Summary
Anabolic-androgenic steroids (AAS) increase impulsive and uncontrolled aggressive (‘roid rage) in humans and enhance agonistic behavior in animals. However, the underlying mechanisms for AAS-induced aggression remain unclear. Potential contributing elements include an increase risk-taking and/or motor impulsivity due to AAS. This study addressed the effects of chronic high-dose testosterone on risk tolerance using a risky decision-making task (RDT) and motor impulsivity with a go/no-go task in operant chambers. Male Long-Evans rats were treated for at least 4 weeks with testosterone (7.5mg/kg) or vehicle beginning in late adolescence. Testosterone was used because it is popular among human AAS users. In RDT testing, one lever was paired with delivery of a small “safe” food reward, while the other was paired with a large “risky” reward associated with an increasing risk of footshock (0, 25, 50, 75, 100%) in successive test blocks. Three shock intensities were used: 1.0, 1.2, and 1.4 mA/kg. As shock intensity and risk of shock increased, preference for the lever signifying a large reward significantly declined for both vehicle- and testosterone-treated rats (p<0.05). There was also a significant effect of drug (p<0.05), where testosterone-treated rats showed greater preference for the large reward, compared to vehicle- treated controls. Increased preference for the large reward, despite risk of footshock, is consistent with increased risk tolerance. In go/no-go testing, rats were trained to press a single lever if the go cue was presented (stimulus light) or to refrain from pressing during the no-go cue (tone). There was no effect of testosterone on pre-cue responses, or performance in go and no-go trials. These results suggest that AAS may increase risk-tolerance without altering motor impulsivity.
Keywords: anabolic agents, risk, reward, punishment, response inhibition, go/no-go
Introduction
Anabolic-androgenic steroids (AAS) are derivatives of testosterone used by athletes at all levels, from casual to elite competitors, to increase muscle-mass and improve performance. Recently, there is concern about negative psychological side-effects of AAS abuse (Kanayama et al, 2009). In particular, AAS can increase aggression in humans and animals, with manic-like episodes of anger (‘roid rage) (Schulte et al, 1993; Breuer et al, 2001; Midgley et al, 2001; Farrell and McGinnis, 2003; Pagonis et al, 2006). However, underlying mechanisms for AAS-induced aggression remain unclear. In humans, aggression has been classified as hostile (impulsive, with intent to injure) or instrumental (premeditated, with intent for personal benefit; Ramirez and Andreu, 2006). In AAS users, ‘roid rage encompasses irritability, impaired judgment, and feelings of invincibility (Katz and Pope, 1990). This is consistent with hostile, impulsive aggression. Conversely, endogenous testosterone in humans correlates with power motivation and risk-taking in economic and social domains (reviewed in Wood and Stanton, 2012), which would reflect instrumental aggression. Furthermore, to understand how AAS may promote hostile and instrumental aggression, it is difficult to rule out the possibility that AAS users might have a predisposition towards impulsivity and risk-taking. Therefore, the present study investigated the effects of AAS on impulsive behavior and risk assessment in a rodent model. In particular, because impulsive behavior is multi-faceted (Winstanley, 2011), we have focused here on motor impulsivity and risk-taking in response to punishment. Male rats were exposed chronically to high-dose testosterone as young adults and behavior was evaluated by established methods using operant responding for food to measure risk tolerance (Simon et al, 2009; Simon and Setlow, 2012) and motor impulsivity (Fardell et al, 2010; Moschak and Mitchell, 2012).
High-dose testosterone treatment in in young adult male rats offers parallels with human use, and has a precedent in animal studies. While media attention focuses on ‘designer’ steroids used by elite athletes, it appears that a young adult male taking exogenous testosterone reflects a more typical AAS user. The majority of users are men: among American high school students, 4–6% of men have used AAS vs 1–2% of women (Bahrke and Yesalis, 2004). AAS abuse frequently begins in the early 20's (Pope et al, 2013), coincident with the peak in endogenous testosterone production. Many animal studies of AAS on mating and aggression have used adolescent males (Farrell and McGinnis, 2004; McGinnis, 2004; Cunningham and McGinnis, 2006).
Testosterone is used here because it is the prototypical AAS, both for its popularity and for its chemical structure. All AAS are derived from testosterone. Furthermore, testosterone remains a frequent choice for human users, typically in the long-acting forms such as testosterone propionate (Summers, 2003). In 2011, testosterone was the most- common ‘adverse analytical finding’ in urine tests at World Anti-Doping Agency laboratories (WADA, 2012). Testosterone is also popular among rank-and-file users because of its low price and ready availability (Wood and Stanton, 2012). Most AAS users do not limit themselves to a single dose or a single type of steroid (Summers, 2003). Instead, users combine different steroids (“stacking”) in cycles of increasing and decreasing concentrations. AAS stacks also include non-steroidal drugs to counteract side effects (aromatase inhibitors, estrogen receptor antagonists), to enhance fat and water loss (diuretics, thyroid hormones, β2 adrenergic receptor agonists) and to reactivate endogenous steroidogenesis at the end of a cycle (gonadotropins). While testing AAS stacks is clinically relevant, it becomes difficult to evaluate the contributions of any individual element in the stack. To focus and simplify these studies, treatment was limited to one androgen at a single, consistent dose.
Impulsivity incorporates at least three components including impatience, reduced response inhibition (motor impulsivity), and increased risk-taking (Evenden, 1999; Eagle and Baunez, 2010; Winstanley, 2011). Methods in animals using operant responding for food reward have been developed to individually evaluate these behaviors. For impatience, delay-discounting measures preference for a large reward despite delays in delivery. Stop-signal reaction time and go/no-go tasks measure response inhibition: the ability to inhibit a planned action. The rodent gambling task (rGT) and risky decision-making task (RDT) estimate risk-taking, where the risk is either punishment (footshock in RDT) or absence of reward (rGT).
