.
Statistical Analyses
Multilevel Poisson, and Gaussian regression models were used to statistically examine eight dependent variables regarding the frequency, usage, and potential function of SBTs [McCullagh and Nelder, 1989]. First, to examine whether SBT style influences grooming and aggressive interactions, we analyzed: (1) count of grooming per dyad across 11,838 non-kin dyads, which included only individuals observed to use both peaceful and cSBTs at least once, and (2) count of severe aggression per dyad. Next, to examine whether fearful, subservient, or emotional temperament influence SBT usage, we analyzed: (3) the counts of pSBT-leave per individual across 215 individuals, (4) counts of pSBT-stay per individual, and (5) counts of cSBT per individual. Finally, to examine how sex, rank, age, and weight influence SBT usage, we analyzed: (6) count of pSBT-stay signals given from subordinates to dominants across 16,274 non-kin dyads, (7) count of pSBT-leave signals from subordinates to dominants, and (8) count of cSBTs from subordinates to dominants.
For each dependent variable, we ran a series of models and used Akaike’s Information Criterion (AIC) scores to select the best-fit model that is the model with the lowest AIC score. Nested models having a difference in AIC score less than or equal to two were considered equivalent [Burnham and Anderson, 2002]. A random effect for group was included in all models as well as for dominant and subordinate ID when necessary.
RESULTS
Descriptive
We recorded a total of 746 pSBT-stay signals in 622 dyads, 705 pSBT-leave signals in 638 dyads, and 1,967 cSBTs in 1,557 dyads across a total of 16,274 dyads. The rates of pSBTs and cSBTs were 0.0021 and 0.0028 signals per individual per hour, respectively. Bidirectional cSBTs were observed in 43 dyads, whereas bidirectional pSBTs were observed in three dyads. Forty of the 16,274 dyads (0.2%) displayed unidirectional SBTs in the unexpected direction: from dominant to subordinate. Interestingly, 33 of these dyads (82.5%) represented a female giving a pSBT to a male. It is possible that rank assignments were incorrect for these 40 dyads, as male and female hierarchies are thought to be separate.
Most approaches by dominants were not followed by pSBTs. Of the 54,260 dominant approaches recorded, only 3.4% of subordinates gave a pSBT in response. Of the dyads observed to use pSBTs, 38.1% of dominant approaches were followed by subordinates giving pSBTs.
