Abstract
Objectives
This study investigated the hypothesis whether S100A1 gene therapy can improve pathological key features in human failing ventricular cardiomyocytes (HFCMs).
Background
Depletion of the Ca2+-sensor protein S100A1 drives deterioration of cardiac performance toward heart failure (HF) in experimental animal models. Targeted repair of this molecular defect by cardiac-specific S100A1 gene therapy rescued cardiac performance, raising the immanent question of its effects in human failing myocardium.
Methods
Enzymatically isolated HFCMs from hearts with severe systolic HF were subjected to S100A1 and control adenoviral gene transfer and contractile performance, calcium handling, signaling, and energy homeostasis were analyzed by video-edge-detection, FURA2-based epifluorescent microscopy, phosphorylation site-specific antibodies, and mitochondrial assays, respectively.
Results
Genetically targeted therapy employing the human S100A1 cDNA normalized decreased S100A1 protein levels in HFCMs, reversed both contractile dysfunction and negative force-frequency relationship, and improved contractile reserve under beta-adrenergic receptor (β-AR) stimulation independent of cAMP-dependent (PKA) and calmodulin-dependent (CaMKII) kinase activity. S100A1 reversed underlying Ca2+ handling abnormalities basally and under β-AR stimulation shown by improved SR Ca2+ handling, intracellular Ca2+ transients, diastolic Ca2+ overload, and diminished susceptibility to arrhythmogenic SR Ca2+ leak, respectively. Moreover, S100A1 ameliorated compromised mitochondrial function and restored the phosphocreatine/adenosine-triphosphate ratio.
Conclusions
Our results demonstrate for the first time the therapeutic efficacy of genetically reconstituted S100A1 protein levels in HFCMs by reversing pathophysiological features that characterize human failing myocardium. Our findings close a gap in our understanding of S100A1’s effects in human cardiomyocytes and strengthen the rationale for future molecular-guided therapy of human HF.
Keywords: calcium, gene therapy, heart failure, S100A1, sarcoplasmic reticulum
Heart failure (HF) remains a major cause of morbidity and mortality (1), and a currently unmet need for novel therapeutic strategies requires clinically applicable treatment options targeting the underlying molecular causes of the disease. A promising innovation entering the clinical stage is genetically targeted therapy of human HF (2). Ongoing early stage phase I/II human clinical trials by Hajjar et al. (3) determined safety and therapeutic efficacy of gene-based restoration of an enzyme improving cardiomyocyte Ca2+handling in patients with advanced HF. These studies, therefore, pave the way for the development of molecular-guided treatments for HF aiming at the roots of the disease.
The EF-hand Ca2+-sensor protein S100A1 has emerged as an attractive target for genetically targeted HF therapy because the association between decreased S100A1 expression in cardiomyocytes and the progression to systolic HF has been well documented. Translational studies in small- and large-animal HF models identified S100A1 as a unique regulator of an integrative and Ca2+-controlled network in cardiomyocytes improving sarcoplasmic reticulum (SR), sarcomeric, and mitochondrial function (reviewed in Rohde et al. [4]). Comprehensive actions of S100A1 are modulation of ryanodine receptor (RyR2), SR Ca2+-ATPase (SERCA2), cardiac titin, and mitochondrial F1-ATPase activity (5–9). As a result, hearts with increased S100A1 expression exhibit superior systolic and diastolic performance (6,10,11), as well as mitochondrial high-energy phosphate generation (8,9), whereas mice with cardiac S100A1 deficiency show accelerated HF and fatal arrhythmias in response to chronic hemodynamic stress and ischemic damage (12– 15). Mechanistically, S100A1 improves cardiomyocyte performance by augmenting Ca2+ transient amplitudes and decreasing diastolic Ca2+ overload based on increased Ca2+-induced SR Ca2+ release together with decreased diastolic SR Ca2+ leak and accelerated Ca2+ resequestration (5–11,14,16).
Preclinical studies have clearly documented the association of decreased cardiomyocyte S100A1 expression with HF and show that S100A1 gene-targeted therapy reversed experimental HF and improved cardiac performance in small and large animal studies (17,18).
This study investigates restoration of S100A1 expression in human isolated cardiomyocytes, revealing improved pathological key features of human failing myocardium and phenotyping the molecular and cellular effects of targeted S100A1 gene therapy for the first time in a valuable in vitro model of human systolic HF. Our findings close a gap in the current knowledge of ways in which S100A1 modifies several molecular endpoints of HFCMs, and further translation might pave the way for S100A1 genetically targeted human HF therapy.
Materials and Methods
Human ventricular myocardium was obtained from 27 patients with severe ischemic systolic HF undergoing heart transplantation. Protocols for cardiomyocyte isolation, generation of the adenovirus used to express S100A1 in cultured HFCMs, analysis of cardiomyocyte contractility and Ca2+ handling, RNA and protein expression, SR Ca2+ fluxes, mitochondrial function, and high-energy metabolites are detailed extensively in the Online Appendix. Results are presented as mean ± SEM. Comparisons between treatment groups were made using unpaired 2-tailed Student t test and analysis of variance followed by the Student-Newman-Keuls method for post hoc analysis. Fisher exact test was used to compare percentage of diastolic Ca2+ waves between groups by comparing paced cardiomyocytes without, to cardiomyocytes with diastolic Ca2+ waves. For all tests, a probability value <0.05 was considered significant.