A recent study from our laboratory (Wood et al, 2013) showed that testosterone treatment at pharmacologic doses in male rats decreased impulsive behavior in a delay-discounting task (Winstanley et al, 2006). Compared with controls, testosterone-treated rats showed greater preference for a large delayed reward. While testosterone may not increase impatience, it could affect other aspects of impulsivity. Thus, the present study investigated testosterone's effects on risk-taking with RDT (Simon et al, 2009; Simon and Setlow, 2012) and response inhibition using the go/no-go task (Fardell et al, 2010; Moschak and Mitchell, 2012). Our hypothesis is that chronic high-dose testosterone increases impulsive behavior by increasing risk tolerance and impairing response inhibition.
Methods
Animals
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA) were individually housed under a reversed 14L:10D photoperiod. RDT was tested in 10 vehicle- and 10 testosterone-treated rats. The go/no-go test included a separate group of 8 vehicle-treated rats and 10 rats treated with testosterone. All rats remained gonad-intact to approximate AAS use in humans. Injection, training and testing were conducted 5x/week under dim illumination during the first 4h of the dark phase. To facilitate operant responding, rats were weighed daily and food availability was adjusted to maintain a slow rate of growth (1-2 g/day). Experimental procedures were approved by USC's Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Ed (National Research Council, National Academies Press, Washington DC; 2011).
AAS treatment
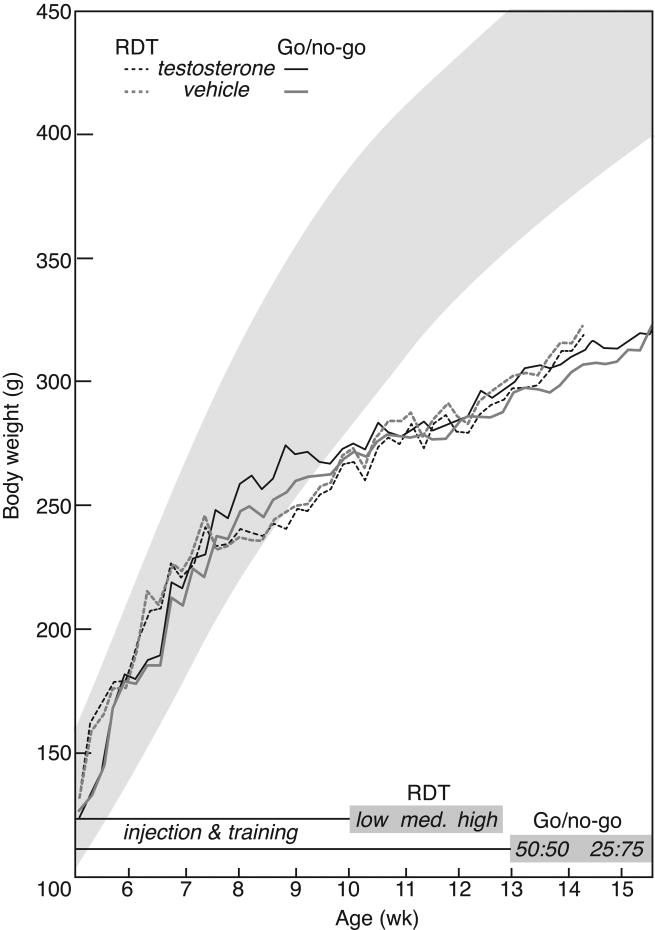
Rats were injected SC with 4-androsten-17β-ol-3-one (testosterone; 7.5 mg/kg; Steraloids, RI) or aqueous vehicle (13% cyclodextrin with 3% ethanol; RBI, MA) immediately before daily behavioral training and testing. This dose approximates a heavy steroid dose in humans adjusted to body surface area according to US Federal Drug Administration guidelines (FDA, 2005), and has been used previously to demonstrate AAS effects on mating and aggression in rats (Clark and Fast, 1996; Clark et al, 1998; Wood et al, 2013). Rats were received at 5 weeks of age, and were 6 weeks of age when injections began, at the end of adolescence as defined by Spear (2000). They received daily injections during training for RDT or go/no-go tasks. For RDT, behavior testing began at an average of 10 weeks of age, after at least 4 weeks of exposure to testosterone or vehicle, and continued until rats were 16 weeks of age. The go/no-go test required more extensive training, until rats averaged 13 weeks of age. Go/no-go testing was complete when rats were 15 weeks of age (Figure 1).
Figure 1.
Body weights in testosterone (black lines) and vehicle-treated rats (gray lines) in relation to training and testing for the footshock RDT (dashed lines) and go/no-go tasks (solid lines). Shading represents body weights (mean ± 1 SD) for Long-Evans rats fed ad libitum. See methods for details.
Operant Chambers
Training and testing were conducted in operant conditioning chambers controlled by WMPC software (Med Associates, VT). Each chamber was equipped with a house light, food pellet dispenser with trough to deliver 45-mg sucrose pellets (Bioserve Inc, NJ), two retractable levers with stimulus lights, a nose-poke with stimulus light, and Sonalert module (2,900Hz at 65db). Levers flanked the pellet trough, and the nose poke was located on the opposite wall. Footshock was administered through the cage floor using a constant current aversive stimulation module. Chambers were enclosed in a sound-attenuating cubicle.
Footshock: RDT
The experimental design is modified from a previously published protocol (Simon et al, 2009; Simon and Setlow, 2012), and is summarized in Figure 2A. Rats were trained to press both levers to receive sucrose pellets. One lever was paired with delivery of one pellet (small reward), while the opposite lever was paired with delivery of three pellets (large reward). Response for the large reward was paired with a mild footshock, with an increasing shock risk (0, 25, 50, 75, and 100%) across each daily session. Rats were tested with three shock intensities, beginning at the lowest intensity. Risk tolerance was evaluated as continued preference for the large “risky” reward over the small “safe” (no- shock) reward across the range of shock risk.
Figure 2.