Comparing Context Modulation, Formal Signal, and Preempt Hypotheses
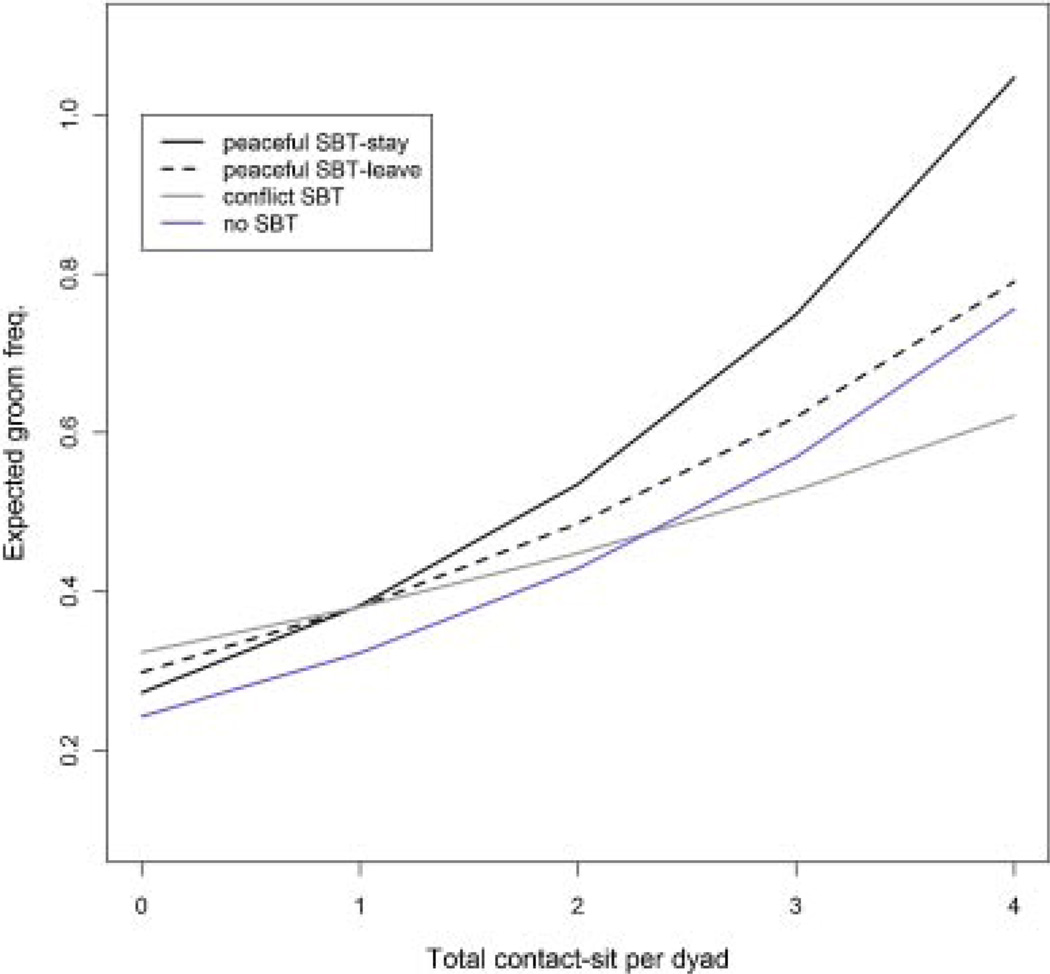
We fit a multi-level Poisson regression model to the count of grooming events per dyad to investigate whether grooming is more frequent among dyads that use pSBTs than those using only cSBTs (AIC =10,481, compared to second best-fit model ΔAIC =5, N=11,838 dyads). The definition of SBT style in the best-fit model distinguished four categories (peaceful-stay, peaceful-leave, conflict, none) such that dyads using pSBT-stay were categorized as such, regardless of whether they also used pSBT-leave or cSBT. The model showed that (a) grooming was more frequent among dyads that affiliated frequently (contact sit frequency: β=0.16, P<0.001), and (b) among dyads that affiliated frequently, those who used pSBT-stay groomed more than dyads that used cSBTs or pSBT-leave (SBT [peaceful-stay] vs. [conflict]: β =−0.39, P=0.008; SBT [peaceful-leave] vs. [conflict]: β =−0.55, P=0.001; contact sit × SBT [peaceful-stay]: β =0.17, P<0.001; contact sit × SBT [peaceful-leave]: β =0.08, P=0.004; contact sit × SBT [none]: β =0.12, P<0.001; Fig. 3). The interaction rank difference × SBT style also affected dyadic groom frequency. Generally, grooming was more frequent when the dominant and subordinate were closer in rank (rank difference [dominant × subordinate]: β =0.02, P<0.001). Among dyads close in rank, grooming was much more frequent among dyads using pSBT-stay than those using pSBT-leave whereas among dyads distant in rank, grooming was equally frequent in dyads using pSBT-stay and pSBT-leave (rank difference × SBT × [peaceful-stay]: β =−0.007, P=0.09; rank difference × SBT × [ peaceful-leave]: β =−0.02, P=0.0007; rank difference × SBT × [none]: β =−0.006, P=0.04).
Fig. 3.
Expected dyadic groom frequency plotted by SBT style (pSBT-stay, pSBT-leave, cSBT, or no SBT) and frequency of contact sitting (i.e., interaction frequency). Groom frequencies were calculated from coefficients from the best-fit model of groom frequency per dyad.
Grooming was most frequent between males and females (dominant [male]: β =0.66, P<0.001; subordinate [male]: β =0.39, P<0.001) and least frequent in male–male dyads (dominant [male] × subordinate [male]: β =−1.7, P<0.001).