Results
Genetically targeted therapy reconstitutes S100A1 expression in human failing cardiomyocytes
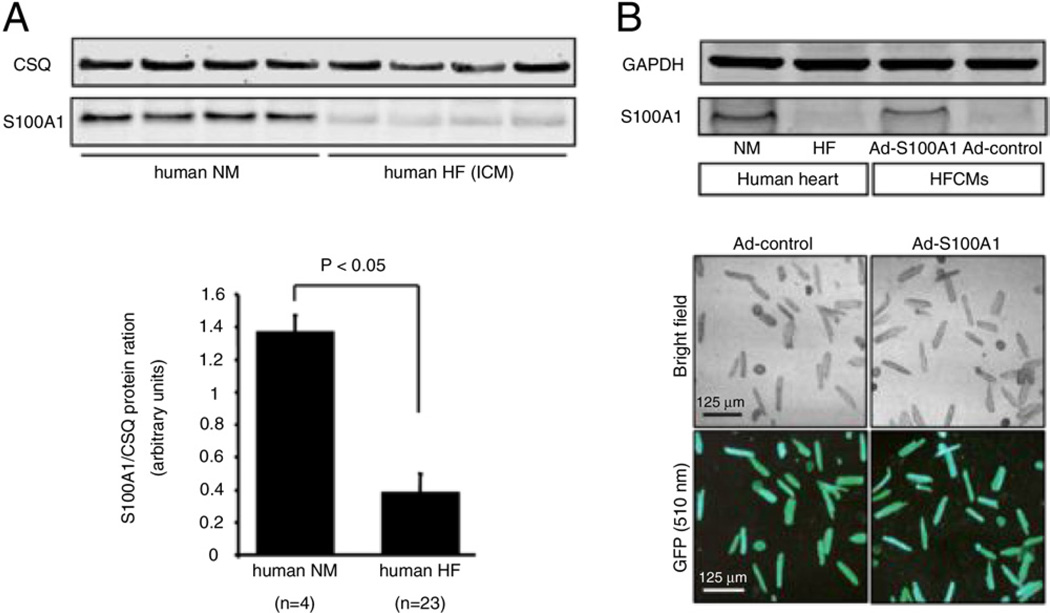
S100A1 protein and mRNA levels were decreased in ischemic cardiomyopathy by 3.5-fold (Fig. 1A) and 5.0-fold (data not shown), respectively, compared with healthy normal myocardium. These findings suggest that abnormal transcriptional regulation causes aberrant S100A1 protein expression in advanced stages of HF, and corroborate earlier results by Remppis et al. (19). Human S100A1 cDNA was delivered to isolated HFCMs by adenoviral infection (Ad-S100A1), resulting in significantly enhanced S100A1 protein levels 24 h after transfection (Fig. 1B) ( +3.2-fold, p < 0.05, n = 5). Our in vitro gene therapy protocol apparently restored S100A1 protein levels to approximately normal levels in HFCMs, whereas the Ad-GFP control virus did not change diminished S100A1 protein levels (Fig. 1B). Expression of other Ca2+-handling proteins in HFCMs, such as down-regulated SERCA2a and increased NCX were unchanged (Online Fig. 1).
Figure 1. Diminished S100A1 Expression in End-Stage Human Failing Myocardium Is Reconstituted by S100A1 Gene Therapy.
(A) Significantly diminished S100A1 protein levels in left ventricular myocardium from human failing hearts with severe systolic failure (ejection fraction 18 ± 9%, mean ± SEM) compared with normal myocardial (NM) samples (p < 0.05 vs. NM; n = 4 NM vs. HF ICM n = 23). (B) S100A1 protein levels can be restored in left ventricular HFCMs to levels seen in normal myocardium by adenoviral-mediated gene transfer shown by a green fluorescent reporter gene expression within Ad-S100A1 (human S100A1 cDNA) and Ad-control-transfected cells. Ad = adenoviral; CSQ = calsequestrin; GFP = green fluorescent protein; HF = heart failure; HFCM = human failing cardiomyocyte; ICM = ischemic cardiomyopathy.
S100A1 gene replacement improves contractility and reverses negative force-frequency relation
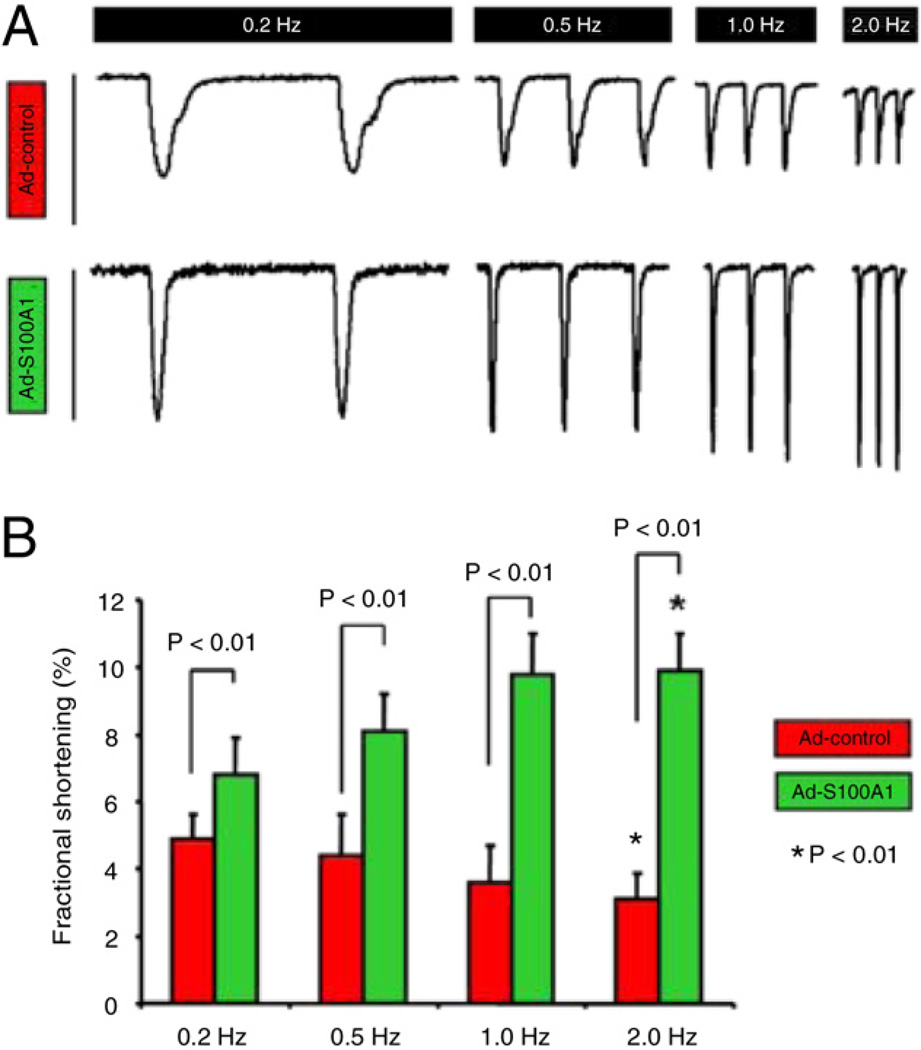
HFCMs with restored S100A1 protein levels showed significant improvement of fractional shortening (FS) (Figs. 2A and 2B) at all tested frequencies (0.2 to 2 Hz), indicating concurrent enhancement of systolic and diastolic function compared with control cells. Normalization of S100A1 even reversed the negative force-frequency response (FFR) seen in control HFCMs, which is a hallmark of human failing hearts (20). In vitro S100A1 therapeutic effects were most prominent at Abbreviations 1 and 2 Hz, reflecting clinically relevant human heart rates in vivo (Figs. 2A and 2B).