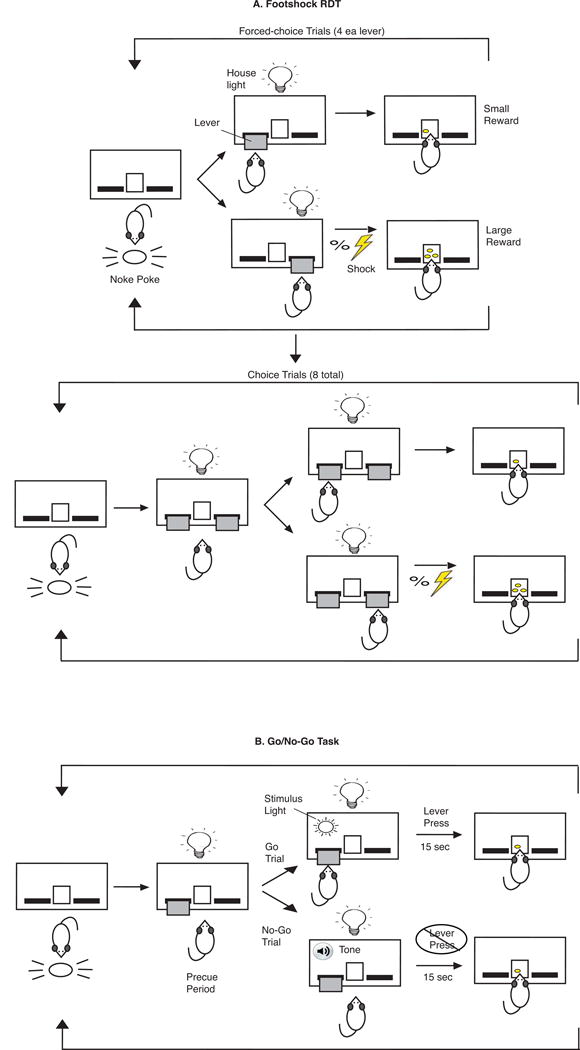
Diagram of footshock RDT (A) and go/no-go (B) tests. A shows 1 block of 8 forced-trials (top), followed by 8 choice trials (bottom) where the rat initiates each trial with a response on the nose-poke, followed by extension of 1 (forced-choice trials) or 2 levers (choice trials). Selection of the lever paired with the large reward has a probability of footshock (lightning bolt). At the end of each trial, the levers retract and the house-light is extinguished. B shows the sequence of events in go and no-go trials, where a stimulus light reflects the onset of a go trial in which the rat must press the lever within 15 sec to receive a food reward, while a tone signifies a no-go trial in which the rat must refrain from pressing the lever for 15 sec to receive a food reward. See methods for details.
Training
Beginning at least two weeks after the first testosterone or vehicle injection, rats were trained to press both levers to obtain a sucrose pellet on a continuous reinforcement schedule. Rats initiated each trial by responding on the nose poke to trigger extension of one lever into the operant chamber. Rats had 30s to respond before the lever retracted and the trial was scored as an omission. A 2s intertrial interval (ITI) in darkness with both levers retracted began immediately after a lever press or trial omission, after which the nose-poke light was re-illuminated and active. Rats completed >50 trials/20-min session before training for reward discrimination.
In this next phase of training, one lever (left or right, counter-balanced between animals) was paired with delivery of three pellets, while the other was paired with delivery of one pellet. A session consisted of 80 trials divided into five blocks of 16 trials each. Each block began with eight forced-choice trials where only one lever was available (four trials/lever). The rat could respond on the lever to receive pellet(s) or permit the lever to retract (trial omission). This was followed by eight choice trials where both levers were presented, offering the choice among one pellet, three pellets, or trial omission. After the final choice trial in each block, an inter-block interval was distinguished with levers retracted and lights extinguished for 20s. Criteria for reward discrimination was completion of 80 trials within 60 min, with >90% preference for the large reward during choice trials.
Testing
Footshock test sessions were identical to reward discrimination except selection of the large reward, during both forced-choice and choice trials, was now paired with an increasing risk of footshock across test blocks (0, 25, 50, 75, and 100%). Forced-choice trials at the start of each block demonstrated the increased risk. Rats were tested with three shock intensities (1.0, 1.2, and 1.4mA/kg), beginning with the lowest. Testing at each shock intensity continued for at least seven days until responses stabilized. Responses were considered stable when there was no effect of time by repeated-measures ANOVA (RM-ANOVA) comparing choice of the large reward at each risk block over five days of testing, with time as the repeated measure. Shock intensity was comparable to that used previously (Simon et al, 2009; Simon et al, 2012), except that values were normalized to body weight to minimize confounding effects of weight on response to footshock.
Go/No-go: Response inhibition
Figure 2B depicts the experimental design modified from previous studies (Fardell et al, 2010; Moschak and Mitchell, 2012). Only one lever was used for training and testing. When the lever extended at the start of each trial, there was a short pre-cue period, during which responses on the lever were recorded but not reinforced. For go trials, the stimulus light above the lever was illuminated, and pressing the lever within 15s was paired with delivery of a sucrose pellet. During no-go trials, a tone sounded and a pellet was delivered if the lever was not pressed for 15s. Go and no-go trials were presented at a 50:50 ratio, and subsequently at 25:75. Response inhibition was measured as percent successful completion of no-go trials, as well as number of responses during the pre-cue period.
Training
Rats were initially trained to press a single lever (left or right, counterbalanced between animals) in go trials to obtain a sucrose pellet on a continuous reinforcement schedule. Trials were initiated by responding on the nose poke to illuminate both the house light and stimulus light, and trigger extension of the lever. Rats had 15s to respond on the lever to receive a pellet. If not, the lever retracted and the trial was scored as an omission. A 3s ITI followed a lever press or trial omission. Next, rats were trained for no-go trials after successful completion of go trial training (two consecutive days of >100 trials/30-min with 80% success). No-go trials began with a tone sounding for 15s while the stimulus light remained dark and the lever was extended. Any response on the lever terminated the trial and the chamber reverted to ITI conditions. However, if rats refrained from responding during the 15s tone, they received a sucrose pellet. Once rats acquired >80% success over 120 no-go trials, they began testing for go and no-go trials within the same session.