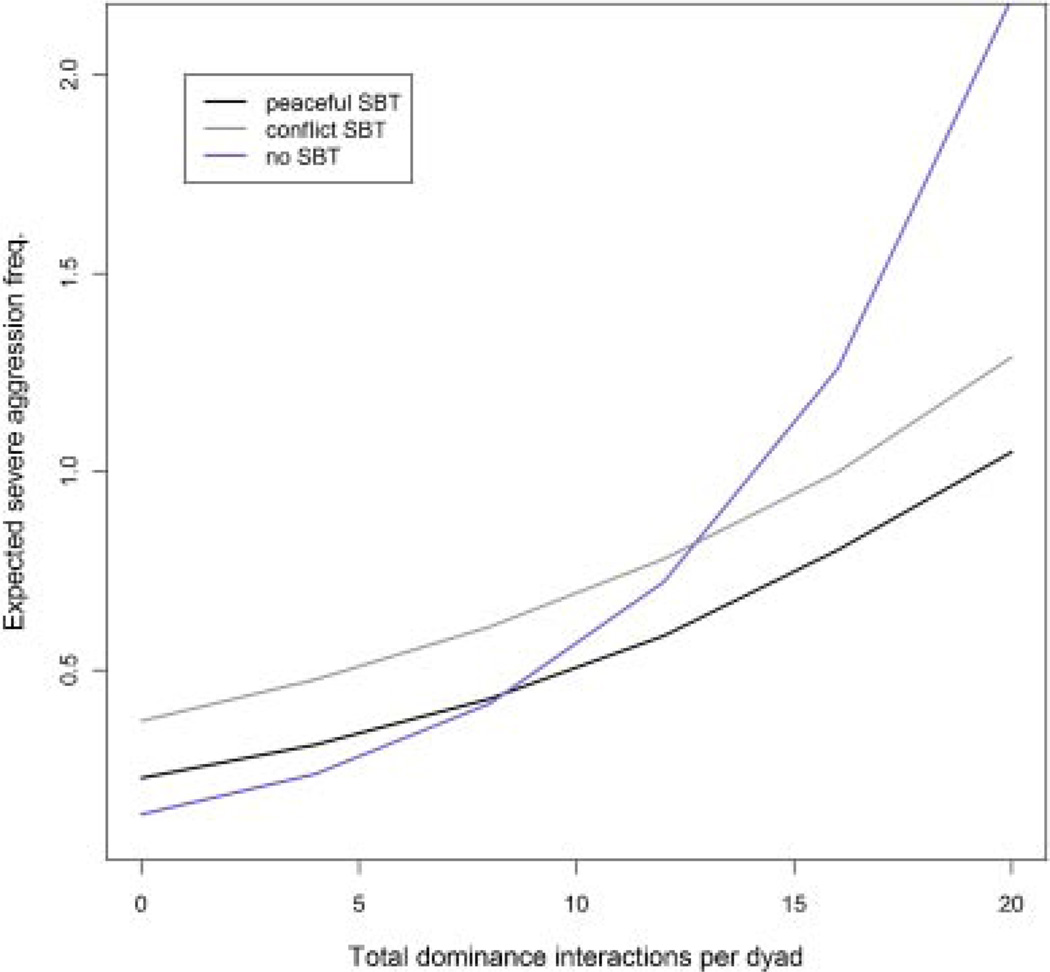
We fit a multi-level Poisson regression model to the count of severe aggression per dyad to investigate whether severe fighting is less frequent among dyads that give pSBTs than those giving only cSBTs (AIC=10,689, compared to the second best-fit model ΔAIC=10, N=11,838 dyads). The definition of SBT style in the best-fit model was categorization by most frequent SBT type and combining pSBT-stay and SBT-leave signals into a single category: pSBT. The model showed that (a) severe fighting was more frequent among dyads that interacted frequently (dominance interaction frequency: β =0.06, P<0.001), and (b) among dyads that interacted frequently, those who used pSBTs fought less than dyads that used cSBTs (SBT [peaceful] vs. [conflict]: β =−0.49, P<0.0001; SBT [none] vs. [conflict]: β =−1.0, P<0.001); dominance interactions × SBT [peaceful]: β =0.017, P=0.005; dominance interactions ×SBT [none]: β =0.077, P<0.001; Fig. 4). The interaction between dominant sex and rank showed that the pattern of greater severe aggression with higher ranking dominants is even more pronounced in males than females (dominant rank: β =−0.004, P=0.09; dominant rank × dominant sex [male]: β = −0.018, P=0.0003). See Table II for a comparative summary of these results relative to each hypothesis.
Fig. 4.
Expected severe aggression frequency plotted by SBT style (pSBT-stay, pSBT-leave, cSBT, or no SBT) and the frequency of other dominance interactions (i.e., interaction frequency). Severe aggression frequencies were calculated from coefficients from the best-fit model of severe aggression frequency per dyad.
TABLE II.
Summary of Competing Hypotheses Regarding the Meaning of Peaceful SBTs
| Hypotheses | SBT function | Predictions | Supported? |
|---|---|---|---|
| Formal signal | All SBTs communicate subordination | All SBT associated with less fighting and more grooming than no SBT | No |
| Context modulation [Flack and de Waal, 2007] | pSBT: subordination; cSBTs: submission | pSBT associated with less fighting and more grooming than cSBT | Yes |
| Context modulation modified [Beisner and McCowan, 2013] | pSBT - stay: subordination; pSBT - leave: submission; cSBT: submission | If withdrawal behavior constitutes fear, only pSBT - stay associated with more grooming then cSBT | Yes |
| Fearful temperament | pSBT: submission, due to fearful temperament | Animals high on nervous scale give more pSBTs than those low on nervous scale | No |
| Preempt | pSBT: submission to prevent a fight; cSBT: submission to stop a fight | pSBT not associated with long - term grooming | No |
Testing the Fearful Temperament Hypothesis: Signaler Temperament Relative to SBT Usage
To investigate the potential influence of temperament on giving pSBT-stay signals, we fit a multilevel negative binomial regression model to the count of pSBT-stay given per subordinate (N=215). There were two best-fit models with ΔAIC<2 and both showed no effect of signaler temperament on frequency of pSBT-stay. According to both models, females gave pSBT-stay more often than males (sex [male]: β =−0.45, P=0.008). The second best-fit model included rank, but it was not significant (β =−0.002, P=0.56). The count of observation days (offset variable) and total status (fixed effect) were included to account for opportunity to record SBTs.