Figure 2. S100A1 Improves Contractile Performance and Reverses the Negative FFR in HFCM.
(A) Representative steady-state twitches for control (Ad-control) and S100A1-treated (Ad-S100A1) human failing cardiomyocytes (HFCMs) ranging from 0.2 to 2 Hz illustrate (B) restoration of a positive FFR and significantly enhanced FS by S100A1 at all tested frequencies compared with controls. Asterisks correspond to comparisons between 0.2 and 2 Hz values for control and S100A1-treated cells. Ad = adenoviral; FFR = force-frequency response; FS = fractional shortening.
S100A1 gene-based therapy improves cellular Ca2+ handling and SR Ca2+ load in HFCMs
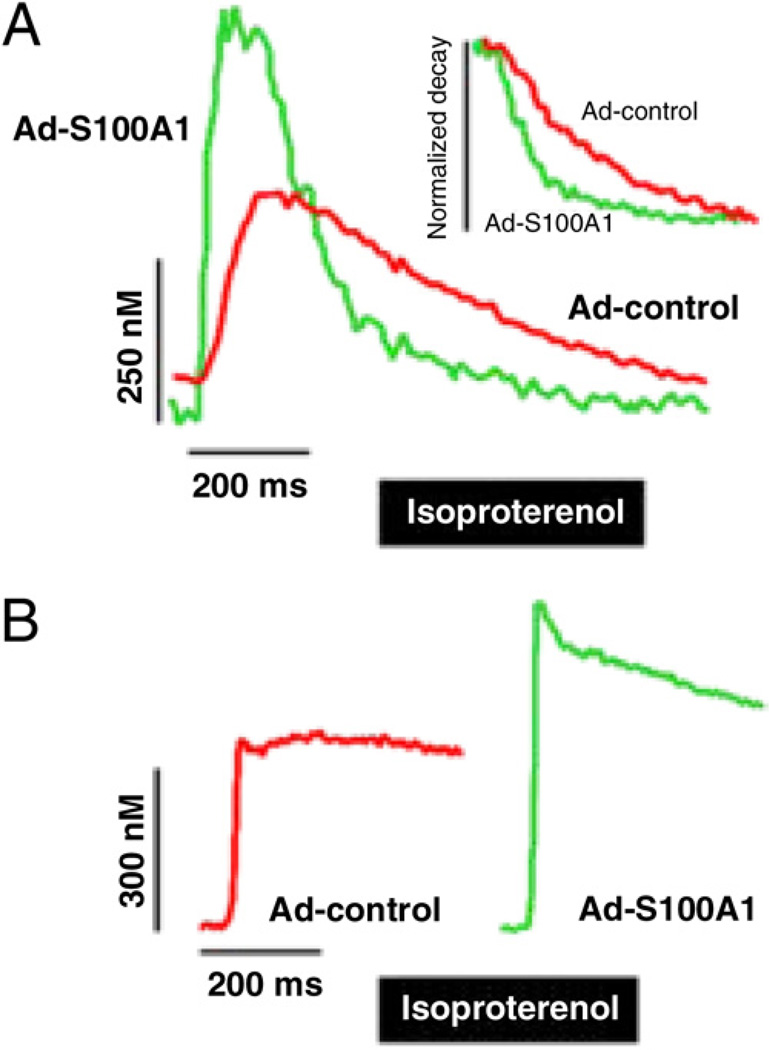
Ca2+ measurements in field-stimulated HFCMs revealed significantly increased systolic Ca2+ transient amplitudes (control 152 ±31 nmol/l vs. S100A1 389 ± 45 nmol/l, p < 0.05) and enhanced SR Ca2+ load (control 314 ± 22 nmol/l vs. S100A1 468 ± 36 nmol/l, p < 0.05) in S100A1-treated cells (Figs. 3A and 3B). Accordingly, diastolic cytosolic Ca2+ concentrations (control 302 ± 44 nmol/l vs. S100A1 187 ± 16 nmol/l, p < 0.05) and the normalized Ca2+ 50% transient decay (control 413 ± 23 ms vs. S100A1 301 ± 24 ms, p < 0.05) were significantly lower in S100A1-expressing HFCMs, which was accompanied by significantly greater enzymatic SERCA2 activity ( +1.51-fold vs. control HFCMs, p < 0.05) (Online Fig. 2). In SR vesicles treated with 100 nmol/l hrS100A1 protein (p < 0.05 vs. vehicle; n = 5), which was associated with a 2.1-fold significantly enhanced S100A1/ SERCA2 interaction (Online Fig. 3), Ca2+ uptake and Ca2+ ATPase activity improved by 1.9- and 2.4-fold, respectively. Overall, these data point toward a direct effect of S100A1 on SERCA2 to stimulate accelerated SR Ca2+ resequestration and improve diastolic function in HFCMs.