Testing
Rats were tested daily with 120 trials, in which go and no-go trials varied randomly. The first five days of testing presented go and no-go trials at a 50:50 ratio, with another five days at 25:75. For both go and no-go trials, operation of the nose-poke caused the lever to extend and the house light to turn on, initiating a variable pre-cue period (9-24s). This variable duration ensured that rats could not predict when the stimulus light or tone would begin. High pre-cue responses are suggestive of impulsive behavior (Gubner et al, 2010). After the pre-cue period, the stimulus light or tone was presented, and the trial continued. The house light was illuminated during the pre-cue period because rats respond very little during a darkened pre-cue period (Moschak and Mitchell, 2012).
Data Analysis
Footshock
Operant responses and trial omissions during forced-choice and choice trials at each risk block were averaged for each rat across the last five days of testing at each shock intensity. In 8 forced-choice trials at each risk block, the lever paired with the large reward was available during 4 trials. Percent response for the large reward during forced-choice trials for each animal was calculated from the average number of trials in which the rat responded on this lever out of 4 trials in each risk block on each day of testing. In 8 choice trials at each risk block, both levers were available during all 8 trials. Percent choice of the large reward during choice trials for each animal was calculated from the average number of trials in which the lever paired with large reward was selected out of 8 choice trials in each risk block on each day of testing. Individual responses were averaged across the two experimental groups (vehicle and testosterone). Data were analyzed by 2-factor RM-ANOVA using JMP 9.0 statistical software (SAS Institute, NC), comparing the overall effects of drug (vehicle vs testosterone) and shock risk across all shock intensities, with shock intensity as the repeated measure. Subsequently, drug and risk effects were compared within each shock intensity by ANOVA.
Go/No-go
Operant responses and trial omissions during go and no-go trials were averaged for each rat across the five days of 50:50 and 25:75 testing. Individual responses were averaged across the two experimental groups (vehicle and testosterone). Data were analyzed by ANOVA for drug (vehicle vs testosterone) and trial (go vs no-go) effects.
Results
RDT: Choice Trials
Analyzing overall preference for the large reward during choice trials by RM-ANOVA across all shock intensities, there were significant effects of drug, risk, and shock intensity, as well as a shock × risk interaction. Specifically, as shock intensity increased, preference for the large reward decreased significantly [F(2,90)=113.283, p<0.05]. Likewise, as the risk of shock increased, there was a significant decrease in preference for the large reward [F(4,90)=14.858, p<0.05]. This effect was more pronounced at higher shock intensity. Thus, the interaction of shock × risk was also significant [F(8,178)=7.410, p<0.05]. Testosterone-treated rats showed a significantly greater preference for the large reward, compared with vehicle controls [F(1,90)=5.249, p<0.05]. This pattern was consistent as risk and shock intensity increased, and there was no interaction of drug with shock and/or risk [n.s., p>0.05]. Trial omission was negligible: only 27 of 12,000 trials (0.2%) were omitted (15 omissions in 4 vehicle-treated rats, 12 omissions in 5 testosterone-treated rats), and over half of the rats had no omissions at any risk block for all shock intensities. Furthermore, there was no correlation between body weight and reward preference by Pearson's correlation across all shock intensities (data not shown).
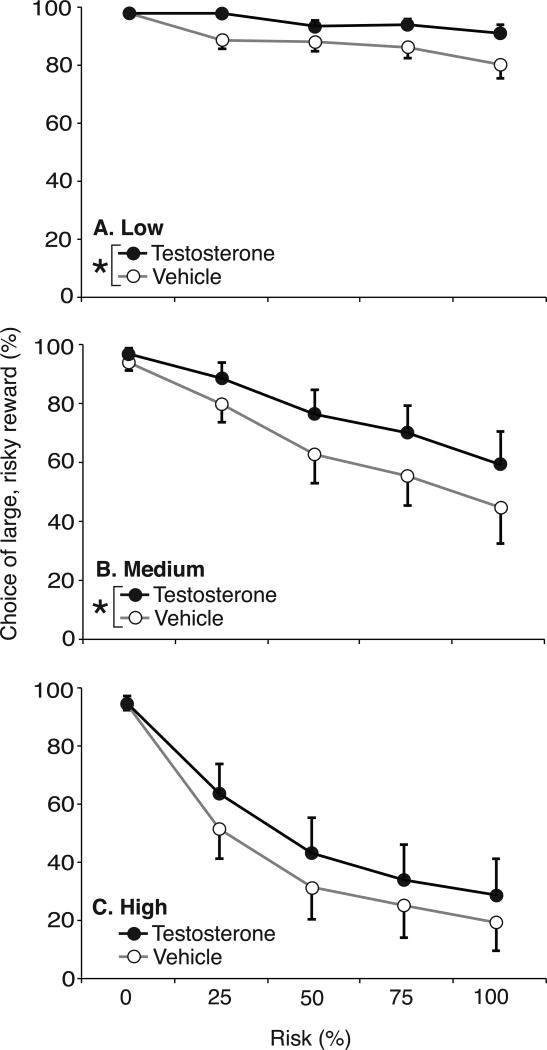
The low shock intensity (1.0 mA/kg: 0.27±0.01 mA) only decreased reward preference to a limited extent (Figure 3A). With no risk of shock, both vehicle- and testosterone-treated rats strongly preferred the large reward, and preference averaged >80% across all risk blocks. Even so, there was a significant effect of risk [F(4,15)=5.876, p<0.05]. In addition, there was a significant effect of drug [F(1,90)=15.524, p<0.05], with testosterone-treated rats showing greater preference for the large reward. At 100% risk, testosterone-treated rats showed 91.0±3.1% preference, compared to vehicle-treated rats at 80.5±4.8%. As shown in Figure 4A, two vehicle-treated rats immediately reduced their preference for the large reward below 80% at the 25% risk block. Testosterone-treated rats did not show a comparable response until 100% risk (Figure 4B).