To investigate the potential influence of temperament on giving pSBT-leave signals, we fit a multilevel negative binomial regression model to the count of pSBT-leave given per subordinate (N=215). The best-fit model (AIC=646.2, ΔAIC=3.6) showed that individuals scoring higher on the gentle scale gave more pSBT-leave signals (β =0.27, P<0.001), as did individuals scoring lower on the confident scale (β =−0.26, P=0.002). In addition, individuals scoring higher on emotionality on Day 2 of BBA gave more pSBT-leave signals (β =0.12, P=0.015). Females and lower ranking individuals also gave more pSBT-leave (sex [male]: β =−0.83, P<0.001; rank: β =0.005, P=0.08). The count of observation days (offset variable) and total status (fixed effect) were included to account for opportunity to record SBTs.
To investigate the potential influence of temperament on giving cSBTs, we fit a multi-level negative binomial regression model to the count of cSBTs given per subordinate (N=215). The best-fit model (AIC=969, ΔAIC=2) did not include any temperament measures, indicating no effect of temperament on giving cSBTs. Females gave cSBTs more frequently than males (sex [male]: β =−1.58, P<0.001) as did lower ranking individuals (b=0.005, P=0.05).
Testing the Attribute Hypothesis: Signaler and Receiver Attributes Relative to SBT Usage
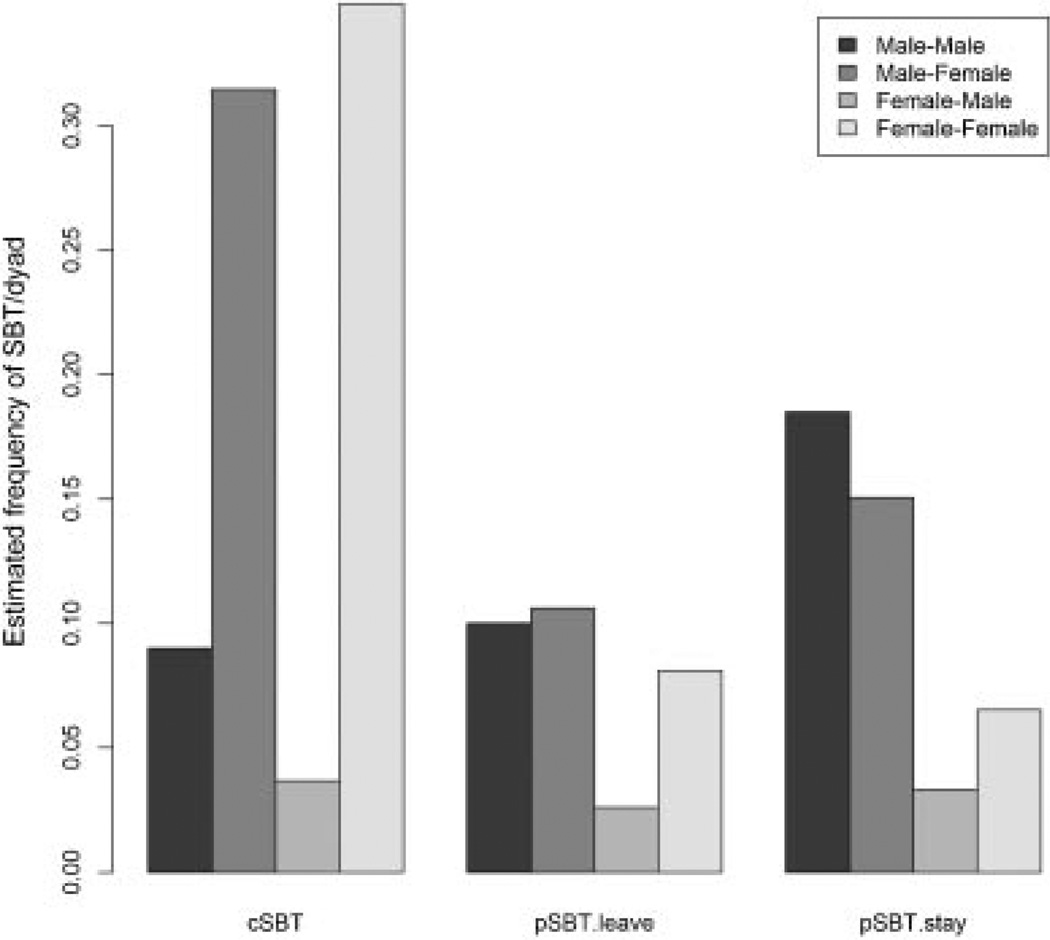
To test the attribute hypothesis that pSBT-stay signals communicate perception of the dominant’s power, we fit a Poisson regression model to counts of pSBT-stay signals across 16,274 non-kin dyads. The best-fit model (AIC=3,741, compared to second bestfit model ΔAIC=2) indicated that sex, rank, and interaction frequency influenced the likelihood of observing a pSBT-stay signal. Peaceful SBT-stay signals were more likely in dyads that had more frequent displacements and more frequent aggressive interactions (total displacement: b=0.13, P<0.001; total aggression: β =0.08, P<0.001). Furthermore, the interaction total displacement × total aggression (β =−0.004, P<0.001) indicated that an increase in total displacement interactions decreased the effect of total aggression, but did not remove its significance. Peaceful SBT-stay signals were most frequent in male–male dyads and least frequent in female–male dyads (dominant sex [male]: β =0.84, P<0.001; subordinate sex [male]: β = −0.41, P=0.04; dominant sex [male] × subordinate sex [male]: β =0.88, P=0.0001; Fig. 5). Finally, pSBT-stay signals were given more frequently to higher ranking individuals (dominant rank: β = −0.02, P=0.0001), larger individuals (dominant weight: β =0.06, P=0.04), and in dyads with little rank disparity (rank diff [dominant - subordinate rank]: β =0.008, P=0.0005).