Figure 3. S100A1 Restores Ca2+ Handling in Failing Human Myocardium.
(A) Representative Ca2+ transients and corresponding normalized transient decays under basal conditions from Ad-control-treated (red line) and Ad-S100A1–treated (green line) HFCMs paced at 1 Hz depict enhanced Ca2+ transient amplitudes and accelerated cytosolic Ca2+ removal by S100A1. (B) Representative tracings of caffeine-induced (10 mmol/l pulse) rise in cytosolic Ca2+ illustrates a greater amplitude in Ad-S100A1–treated cells (red line) compared with control cells (green line) reflecting enhanced SR Ca2+ load by S100A1. SR = sarcoplasmic reticulum; other abbreviations as in Figure 1.
S100A1 improves contractile reserve mechanisms independent of cAMP-dependent and calmodulin-dependent kinase activity
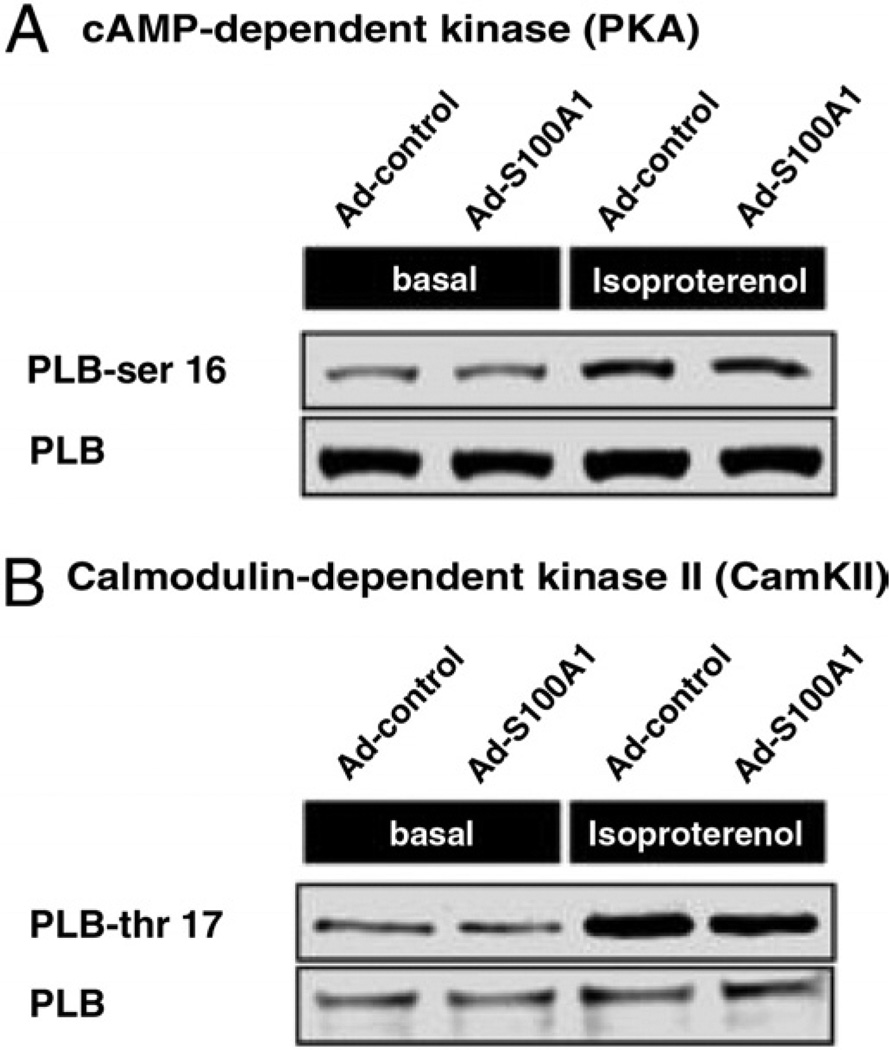
In light of previous studies showing preserved effects of S100A1 in failing myocardium with concurrent beta-adrenergic receptor (β-AR) stimulation, we determined Ca2+ handling in the presence of the β-AR agonist isoproterenol. As shown in Figures 4A and 4B, β-AR-stimulated S100A1-expressing HFCMs exhibited a significantly greater relative increase in systolic Ca2+ transient amplitudes (S100A1 +52 ± 4% vs. control 23 ± 6%, p < 0.05) and preserved gain in SR Ca2+ load over controls (nmol/l; control 367 ± 35 vs. S100A1 551 ± 39, p < 0.05). Despite increased β-AR responsiveness, subsequent analysis of downstream cAMP-dependent protein kinase A (PKA) and calmodulin-dependent protein kinase II (CaMKII) activity revealed a similar increase in phosphorylation levels of phospholamban (PLB) in S100A1-expressing and control HFCMs from equal baseline phosphorylation (relative n-fold increase; PLB-ser 16/PLB +2.1 ± 0.4 S100A1 vs. +2.2 ± 0.6 control, p = NS; PLB-thr 17/PLB +1.7 ± 0.6 S100A1 vs. +1.8 ± 0.5 control, p = NS) (Figs. 5A and 5B). These results indicate that S100A1 therapeutic effects under basal and β-AR stimulation, at least on diastolic SR Ca2+ uptake, do not emanate from altered PKA and CaMKII activity.
Figure 4. S100A1 Improves Contractile Reserve Mechanisms in Failing Human Myocardium.
(A) Representative Ca2+ transients and corresponding normalized transient decays from an isoproterenol-stimulated FURA2-AM loaded Ad-control-treated (red line) and Ad-S100A1–treated (green line) HFCM illustrate preserved gainin-function, enhanced Ca2+ transient amplitude, and accelerated cytosolic Ca2+ removal by S100A1 in response to β-AR stimulation. (B) Representative tracings of caffeine-induced (10 mmol/l pulse) rise in cytosolic Ca2+ depicts a greater amplitude in β-AR-stimulated Ad-S100A1–treated cells (green line) compared with control cells (red line) reflecting increased SR Ca2+ load. Abbreviations as in Figures 1 and 3.
