Abstract
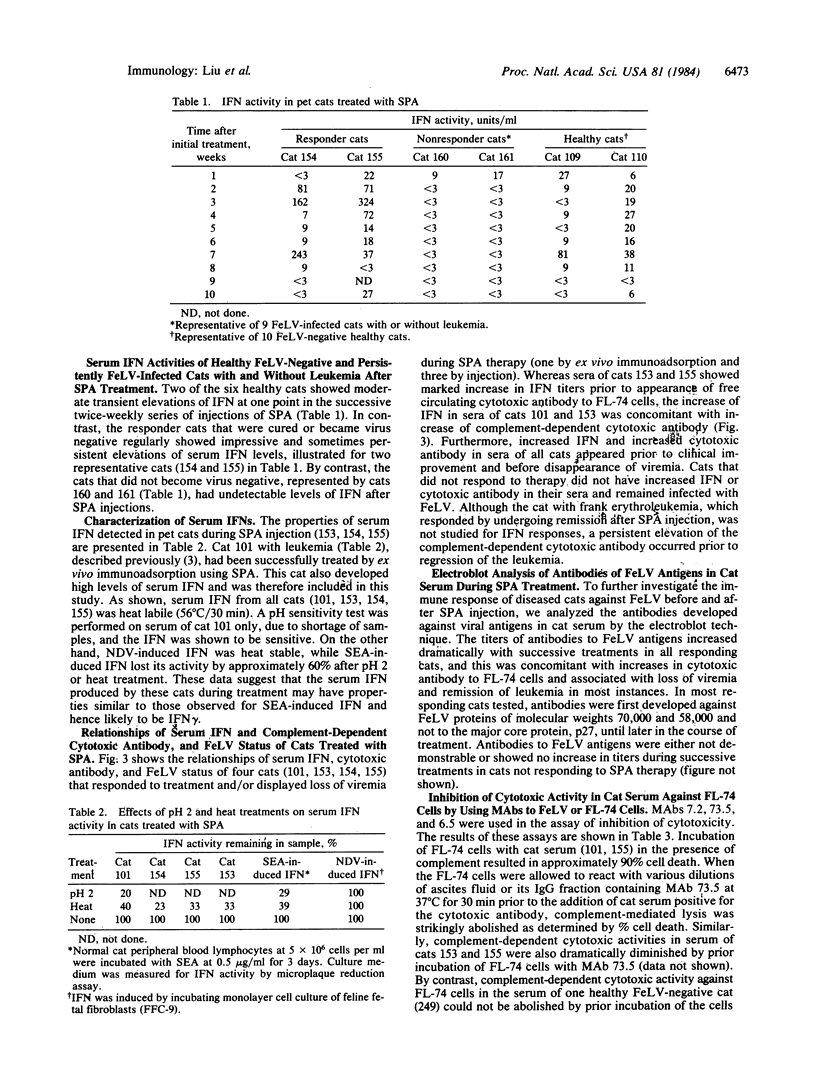
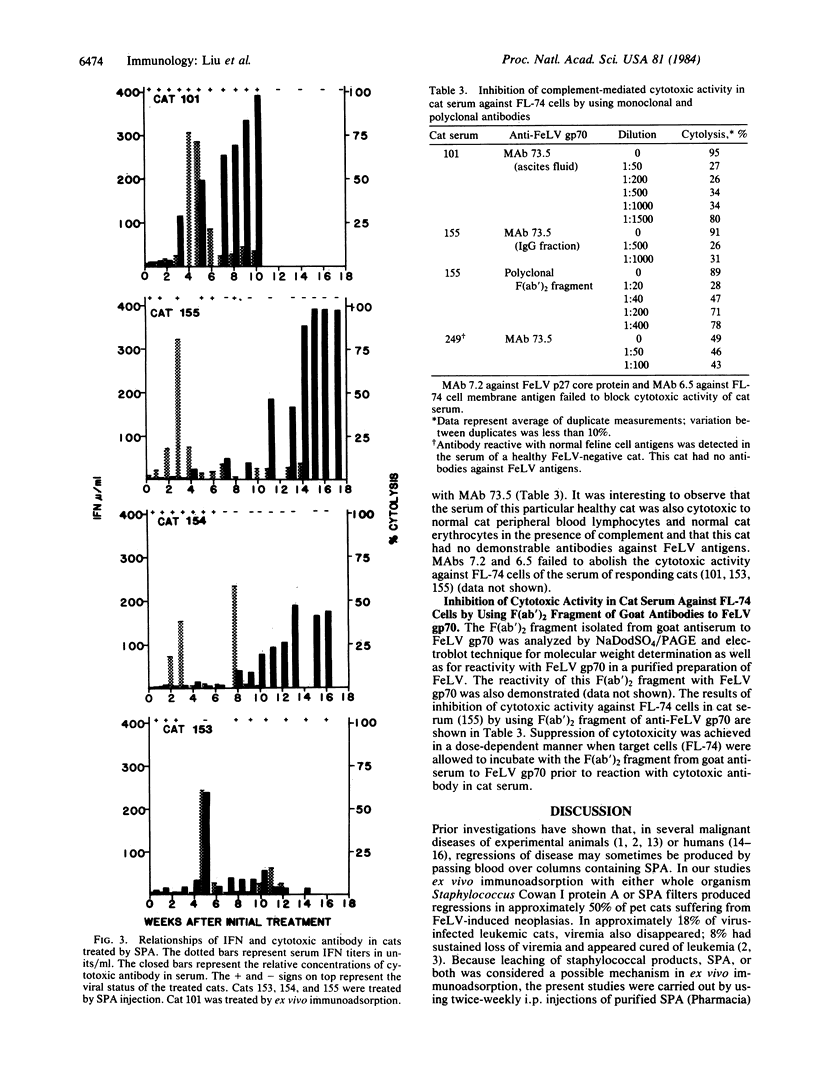
We have injected purified Staphylococcus aureus protein A intraperitoneally into leukemic cats infected with feline leukemia virus, into cats persistently infected with feline leukemia virus but without hematologic or cytologic abnormalities, and into healthy cats without feline leukemia virus infection. Pre- and post-treatment serum samples were evaluated sequentially for interferon activity and for complement-dependent cytotoxic antibody. Our results indicate that serum interferon increased dramatically (less than 3 to 324 units/ml) during treatment only in cats that responded to staphylococcal protein A therapy. Increase of interferon preceded or was closely associated with increasing levels of cytotoxic antibody, loss of viremia, and correction of cytological and hematological abnormalities of three leukemic cats. The cytotoxic antibody was shown to be specific for envelope glycoprotein gp70 of the feline leukemia virus. One persistently feline leukemia virus-infected cat without leukemia that became nonviremic also developed high levels of interferon and specific cytotoxic antibody. By contrast, interferon levels of cats not responding to treatment had levels of less than 3 to 27 units/ml. Normal healthy cats injected with staphylococcal protein A showed moderate transient increases of interferon but no detectable cytotoxic antibodies to FL-74 cells. These data suggest that interferon and cytotoxic antibody may play important, possibly complementary roles in inducing remission of leukemia and loss of viremia in cats treated with staphylococcal protein A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal S. C., Bansal B. R., Thomas H. L., Siegel P. D., Rhoads J. E., Jr, Cooper D. R., Terman D. S., Mark R. Ex vivo removal of serum IgG in a patient with colon carcinoma: some biochemical, immunological and histological observations. Cancer. 1978 Jul;42(1):1–18. doi: 10.1002/1097-0142(197807)42:1<1::aid-cncr2820420102>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Georgiades J. A., Langford M. P., Johnson H. M. Purified human immune interferon has more potent anticellular activity than fibroblast or leukocyte interferon. Cell Immunol. 1980 Feb;49(2):390–394. doi: 10.1016/0008-8749(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Campbell J. B., Grunberger T., Kochman M. A., White S. L. A microplaque reduction assay for human and mouse interferon. Can J Microbiol. 1975 Aug;21(8):1247–1253. doi: 10.1139/m75-186. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Ratliff T. L., McCool R. E. gamma-Interferon induced by S. aureus protein A augments natural killing and ADCC. Nature. 1981 May 7;291(5810):77–79. doi: 10.1038/291077a0. [DOI] [PubMed] [Google Scholar]
- Day N. K., Engelman R. W., Liu W. T., Trang L., Good R. A. Remission of lymphoma leukemia in cats following ex vivo immunosorption therapy using Staphylococcus protein A. J Biol Response Mod. 1984;3(3):278–285. [PubMed] [Google Scholar]