Figure 3.
Percent preference for the large food reward during RDT choice trials across increasing risk of footshock in testosterone- (closed symbols, n=10) and vehicle-treated rats (open symbols, n=10) at 1.0 mA/kg (Low, A), 1.2 mA/kg (Medium, B) and 1.4 mA/kg (High, C) shock intensity. Data are presented as mean±SEM. Asterisks indicate a significant effect of drug by ANOVA at each shock intensity (p<0.05).
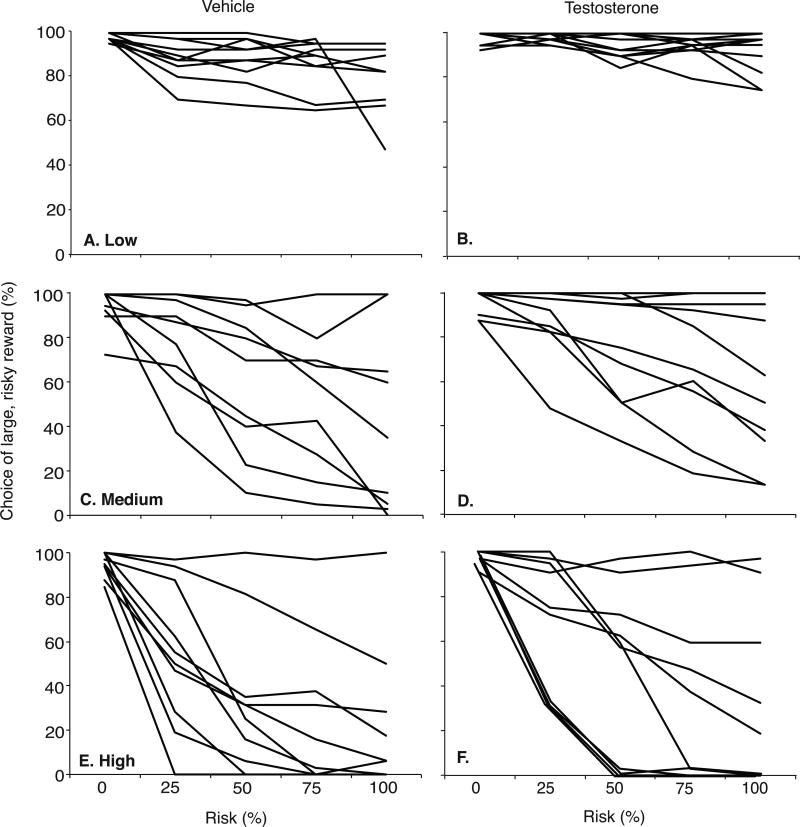
Figure 4.
Percent preference for the large food reward during RDT choice trials across increasing risk of footshock in individual testosterone- (left column, n=10) and vehicle-treated rats (right column, n=10) at 1.0 mA/kg (Low, A,B), 1.2 mA/kg (Medium, C,D) and 1.4 mA/kg (High, E,F) shock intensity. Data are presented as individual means across the final 5 days of testing at each shock intensity.
The medium shock intensity (1.2 mA/kg: 0.35±0.01 mA) significantly decreased large reward preference across the increasing risk blocks (Figure 3B). With no risk of shock, rats preferred the large reward. However, as the risk of shock increased, there was a significant reduction in preference [F(4,90)=8.499, p<0.05] to 44.5±12.4% for vehicle- and 59.0±11.1% for testosterone-treated rats at 100% risk. As with the low shock intensity, there was a significant effect of drug [F(1,90)=4.279, p<0.05], with testosterone-treated rats showing greater preference for the large reward compared with vehicle controls; there was no drug × risk interaction [n.s., p>0.05]. Individual performance varied, as illustrated in Figure 4C & D. Notably, four testosterone-treated rats maintained >80% preference for the large reward across all risk blocks, while only two vehicle-treated rats displayed >80% preference at 100% risk. Furthermore, preference for the large reward at 100% risk decreased to <20% in four vehicle-treated rats, compared to only two testosterone-treated rats.
The highest shock intensity (1.4 mA/kg: 0.43±0.01 mA) significantly decreased large reward preference as footshock risk increased [F(4,90)=17.187, p<0.05] (Figure 3C). At 100% risk, testosterone-treated rats decreased their preference to 28.5±12.2% while vehicle-treated showed only 19.0±9.9% preference. However, the effect of drug was not significant [n.s, p>0.05] and there was no drug × risk interaction [n.s., p>0.05]. Responses of individual rats varied substantially. Among controls, preference for the large reward was <20% in seven of 10 rats by 100% risk, and in four by 50% risk (Figure 4E). In comparison, five of 10 rats with testosterone treatment showed <20% preference at 100% risk, and only two by 50% risk (Figure 4F). Only one vehicle- and two testosterone-treated rats maintained preference >80% across all risk blocks (Figure 4E & F).