Figure 5. S100A1 Functions Independently of PKA and CaMKII in Human Failing Myocardium.
Shown by representative immunoblots of phosphorylated phospholamban at serine 16 (PLB-ser 16) (A) and threonine 17 (PLB-thr 17) (B), S100A1 gene-based therapy changes neither PKA- nor CaMKII-dependent signaling downstream of β-ARs in HFCMs under basal conditions or isoproterenol stimulation. β-AR = β-adrenergic receptor; CaMKII = calmodulin dependent kinase II; cAMP = cyclic adenosine monophosphate; HFCM = human failing cardiomyocyte; PKA = protein kinase A.
S100A1 genetically targeted therapy prevents arrhythmogenic SR Ca2+ leak in HFCMs
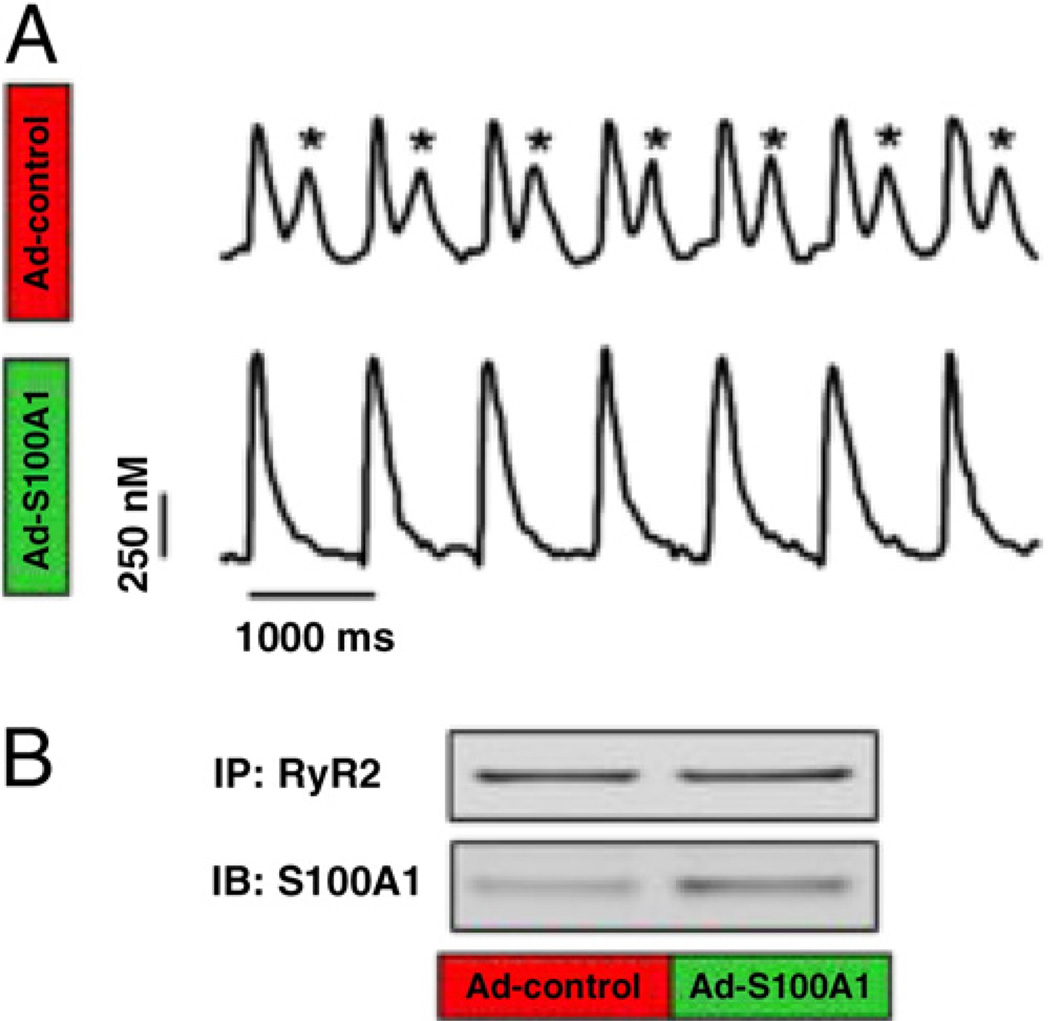
Given the risk of arrhythmogenic Ca2+ leak by reloading the SR with Ca2+, we assessed whether S100A1 effects render HFCMs susceptible to diastolic Ca2+ wave formation. Eighty percent (20 of25) of control but only 13% (3 of 25) of S100A1-expressing field-stimulated (2 Hz) HFCMs exhibited diastolic Ca2+ waves in response to β-AR stimulation (Fig. 6A) ( p < 0.05 control vs. S100A1), indicating protection from a potential arrhythmogenic substrate for delayed after-depolarization (21). The protective effect of S100A1 in HFCMs was associated with augmented binding to the human RyR2 (S100A1/RyR2 ratio; control 0.42 ± 0.11 vs. S100A1 +0.86 ± 0.16, p < 0.05) (Fig. 6B) and occurred despite a significantly higher SR Ca2+ load than control cells (Fig. 4B). Enhanced S100A1/RyR2 interaction (S100A1/RyR2 ratio; control 0.42 ± 0.11 vs. S100A1 +0.86 ± 0.16, p < 0.05) in treated SR vesicles suggests a direct inhibitory effect of S100A1 on RyR2 dysfunction, given a 2.8-fold decrease in Ca2+ leak from S100A1-treated SR vesicles (Online Fig. 3).
Figure 6. S100A1 Interacts With Human RyR2 and Prevents Arrhythmogenic SR Ca2+ Leak.