- Gresser I., De Maeyer-Guignard J., Tovey M. G., De Maeyer E. Electrophoretically pure mouse interferon exerts multiple biologic effects. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5308–5312. doi: 10.1073/pnas.76.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Jones F. R., Yoshida L. H., Ladiges W. C., Kenny M. A. Treatment of feline leukemia and reversal of FeLV by ex vivo removal of IgG: a preliminary report. Cancer. 1980 Aug 15;46(4):675–684. doi: 10.1002/1097-0142(19800815)46:4<675::aid-cncr2820460408>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Georgiades J. A., Stanton G. J., Dianzani F., Johnson H. M. Large-scale production and physicochemical characterization of human immune interferon. Infect Immun. 1979 Oct;26(1):36–41. doi: 10.1128/iai.26.1.36-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. T., Engelman R. W., Trang L. Q., Hau K., Good R. A., Day N. K. Appearance of cytotoxic antibody to viral gp70 on feline lymphoma cells (FL-74) in cats during ex vivo immunoadsorption therapy: quantitation, characterization, and association with remission of disease and disappearance of viremia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3516–3520. doi: 10.1073/pnas.81.11.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio L., Hoffman R., Cotter S. M., Dainiak N., Mooney S., Maffei L. A. Feline preleukemia: an animal model of human disease. Yale J Biol Med. 1978 Jul-Aug;51(4):469–476. [PMC free article] [PubMed] [Google Scholar]
- McCool R. E., Catalona W. J., Langford M. P., Ratliff T. L. Induction of human gamma interferon by protein A from Staphylococcus aureus. J Interferon Res. 1981;1(4):473–481. doi: 10.1089/jir.1981.1.473. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Ray P. K., Idiculla A., Mark R., Rhoads J. E., Jr, Thomas H., Bassett J. G., Cooper D. R. Extracorporeal immunoadsorption of plasma from a metastatic colon carcinoma patient by protein A-containing nonviable Staphylococcus aureus: clinical, biochemical, serologic, and histologic evaluation of the patient's response. Cancer. 1982 May 1;49(9):1800–1809. doi: 10.1002/1097-0142(19820501)49:9<1800::aid-cncr2820490912>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ray P. K., Raychaudhuri S., Allen P. Mechanism of regression of mammary adenocarcinomas in rats following plasma adsorption over protein A-containing Staphylococcus aureus. Cancer Res. 1982 Dec;42(12):4970–4974. [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman D. S., Yamamoto T., Mattioli M., Cook G., Tillquist R., Henry J., Poser R., Daskal Y. Extensive necrosis of spontaneous canine mammary adenocarcinoma after extracorporeal perfusion over Staphylococcus aureus Cowans I. I. Description of acute tumoricidal response: morphologic, histologic, immunohistochemical, immunologic, and serologic findings. J Immunol. 1980 Feb;124(2):795–805. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]