Fig. 5.
Expected frequency of SBT signals of each type across all sex dyads calculated from model coefficients from the best-fit model of three different variables: pSBT-stay frequency per dyad, pSBT-leave frequency per dyad, and cSBT frequency per dyad. To permit comparison across the different models, values were calculated using identical dominance interaction frequency across the three models.
We fit a Poisson regression model to the counts of pSBT-leave signals to further evaluate the attribute hypothesis. The best-fit model (AIC=3,799, compared to second best-fit model ΔAIC=3) showed a similar pattern to pSBT-stay signals. Peaceful SBT-leave signals were more likely in dyads that had more frequent displacements and more frequent aggressive interactions (total displacement: β =0.13, P<0.001; total aggression: β =0.06, P<0.0001). As with SBT-stay, the interaction between total displacement × total aggression showed that an increase in total displacement interactions decreased the effect of total aggression on pSBT-leave, but did not remove its significance (total displacement × total aggression: β =−0.003, P=0.003). Peaceful SBT-leave signals were most frequent in male– female and male–male dyads and least frequent in female–male dyads (dominant sex [male]: β =0.27, P=0.06; subordinate sex [male]: β =−1.14, P<0.001; dominant sex [male] × subordinate sex [male]: β =1.08, P=0.0001; Fig. 5). However, unlike pSBT-stay signals, the weight difference (not absolute weight) influenced pSBT-leave signals such that subordinates gave more pSBT-leave signals to dominants that were both high ranking and larger than themselves (weight difference [dominant - subordinate]: β =0.08, P=0.002; dominant rank × weight difference: β =−0.002, P=0.05).
We fit a Poisson regression model to dyadic counts of cSBTs. The best-fit model (AIC=7,026, compared to second best-fit model ΔAIC=4) showed a different pattern from pSBTs. The interaction total aggressive interactions × subordinate sex showed that female subordinates gave more cSBTs when they had frequent aggressive interactions, but male subordinates did not (total aggression: β =0.21, P<0.001; total aggression × subordinate [male]: β = −0.15, P<0.001; Fig. 5). Furthermore, among those who fought frequently, cSBTs were less likely among male–male dyads than female–female dyads and male–female dyads (dominant [male]: β =−0.10, P=0.27; subordinate [male]: β =−0.92, P<0.001; dominant [male] × subordinate [male]: β =0.99, P<0.001). The interaction dominant sex × dominant rank showed that high-ranking males received more cSBTs than high-ranking females (dominant rank: β =−0.007, P=0.004; dominant sex × dominant rank: β =−0.01, P=0.01).