(A) Representative tracings of Ca2+ transients in an isoproterenol/caffeine-stimulated control (Ad-control) and S100A1-treated (Ad-S100A1) HFCM illustrate the protective effect of S100A1 against β-AR-triggered diastolic SR Ca2+ leak (asterisks). (B) S100A1 interaction with the RyR2 is enhanced after S100A1 gene-based therapy (Ad-S100A1) compared with control (Ad-control). IB = immunoblot; IP = immunoprecipitation; other abbreviations as in Figures 1, 3, and 5.
Compromised energy homeostasis and mitochondrial function in HFCMs is ameliorated through S100A1 gene replacement
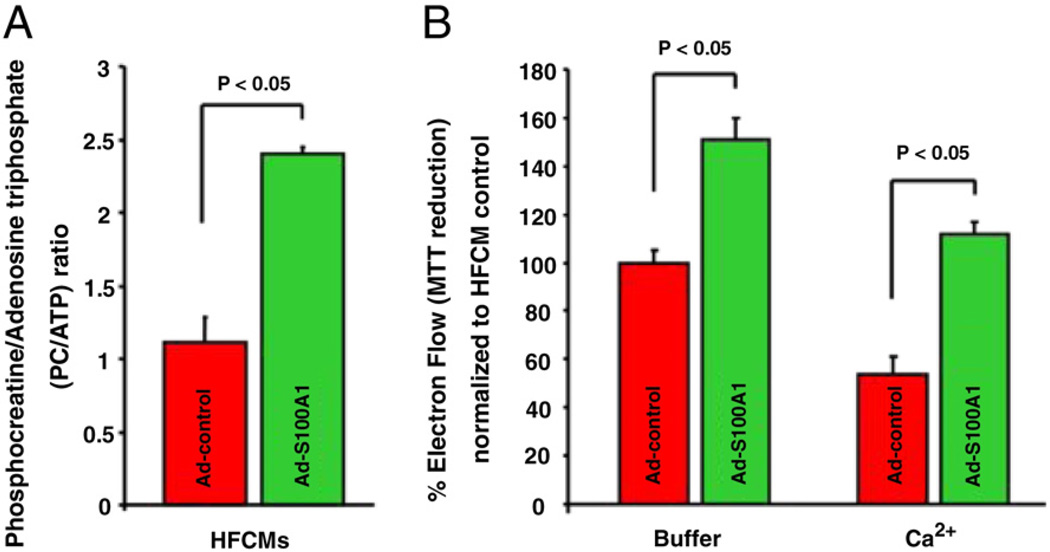
Since elevated diastolic Ca2+ levels and SR Ca2+ leak can disturb cardiomyocyte energy homeostasis (22), we assessed the impact of S100A1 on high-energy phosphate content. The phosphocreatine/adenosine-triphosphate (PC/ATP) ratio was significantly elevated after S100A1 gene delivery (2.4 ± 0.05) to previously published near-normal levels (5,23) as opposed to control with a ratio of 1.1 ± 0.2 (p < 0.05 vs. S100A1) (Fig. 7A). Functional analysis of isolated mitochondria from S100A1-expressing HFCMs revealed superior electron flow even under Ca2+ stress (Fig. 7B) and enhanced mitochondrial S100A1 protein content (Online Figure 4) compared with controls. S100A1 restoration in HFCMs was further accompanied by preserved Δψm basally and in response to Ca2+ treatment (Online Fig. 5). Isolated mitochondria from S100A1-expressing HFCMs further unveiled diminished susceptibility to mitochondrial permeability transition in response to experimental Ca2+ overload and phosphate stress (Online Fig. 6). Overall, these findings suggest a potentially direct effect of S100A1 and further corroborate S100A1’s beneficial actions on mitochondrial function and energy homeostasis in HFCMs.
Figure 7. S100A1 Ameliorates Abnormal High-Energy Phosphate Content and Mitochondrial Electron Flow in HFCMs.
(A) Normalization of S100A1 in HFCMs resulted in a restored PC/ATP ratio reflecting ameliorated cellular energy homeostasis. (B) Superior electron flow in isolated mitochondria from S100A1-expressing HFCMs under basal conditions (buffer) and experimental Ca2+ stress (CaCl2 100 µmol/l) as assessed by MTT reduction. Abbreviations as in Figure 1.
Discussion
This study shows the profound effects of S100A1 genetically targeted therapy in HFCMs and translates previous mechanistic and therapeutic findings in animal HF models towards S100A1 gene-based treatment of human failing myocardium in vitro (for synopsis, see Online Fig. 7).
Therapeutic efficacy of restored S100A1 protein expression in human HF
A key feature of HF seems to be depletion of S100A1 in cardiomyocytes, which results in defective intracellular Ca2+ cycling and poor contractility (4). Our study shows that therapeutic actions of S100A1 in HFCMs might require only modest changes in S100A1 expression obtainable by myocardial-targeted, viral-based gene addition. Sustained therapeutic consequences of similarly modest changes in cardiomyocyte S100A1 expression in various experimental HF models (3,4) prompt speculation that even a moderate S100A1 gene dosage may achieve therapeutically effective changes in human failing myocardium in vivo. Further proof of in vitro therapeutic efficacy stems from the observation that S100A1 can rescue defective SR function and Ca2+ handling in HFCMs despite continued abnormalities in the expression of key sarcoplasmic (SERCA2) and sarcolemmal (NCX) Ca2+-regulator proteins. Similar findings in experimental HF (5,17,18) corroborate the notion that S100A1 neither requires nor depends on normalized SERCA2 and NCX expression, at least for acute therapeutic actions in vitro.
Key role of restored SR function and Ca2+ homeostasis
The SR is critical in the regulation of human cardiomyocyte Ca2+ homeostasis (24). S100A1 seems capable of restoring SR function in HFCMs, which is most likely key to improved systolic SR Ca2+ release being translated into augmented intracellular Ca2+ transients with accelerated removal of cytosolic Ca2+ and reversal of diastolic Ca2+ overload. These results are in line with previously characterized molecular mechanisms in animal HF models relating S100A1 salutary actions to improved RyR2 and SERCA2 function with subsequent rescue of systolic and diastolic contractile function, prevention of arrhythmogenic Ca2+ leak and ameliorated energy homeostasis (5,6,10,14,17,25).