RDT: Forced-Choice Trials
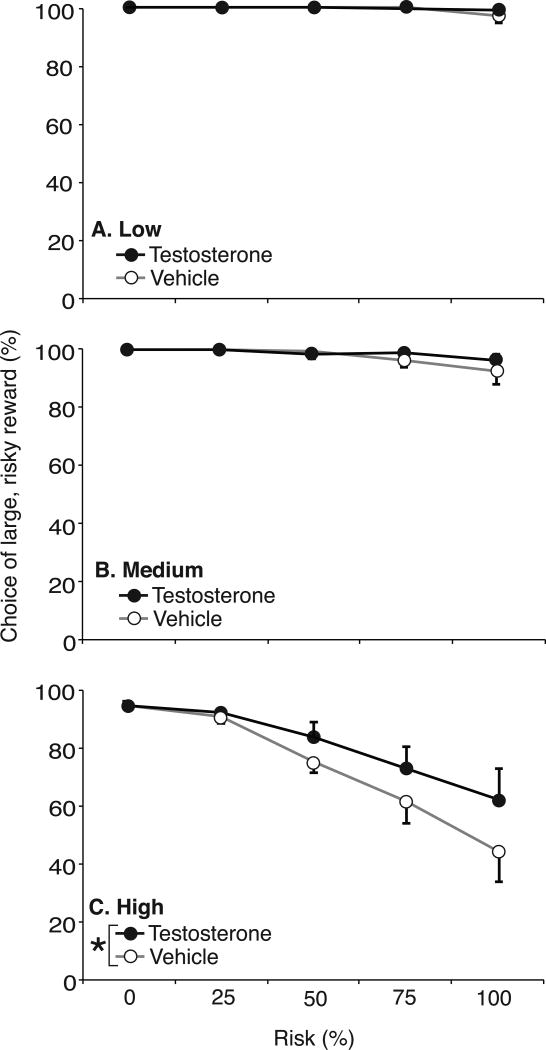
Analyzing overall responses for the large reward during forced-choice trials by RM-ANOVA across all shock intensities, there was an overall effect of risk [F(4,90)=15.847, p<0.05] and shock [F(2,89)=40.512, p<0.05], as well as a shock × risk interaction [F(8,178)=6.545, p<0.05]. Responses for the large reward remained high (>80%) at low and medium intensities, but declined significantly at the highest intensity. There was also a significant overall effect of drug [F(4,90)=4.098, p<0.05], where testosterone-treated rats showed greater response for the large reward. This difference was especially prominent at the highest shock intensity.
At low intensity, footshock did not affect reward response in forced-choice trials: both vehicle- and testosterone-treated rats responded on the lever paired with large reward throughout increasing risk blocks, and there was no effect of risk [n.s., p>0.05] or drug [n.s., p>0.05] (Figure 5A). For vehicle- and testosterone-treated rats, there were 6 and 3 trial omissions, respectively, in 1000 trials each (<1%). With medium shock intensity, response for the large reward remained above 90% even at 100% risk (Figure 5B). Nonetheless, increasing risk significantly decreased responses for the large reward [F(4,90)=3.479, p<0.05]. There was no significant effect of drug and no drug × risk interaction [n.s., p>0.05]. Vehicle-treated rats made 23 trial omissions in 1000 trials (2.3%), vs 12 omissions for testosterone-treated rats (1.2%). By contrast, for the highest shock intensity, increasing risk significantly decreased responding for the large reward in both vehicle- and testosterone-treated rats [F(4,4)= 15.429, p<0.05] (Figure 5C). There was also a significant effect of drug [F(1,1)=4.000, p<0.05], with vehicle-treated rats responding less frequently for the large reward, compared to testosterone-treated rats. Vehicle-treated rats omitted 21.7% of 1000 trials across all risk blocks, including 51% of 200 trials at the 100% risk block. Testosterone-treated rats omitted 11.7% of all trials, and 33.5% of trials at 100% risk. There was no drug × risk interaction [n.s., p>0.05].
Figure 5.
Percent responses for the large food reward during RDT forced-choice trials across increasing risk of footshock in testosterone- (closed symbols, n=10) and vehicle-treated rats (open symbols, n=10) at 1.0 mA/kg (Low, A), 1.2 mA/kg (Medium, B) and 1.4 mA/kg (High, C) shock intensity. Data are presented as mean±SEM. Asterisks indicate a significant effect of drug by ANOVA at each shock intensity (p<0.05).
Go/No-go: Response acquisition and pre-cue response rate
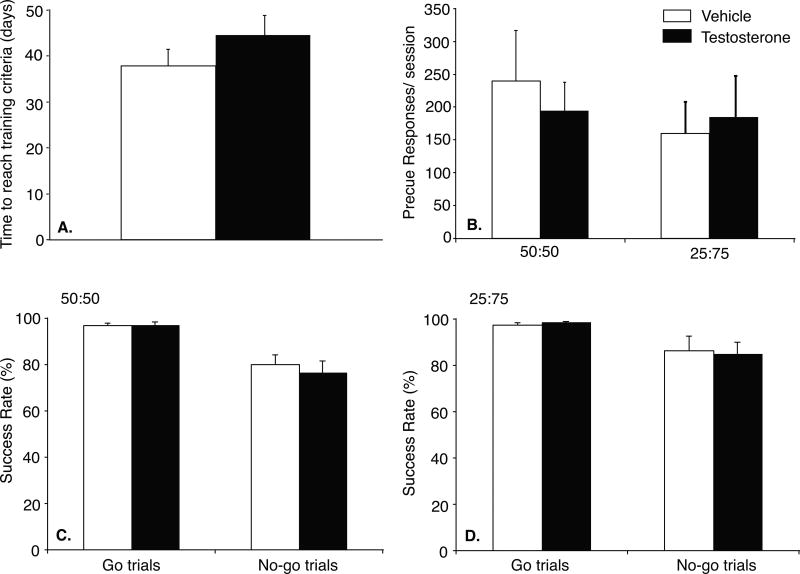
Although testosterone-treated rats took 44.6±4.3 days to master the training steps (Figure 6A), vs 37.9±3.6 days for vehicle controls, this difference was not significant. Likewise, pre-cue responses were not affected by testosterone (Figure 6B). During 50:50 test sessions, vehicle- and testosterone-treated rats made similar pre-cue responses/session. This was sustained at slightly lower rates during 25:75 test sessions.
Figure 6.
A: Time (days) for testosterone- (closed bar, n=9) and vehicle- (open bar, n=8) treated rats to complete training criteria for go/no-go task. B: Pre-cue responses/session during 50:50 and 25:75 test sessions. Percent successful completion of go and no-go trials during 50:50 (C) and 25:75 (D) test sessions. Data are presented as mean±SEM.