DISCUSSION
In this study, we examined whether signaling context (peaceful vs. conflict) affects the apparent meaning of SBTs rhesus macaques. Our results generally support context modulation hypothesis, but also reveal that a modification of this hypothesis is necessary in rhesus.
Our analyses of severe aggression and grooming relative to SBT style support the context modulation hypothesis that pSBTs facilitate the development of positive relationships. Peaceful SBTs were associated with less severe aggression and more grooming. Importantly, interaction frequency mattered—only among dyads that interacted frequently do pSBTs appear to facilitate a more positive relationship.
Consistent with Flack and de Waal’s [2007] context modulation hypothesis, rhesus dyads that used pSBTs most often, regardless of withdrawal behavior, had less frequent severe aggression than those using mostly cSBTs and those that did not use SBTs. First, these results indicate that a pSBT communicates willingness to commit to a subordinate role, thereby reducing the need to reinforce dominance with severe aggression. Second, these results indicate that subordinate rhesus macaques do not use pSBTs with dominants they do not interact with very much. This makes logical sense—there is no benefit to signal commitment to a subordinate role to a dominant with whom you rarely interact. The subordinate cannot benefit from reduced fighting and the low rate of interaction might actually reflect an unresolved relationship, in which case signaling is not expected. Third, the fact that lumping pSBT-stay and -leave signals into a single category best explained the data further indicates that pSBT-leave is not a preemptory cSBT, but a type of pSBT. That both types of pSBT were associated with lower severe aggression than cSBTs (among frequently interacting dyads) demonstrates that the withdrawal behavior does not change the meaning of the pSBT with respect to their dominance relationship. Thus, while cSBTs reduce aggression immediately that is get the aggressor to stop [Preuschoft, 1992], pSBTs reduce aggression over the long term.
The relationship between grooming and SBT style supports our modified context modulation hypothesis with regard to distinguishing pSBTs into stay versus leave. Dyads with at least one pSBT-stay groomed more often than those using only cSBTs or no SBTs, whereas pSBT-leave dyads did not differ from cSBT or no SBT dyads in grooming frequency. The fact that pSBTs did not follow most approaches by dominants, and is therefore unlikely to be a signal to preempt a fight, is also consistent with the groom results. These results suggest that remaining in proximity of the dominant after signaling is an expression of interest in developing a more affiliative relationship, whereas withdrawal behavior after signaling communicates lack of interest in having a grooming relationship. These results support Flack and de Waal’s finding that pSBT dyads groomed more frequently than cSBT dyads in pigtail macaques [Flack and de Waal, 2007], particularly because pSBTs in pigtails were not accompanied by withdrawal.
Analyses of signaler temperament show that use of pSBT-leave signals was influenced by temperament, but not fearful temperament. Instead, individuals with gentle, not-confident, or emotionally reactive temperament gave pSBT-leave signals most frequently. The effect of not confident and emotionally reactive temperaments suggests the signals are preemptive cSBTs. In contrast, signaler-receiver attribute analyses suggest that they are a type of pSBT. These contradictory results, however, appear to be reconciled by the grooming and severe aggression analyses, which we discuss below. As predicted, temperament was not associated with pSBT-stay or cSBTs.
In light of the relationship between SBT style and grooming and aggression, the temperament analyses suggest that pSBT-leave signals communicate disinterest in forming a grooming relationship with the dominant. First, individuals with less confidence or with higher emotional reactivity gave pSBT-leave signals frequently. Furthermore, the weight difference between dominant and subordinate influenced the dyadic frequency of pSBT-leave signals such that these signals are most frequently given to larger individuals that are more capable of using force. Taken together, these results suggest that subordinates with low confidence or high emotional reactivity may feel anxiety about attempting an affiliative relationship with a high-ranking, powerful dominant that is larger than them.
In total, the relationship between pSBT usage and long-term fighting and grooming behavior, and the lack of association with fearful temperament not only supports the hypothesis that pSBTs are subordination signals but also suggests that these signals convey information [Seyfarth et al., 2010]. An association between a short-term biological response signal [Rendall et al., 2009] and long-term behavior might only be produced if the response (pSBT) occurs as frequently as the behavior it is associated with (aggression). However, pSBTs were much less frequent (0.0021 signals/individual/hr) than aggression (0.07 aggressive events/individual/hr).