Therapeutic impact on contractile dysfunction
The ability of the SR to transport larger amounts of Ca2+appears critical for frequency-dependent enhancement in contractile strength (20). Hence, reversal of the negative FFR in HFCMs may be attributed to S100A1-mediated frequency-dependent improvement of SR Ca2+load and, subsequently, augmented intracellular systolic Ca2+transient amplitudes. Of note, the S100A1-mediated effect on peak systolic Ca2+levels did not prolong relaxation but rather improved diastolic function despite persistent abnormalities in SERCA2 and NCX expression, likely due to its concurrent impact on diastolic SR Ca2+uptake in HFCMs. The latter indicates that a previously described interaction of S100A1 with SERCA2 both in human (7) and rodent myocardium (5,10) might actually reflect a relevant stimulus of diastolic SR Ca2+ resequestration.
The therapeutic effect of S100A1 on contractile performance of HFCMs appeared most prominent at frequencies approximating actual beating rates of normal and diseased human hearts (60 to 120 beats/min). Since rodent myocardium per se exhibits a negative contractile response at human heart rates (26) and does not faithfully mirror the human phenotype with respect to Ca2+handling, discovery of this potentially relevant in vitro therapeutic effect is dependent on the use of HFCMs.
Prevention of arrhythmogenic SR Ca2+ leak by S100A1 gene-based therapy
A potentially fatal side effect of drugs or molecular interventions that reload the SR with Ca2+, such as β-AR agonists, is arrhythmogenic diastolic Ca2+leak (21). Our data show that S100A1 effectively prevents diastolic Ca2+ wave formation in experimentally forced SR Ca2+ leakage despite enhanced SR Ca2+load. Increased S100A1 interaction with the RyR2 under these conditions prompts speculation that it directly modulates RyR2 diastolic function, because similar findings in animal HF models corroborate these data (5,6,10,14,17,25). Thus, although the molecular mechanism remains to be determined, S100A1 seems to be a unique molecular therapeutic with respect to SR Ca2+ handling, potentially superior to β-AR inotropic agonists by combining enhanced contractile function with prevention of arrhythmogenic Ca2+ leak.
β-AR-independent improvement of contractile reserve
The S100A1-mediated gain in contractile performance and SR Ca2+handling in HFCMs was preserved under β-AR stimulation and, subsequently, improved β-AR reserve. Further investigation of putative downstream signaling seems to suggest that S100A1 therapeutic actions under basal and stimulated conditions in HFCMs neither depend on nor include detrimental and pro-arrhythmogenic PKA and CaMKII activity. This is in line with previous studies showing that S100A1-mediated improvement of cardiac function in experimental HF models is additive to stimulation with β-AR agonists but independent of downstream PKA and CaMKII activity (5,6,10,14,17,18). These findings support the notion that targeted S100A1 restoration in failing myocardium acts synergistically with catecholamine therapy and could even lower the dosage and duration of toxic, pro-arrhythmogenic cAMP-targeted therapies in systolic HF. These issues are the subject of ongoing investigations.
Amelioration of aberrant cardiomyocyte energy homeostasis
Defects in cardiomyocyte Ca2+ handling such as diastolic Ca2+ overload can adversely affect mitochondrial integrity, initiating a vicious cycle of contractile dysfunction and high-energy phosphate depletion (22). Normalization of S100A1 protein in HFCMs restored the depressed PC/ ATP ratio to previously reported normal values in human hearts (23). The improvement of PC/ATP, being an independent predictor of cardiovascular mortality (23), might be in part the result of reconstituted diastolic Ca2+ handling as previously shown by S100A1 gene therapy in experimental HF models (5). Our results further unveil improved electron flow and Δψm, as well as protection from mitochondrial permeability transition under experimental Ca2+ stress in mitochondria either treated with S100A1 or isolated from S100A1-expressing HFCMs. These findings support the notion that S100A1’s previously reported interaction with mitochondrial proteins in the inner and outer matrix such as isocitrate dehydrogenase, F1-ATPase, and adenine nucleotide translocator, among others (9), might be of therapeutic relevance and mechanistically contribute to restoration of the PC/ATP ratio. Hence, it is tempting to speculate that effects on diastolic Ca2+ levels as well as direct modulation of mitochondrial targets through S100A1 each account in part for preserved mitochondrial function and restoration of HFCM energy homeostasis. However, further studies are clearly needed delineating underlying molecular mechanisms as well as the potential route of mitochondrial uptake for S100A1.
Study limitations
Our approach testing therapeutic actions of S100A1 in HFCMs has advantages over isolated cardiomyocytes from small-animal HF models due to fundamental differences in cellular Ca2+ handling compared with human cardiomyocytes. However, neither can our in vitro results readily be extrapolated to the intact organ nor predict outcome of S100A1 genetically targeted therapies in human failing hearts under clinical conditions. Moreover, absence of mechanical load can significantly alter signaling and contractile characteristics, and most importantly, therapeutic effects of molecular interventions in isolated cardiomyocytes. Future studies employing loaded human trabecular muscle fibers or muscle strips isolated from failing hearts might be desirable to determine S100A1’s impact under isometric stress.
Clinical implications
Our study demonstrates that modest changes of S100A1 protein by genetically targeted gene addition are actually therapeutic in failing human myocardium in vitro. The unique molecular profile of S100A1 originates from reversing clinically relevant key features in HFCMs. S100A1 might, therefore, enable long-term therapy of human failing myocardium without pro-arrhythmic side effects and increased energy consumption typically seen with the use of conventional inotropic agents such as sympathomimetic agents and phosphodiesterase inhibitors. Given the proven therapeutic efficacy of S100A1 in preclinical studies, these findings might well present the rationale for a new treatment targeting a key deficiency in advanced human HF. Since synergistic effects of S100A1 with β-AR blockers were observed in experimental HF (4), it is tempting to speculate that future targeted S100A1 gene therapy may complement conventional drug regimens for systolic HF treatment.