Both vehicle- and testosterone-treated rats were highly successful in go-trials, completing at least 97% of trials with reinforcement. No-go trial success was significantly lower. During 50:50 sessions, vehicle-treated rats successfully completed 80.1±4.2% of no-go trials, and testosterone-treated rats completed 76.6±5.2%, with similar results at the 25:75 ratio. By ANOVA, there was a significant effect of trial type (go vs. no-go) [F(1,15)=26.952, p<0.05], but no drug effect nor drug × trial interaction [n.s., p>0.05].
Discussion
The present study investigated the effects of chronic high-dose testosterone on impulsive behavior in rats, specifically with respect to risk-taking and response inhibition. With RDT, testosterone-treated rats showed a greater preference for the large risky reward over a smaller safe reward at low and moderate shock intensities, compared with vehicle-treated controls. By contrast, the go/no-go test of response inhibition was unaffected by testosterone. There was no difference between vehicle- and testosterone-treated rats on their ability to refrain from pressing a lever to receive a food reward. In combination with our earlier study of delay-discounting, these findings suggest that AAS may selectively alter aspects of impulsivity. In particular, testosterone appears to increase risk-tolerance, while simultaneously reducing non-planning impulsive choice measured by delay- discounting. However, there was no effect on motor impulsivity.
Within the normal physiologic range, testosterone has adaptive effects on animal behavior to enhance competition for resources, such as food, territory, and mating opportunities. In humans, testosterone offers similar competitive advantages in business, law, and political arenas (reviewed in Wood and Stanton, 2012). However, AAS users consume androgens up to 100x normal endogenous levels. Under these circumstances, it is reasonable to expect pathologic changes in physiology (on the reproductive, hepatic, and cardiovascular systems) and behavior (social and cognitive behavior; Kanayama et al, 2010). In the present study, testosterone treatment at 7.5 mg/kg aims to model excessive androgen intake among human AAS abusers. As a comparison, testosterone replacement at 2 g/kg in castrated males restores circulating androgens to the level of gonad-intact males (Liu et al, 2006).
Understanding AAS-induced aggression was a primary motivation to investigate AAS-induced impulsive behavior. In both human and animal studies, AAS increase aggressive behavior (Schulte et al, 1993; Breuer et al, 2001; Midgley et al, 2001; Farrell and McGinnis, 2003; Pagonis et al, 2006). This has given rise to the popular image of ‘roid rage: a swift loss of control, often leading to crimes of passion (McGinnis, 2004). ‘Roid rage has been implicated in several violent crimes, including murder (Pope and Katz, 1990; Pope et al, 1996). Horace Williams submitted the first steroid insanity plea in 1988 (Greenbaum, 1988), and steroids have been implicated in the trial of Oscar Pistorius in 2013. While the steroid defense has yet to be successful, the role of AAS in behavior and the validity of ‘roid rage as an uncontrolled and impulsive response are important aspects of these cases.
Therefore, the focus of the present study was to explore testosterone-induced impulsivity as a potential contributing factor to testosterone-induced aggression. This has implications for whether ‘roid rage necessarily involves a loss of control. Already, studies in rats suggest that AAS-induced aggression is not insensitive to social and environmental cues. With provocation (tail pinch), AAS-treated male rats will attack atypical conspecific targets, including castrated males and anestrous females (McGinnis et al, 2002; Cunningham and McGinnis, 2006).
However, they will not attack estrous females. The results of the present study build on these earlier reports to suggest that while AAS do alter behavior, it is a more calculated response, rather than an uncontrolled reaction.
Impulsivity covers a broad category of behaviors loosely defined as premature actions made without forethought or planning and with disregard to risk, which often result in undesirable consequences (Evenden, 1999). As such, impulsive behavior incorporates at least three dimensions: impatience (non-planning), reduced response inhibition (motor impulsivity), and increased risk-taking. In a previous study, we evaluated the effect of testosterone on non-planning behavior using a delay-discounting test for food reward (Wood et al, 2013). In this task, rats choose between two levers: one paired with a small reward immediately, the other paired with a larger reward after an increasing delay (up to 45s). Preference for the small immediate reward reflects impatience. Somewhat surprisingly, testosterone-treated rats preferred the large delayed reward more frequently than vehicle-treated controls, suggesting that testosterone may actually reduce impatience. The current study extends our understanding of testosterone's effects on impulsive behaviors by investigating response inhibition and risk assessment. The results suggest that testosterone exposure at pharmacologic doses increases risk tolerance, but does not alter response inhibition. Together, these findings suggest that androgens modify impulsive choice without influencing impulsive action. This is consistent with the concept that testosterone-induced aggression does not reflect a loss of control (McGinnis, 2004). Nonetheless, it is important to note here that testosterone-treated rats had extensive exposure to testosterone prior to training and testing; baseline levels of risk-taking prior to testosterone treatment were not measured. In this regard, we did observe substantial within-group variability in response to footshock among both vehicle- and testosterone-treated rats. Future studies could evaluate measures of anxiety- and depression-related behaviors, as potential contributors to this individual variability.
The go/no-go test is one of several tests of motor impulsivity. Motor impulsivity is an inability to inhibit prepotent motor responses, and is a characteristic feature of attention-deficit hyperactivity disorder (Chamberlain and Sahakian, 2007). In particular, motor impulsivity is often thought to reflect impaired action inhibition, rather than enhanced action initiation (Kalis et al, 2008). The go/no-go test measures the ability to inhibit a planned action. Tests of motor impulsivity can emphasize ‘action cancellation’ (inhibition of an on-going action, as in the stop-signal task) vs ‘action restraint’ (withholding of a planned action, as in the go/no-go test; Winstanley, 2011). Acute ethanol administration and chronic treatment with the chemotherapeutic agent methotrexate impair performance on the go/no-go test (Fardell et al, 2010; Moschak and Mitchell, 2012). Nonetheless, we cannot rule out the possibility that AAS might selectively impair action cancellation, rather than action restraint. This could be addressed using the stop-signal or five-choice serial reaction time tasks. Motor impulsivity is present in a number of conditions with a strong male bias, including attention-deficit-hyperactivity disorder, Parkinson's Disease, and schizophrenia (Eagle and Baunez, 2010). This might suggest a role for androgens in motor impulsivity, and thus impaired performance on the go/no-go task. Furthermore, serotonin is critical for action restraint (Eagle and Baunez, 2010; Winstanley, 2011), and AAS inhibit the serotonergic system. AAS reduce serotonin receptors in the anterior hypothalamus and medial amygdala of male Syrian hamsters (Grimes and Melloni, 2002), and serotonin receptor mRNA in the PFC and amygdala of mice (Ambar and Chiavegatto, 2009). However, our study did not find evidence of AAS-induced impairment of action inhibition.