Our analyses of sender and receiver usage patterns support the attribute hypothesis that pSBTs communicate acknowledgment of the receiver’s power. First, the similar pattern of signaler-recipient sex between pSBT-stay and -leave signals suggests that both are pSBTs, meaning that pSBT-leave signals are not preemptive cSBTs. Second, as predicted, males received more pSBTs (both SBT-stay and SBT-leave) than females, and males rarely gave pSBTs to females. In fact, 85% of pSBTs given in the unexpected direction were female dominants signaling to male subordinates. Further evidence that pSBTs signal perception of ability to use force comes from the effect of body size difference. Dominants that were absolutely larger and relatively larger received more pSBT-stay and SBT-leave signals, respectively. Body size did not influence receipt of cSBTs. In sum, these results show that pSBTs, but not cSBTs, communicate the signaler’s acknowledgment of the receiver’s ability to use force against them, which appears to be determined by sex and body size rather than by rank or kin alliances.
High-ranking adult males perform policing interventions to control group conflict more often than females [Flack et al., 2005b; McCowan et al., 2011; Petit and Thierry, 1994], which may explain why males receive more pSBTs than females. Though physical size and weaponry are correlates of policing, subordinates may give more pSBTs to those that frequently demonstrate their ability to successfully introduce force in polyadic fights [Flack et al., 2005a].
Peaceful SBTs as Formal Signals of Subordination?
de Waal [1986] and others [Preuschoft and van Schaik, 2000] have referred to SBTs as formal dominance signals because they remain unidirectional across a variety of social contexts (e.g., presence of supporters, proximity to resources) [but see Maestripieri, 1999]. de Waal [1986] developed the concept of a “double-layered” hierarchy in which primates distinguish between current interactions and long-term relationships. Flack and de Waal [2007] later modified this concept by showing that unidirectional SBTs in peaceful contexts are subordination signals which communicate the long-term relationship state, thereby allowing actual expression of the agonistic relationship to vary [Flack and de Waal, 2004]. Our findings of less severe aggression in dyads using pSBTs support Flack and de Waal’s claim.
Evolution of Peaceful SBTs
In the aggressive context, the SBT increases receiver certainty about willingness to submit in the current fight. In order to communicate a long-term commitment to a subordinate pattern of behavior, a contextual shift in usage was necessary [Flack and de Waal, 2007]. Four lines of evidence suggest this contextual shift in SBT usage occurred before the divergence of the modern lineages of macaques. First, the macaque common ancestor is tentatively identified as Grade 3, according to a reconstruction of ancestral social characters derived from an analysis of the relationship between macaque social styles and their phylogeny [Thierry et al., 2000]. This is consistent with the fact that Macaca sylvanus and Macaca silenus, both Grade 3 species, come closest to the root of phylogenetic trees constructed from morphological and molecular data [Delson, 1980; Morales and Melnick, 1998]. Second, the SBT is primarily submissive and unidirectional [Thierry, 2000] and is used in peaceful contexts in at least some Grade 3 species [M. arctoides: de Waal and Luttrell, 1989; M. sylvanus: Preuschoft, 1992]. Third, the contextual shift in SBTs was first identified in pigtail macaques, a member of the most ancient macaque lineage [Flack and de Waal, 2007], suggesting that peaceful use of SBTs may have been inherited from the common ancestor. Finally, SBTs are used only in peaceful contexts in the fourth grade of macaques, all of which are members of the most ancient silenus-sylvanus lineage. These lines of evidence all suggest peaceful use of SBTs being present in the common ancestor.
The fact that Grade 4 macaques use SBTs bidirectionally to communicate peaceful intentions [Petit and Thierry, 1992; Thierry et al., 1989] whereas more despotic grades use SBTs unidirectionally to communicate dominance suggests a correlation between dominance style and SBT usage. Dominance style, according to Thierry [2004], refers to the covariation among conflict asymmetry, severity of aggression, conciliatory tendency, and kin bias within the macaque genus. Thierry says that greater symmetry and uncertainty about aggression outcomes create room for negotiation in the less despotic grades. Rhesus are the most despotic of the macaques, and presumably have the lowest degree of dominance uncertainty, suggesting that using pSBTs to reduce uncertainty is minimally important. Our data support this interpretation. Across our study groups, pSBTs were less frequent (0.0021 signals/ individual/hr) than reported for the less despotic pigtail macaques (0.15 signals/individual/hr [Flack and de Waal, 2007]).
ACKNOWLEDGMENTS
This project was supported by NIH grants R24 RR024396 (BM) and PR 51 RR000169 (CNPRC base grant). We thank Darcy Hannibal, Allison Heagerty, Megan Jackson, and Shannon Seil for helpful suggestions and conversations throughout the study.
Contract grant sponsor: NIH; contract grant numbers: R24 RR024396, PR 51 RR000169.