Conclusions
This study is the first to document the therapeutic effectiveness of restored S100A1 protein expression in human failing myocardium in vitro. Given the recent evolution of increasingly efficient gene transfer technologies, which have placed congestive HF within the reach of gene-based clinical therapy, these promising results provide the necessary insight into the molecular and cellular mechanisms of S100A1 action in isolated cardiomyocytes, building a strong rationale for its further translation.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health, Bethesda, Maryland (RO1 HL92130 and RO1 HL92130-02S1 to Dr. Most; P01 HL075443 [Project2], R01 HL56205, and R01 HL061690 to Dr. Koch); Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (562 to Drs. Most and Pleger; 1659 to Dr. Voelkers), the Bundesministerium für Bildung und Forschung (BMBF), Berlin, Germany (01GU0572 to Dr. Most); and Schweizerische Nationalfonds (SNF), Bern, Switzerland (PBSK33-118914 to Dr. Brinks). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Brinks and Rohde contributed equally to this work.
Abbreviations and Acronyms
- β-AR
beta-adrenergic receptor
- Ad
adenoviral
- CaMKII
calmodulin-dependent protein kinase II
- FFR
force-frequency response
- FS
fractional shortening
- HF
heart failure
- HFCM
human failing cardiomyocyte
- PC/ATP
phosphocreatine adenosine-triphosphate
- PKA
cAMP-dependent protein kinase A
- RyR2
Ryanodine Receptor 2
- SERCA2
Sarcoplasmic Reticulum Ca2+ ATPase 2
- SR
sarcoplasmic reticulum
Footnotes
APPENDIX
For an expanded Methods section and supplemental figures and their legends, please see the online version of this article.
REFERENCES
- 1.American Heart Association. Heart disease and stroke statistics: 2010 update. Circulation. 2010;121:e1–e170. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Vinge LE, Raake PW, Koch WJ. Gene therapy in heart failure. Circ Res. 2008;102:1458–1470. doi: 10.1161/CIRCRESAHA.108.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajjar BE, Jessup ML, Mancini DM, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohde D, Brinks H, Ritterhoff J, Qui G, Ren S, Most P. S100A1 gene therapy for heart failure: a novel strategy on the verge of clinical trials. J Mol Cell Cardiol. 2011;50:777–784. doi: 10.1016/j.yjmcc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Most P, Pleger ST, Völkers M, et al. Cardiac adenoviral S100A1 gene transfer rescues failing myocardium. J Clin Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Most P, Remppis A, Pleger ST, et al. Transgenic overexpression of the Ca2+ binding protein S100A1 in the heart leads to increased in vivo myocardial contractile performance. J Biol Chem. 2003;278:33809–33817. doi: 10.1074/jbc.M301788200. [DOI] [PubMed] [Google Scholar]
- 7.Kiewitz R, Acklin C, Schafer BW, et al. Ca(2+)-dependent interaction of S100A1 with the sarcoplasmic reticulum Ca(2+)-ATPase2a and phospholamban in the human heart. Biochem Biophys Res Commun. 2003;306:550–557. doi: 10.1016/s0006-291x(03)00987-2. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki R, Berri M, Wu Y, et al. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/ S100A1. Biophys J. 2001;81:2297–2313. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerries M, Most P, Gledhill JR, et al. Ca2 +-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol Cell Biol. 2007;27:4365–4373. doi: 10.1128/MCB.02045-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Most P, Bernotat J, Ehlermann P, et al. S100A1: a regulator of myocardial contractility. Proc Natl Acad Sci U S A. 2001;98:13889–13894. doi: 10.1073/pnas.241393598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Most P, Eicher C, Börries M, et al. Distinct subcellular location of the Ca2 +-binding protein S100A1 differentially modulates Ca2+-cycling in ventricular rat cardiomyocytes. J Cell Sci. 2005;118:421–431. doi: 10.1242/jcs.01614. [DOI] [PubMed] [Google Scholar]
- 12.Desjardins JF, Pourdjabbar A, Quan A, et al. Lack of S100A1 in mice confers a gender-dependent hypertensive phenotype and increased mortality after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1457–H1465. doi: 10.1152/ajpheart.00088.2008. [DOI] [PubMed] [Google Scholar]
- 13.Du XJ, Cole TJ, Tenis N, et al. Impaired cardiac contractility response to hemodynamic stress in S100A1- deficient mice. Mol Cell Biol. 2002;22:2821–2829. doi: 10.1128/MCB.22.8.2821-2829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Most P, Seifert H, Gao E, et al. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann GE, Domenighetti AA, Deten A, et al. S100A1 deficiency results in prolonged ventricular repolarization in response to sympathetic activation. Gen Physiol Biophys. 2008;27:127–142. [PubMed] [Google Scholar]
- 16.Voelkers M, Weidenhammer C, Herzog N, et al. S100A1 prevents arrythmogenic diastolic sarcoplasmic reticulum calcium leak in ventricular cardiomyocytes. Circulation. 2008;118:S_527. [Google Scholar]
- 17.Pleger ST, Most P, Boucher M, et al. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 18.Pleger ST, Shan C, Kszienck J, et al. Cardiac AAV9-S100A1 rescues postischemic heart failure and reverses remodeling in a preclinical large animal model. Sci Transpl Med. 2011 doi: 10.1126/scitranslmed.3002097. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remppis A, Greten T, Schafer BW, et al. Altered expression of the Ca(2+)-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 20.Pieske B, Kretschmann B, Meyer M, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 21.Venetucci LA, Trafford AW, O’Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 22.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatineto-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 24.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Most P, Koch WJ. Sealing the leak, healing the heart. Nat Med. 2003;9:993–994. doi: 10.1038/nm0803-993. [DOI] [PubMed] [Google Scholar]
- 26.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43:523–531. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.