REFERENCES
- Beisner BA, Jackson ME, Cameron A, McCowan B. Effects of natal male alliances on aggression and power dynamics in rhesus macaques. Am J Primatol. 2011;73:790–801. doi: 10.1002/ajp.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierstedt R. An analysis of social power. Am Sociol Rev. 1950;15:161–184. [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information theoretic approach. New York: Springer - Verlag; 2002. [Google Scholar]
- Datta SB. The role of alliances in the acquisition of rank. In: Else JG, Lee PC, editors. Primate ontogeny, cognition, and social behaviour. New York: Cambridge University Press; 1986. pp. 219–225. [Google Scholar]
- de Waal FBM. The integration of dominance and socialbonding in primates. Q Rev Biol. 1986;61:459–479. doi: 10.1086/415144. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Luttrell LM. The formal hierarchy of rhesus macaques: an investigation of the bared - teeth display. Am J Primatol. 1985;9:73–85. doi: 10.1002/ajp.1350090202. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Luttrell LM. Toward a comparative socioecology of the genus Macaca: different dominance styles in rhesus and stumptail macaques. Am J Primatol. 1989;19:83–109. doi: 10.1002/ajp.1350190203. [DOI] [PubMed] [Google Scholar]
- Delson E. Fossil macaques, phyletic relationships and a scenario of development. In: Lindburg DG, editor. The macaques: studies in ecology, behavior and evolution. New York: Van Nostrand Reinhold; 1980. pp. 10–30. [Google Scholar]
- Flack JC, de Waal FBM. Dominance style, social power, and conflict. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. Cambridge: Cambridge University Press; 2004. pp. 157–182. [Google Scholar]
- Flack JC, de Waal FBM. Context modulates signal meaning in primate communication. Proc Natl Acad Sci. 2007;104:1581–1586. doi: 10.1073/pnas.0603565104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC. Encoding power in communication networks. Am Nat. 2006;168:E87–E102. doi: 10.1086/506526. [DOI] [PubMed] [Google Scholar]
- Flack JC, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. Am Nat. 2005a;165:E000. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proc R Soc Biol Sci B. 2005b;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushing H, McAssey M, Beisner BA, McCowan B. Ranking network of a captive rhesus macaque society: a sophisticated corporative kingdom. PLoS ONE. 2011;6:e17817. doi: 10.1371/journal.pone.0017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio J. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Formal dominance: the emperor’s new clothes? J Comp Psychol. 1999;113:96–98. doi: 10.1037/0735-7036.113.1.96. [DOI] [PubMed] [Google Scholar]
- McCowan B, Beisner BA, Capitanio JP, et al. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS ONE. 2011;6:e22350. doi: 10.1371/journal.pone.0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1989. [Google Scholar]
- Morales JC, Melnick DJ. Phylogenetic relationships of the macaques (Cercopithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal genes. J Hum Evol. 1998;34:1–23. doi: 10.1006/jhev.1997.0171. [DOI] [PubMed] [Google Scholar]
- Petit O, Thierry B. Affiliative function of the silent baredteeth display in moor macaques (Macaca maurus): further evidence for the particular status of Sulawesi macaques. Int J Primatol. 1992;13:97–105. [Google Scholar]
- Petit O, Thierry B. Aggressive and peaceful interventions in conflicts in tonkean macaques. Anim Behav. 1994;48:1427–1436. [Google Scholar]
- Preuschoft S. Laughter and smile in barbary macaques (Macaca sylvanus) Ethology. 1992;91:220–236. [Google Scholar]
- Preuschoft S, van Schaik CP. Dominance and communication: conflict management in various social settings. In: Aureli F, de Waal FBM, editors. Natural conflict resolution. Berkeley: University of California Press; 2000. pp. 77–105. [Google Scholar]
- Rendall D, Owren MJ, Ryan MJ. What do animal signals mean? Anim Behav. 2009;78:233–240. [Google Scholar]
- Seyfarth RM, Cheney DL, Bergman T, et al. The central importance of information in studies of animal communication. Anim Behav. 2010;80:3–8. [Google Scholar]
- Smith RC, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- Thierry B. Covariation of conflict management patterns across macaque species. In: Aureli F, de Waal FBM, editors. Natural conflict resolution. Berkeley: University of California Press; 2000. pp. 106–128. [Google Scholar]
- Thierry B. Social epigenesis. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies. Cambridge: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- Thierry B, Demaria C, Preuschoft S, Desportes C. Structural convergence between silent bared-teeth display and relaxed open-mouth display in the Tonkean macaque (Macaca tonkeana) Folia Primatol. 1989;52:178–184. doi: 10.1159/000156396. [DOI] [PubMed] [Google Scholar]
- Thierry B, Iwaniuk AN, Pellis SM. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithedicdae, genus Macaca) Ethology. 2000;106:713–728. [Google Scholar]