The disparate effects of testosterone on impatience, motor impulsivity and risk tolerance are consistent with the concept that impulsivity is not a single behavior. Although impatience and risky decision-making may be correlated (e.g. pathologic gambling), sometimes they are not (as in schizophrenia) (Winstanley, 2011). Looking at the data in another way, the effects of testosterone on the RDT and delay-discounting tasks are not dissimilar. In both tests, testosterone-treated rats maximized food reward despite delay (delay-discounting) or punishment (RDT). It is unlikely that this is due to proximate causes of increased hunger or insensitivity to pain. Chronic treatment with testosterone and other AAS at high doses in male rats does not increase food consumption (Bisschop et al, 1997; Lindblom et al, 2003) or reduce responsiveness to pain, including acute thermal and mechanical nociceptive stimuli applied to the paws as in the present study (Celerier et al 2003; Tsutsui et al, 2011). Instead, it might appear that testosterone reduces aspects of cognitive flexibility, to favor a “win-at-all- costs” approach to maximizing gains. To our knowledge, no published studies have evaluated the effects of high-dose testosterone on progressive ratio (PR) schedules of reinforcement for food or drug reward. In studies of nicotine and cocaine self-administration, there was no relationship between endogenous testosterone and levels of responding under a PR schedule in adolescent male rats (Lynch 2008, 2009). However, this does not rule out the potential for exogenous testosterone at high doses to increase effort for reward.
In terms of brain targets and mechanisms for androgen action, the present study cannot resolve whether the effects of testosterone on performance in the RDT are due to acute vs chronic actions. Although testosterone is classically thought to act over a relatively slow time-course (days to weeks), the rewarding effects of high-dose androgens can be rapid (minutes to hours) and do not require classical genomic androgen receptors (Sato et al, 2010). In this regard, brain circuits for reward and executive function, including the ventral striatum and frontal cortex, have relatively few androgen receptors. However, dopaminergic projections to nucleus accumbens from androgen-responsive neurons in the ventral tegmental area have been described (Creutz and Kritzer, 2004).
Neural circuitry for impulsivity involves not only serotonin, but also dopaminergic activity, particularly the connections of prefrontal and orbitofrontal cortex with dorsal striatum (Mobini et al, 2002; Winstanley et al, 2004; Rudebeck et al, 2006). In rGT, lesions of orbitofrontal cortex increased preference for the high-risk/large reward over the low-risk/small reward (Pais-Vieira et al, 2007). Other studies have implicated the nucleus accumbens (Cardinal and Howes, 2005), basolateral amygdala (Ghods-Sharifi et al, 2009), and dopamine (St. Onge and Floresco, 2009) in biasing choice toward larger probabilistic rewards. Treatments that increase dopamine (stimulants, inhibitors of dopamine reuptake) increase choice of a large uncertain reward (Young et al, 2011; Cocker et al, 2012). Conversely, antagonists of dopamine (St. Onge et al, 2010), dopamine D1 receptors (St. Onge et al, 2011) and D2 receptors reduce this response (Cocker et al, 2012). However, dopamine has opposite effects on the response to punishment. In particular, either amphetamine or the dopamine D2 receptor agonist bromocriptine reduced responding for large reward paired with footshock, while the D2 receptor antagonist eticlopride reversed the suppressive effect of amphetamine (Simon et al, 2011). Previous studies have found that AAS reduce dopamine and its D1 receptor in the nucleus accumbens and dorsal striatum, though D2 receptors may be increased (Kindlundh et al, 2003). The effects of androgens in the present study are consistent with diminished activity of dopamine D2 receptors in the nucleus accumbens.
Acknowledgments
The authors thank Mary Rivas for assistance with animal handling.
Role of the funding source: This work was supported by NIH RO1-DA029613.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Contributors: Sarah E. Cooper
Sydney P. Going
Jessica Y. Kim
Ruth I. Wood
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and down-regulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8(2):161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE. Abuse of anabolic-androgenic steroids and related substances in sport and exercise. Curr Opin Pharmacol. 2004;4(6):614–620. doi: 10.1016/j.coph.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bisschop A, Gayan-Ramirez G, Rollier H, Dekhuijzen PN, Dom R, de Bock V, Decramer M. Effects of nandrolone decanoate on respiratory and peripheral muscles in male and female rats. J Appl Physiol. 1997;82(4):1112–1118. doi: 10.1152/jappl.1997.82.4.1112. [DOI] [PubMed] [Google Scholar]
- Breuer ME, McGinnis MY, Lumia AR, Possidente BP. Aggression in male rats receiving anabolic-androgenic steroids: effects of social and environmental provocation. Horm Behav. 2001;40(3):409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 2005;6:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Yazdi MT, Castane A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol. 2003;465(1-2):69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Clark AS, Blasberg ME, Brandling-Bennett EM. Stanozolol, oxymetholone, and testosterone cypionate effects on the rat estrous cycle. Physiol Behav. 1998;63(2):287–295. doi: 10.1016/s0031-9384(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Clark AS, Fast AS. Comparison of the effects of 17 alpha-methyltestosterone, methandrostenolone, and nandrolone decanoate on the sexual behavior of castrated male rats. Behav Neurosci. 1996;110(6):1478–1486. doi: 10.1037//0735-7044.110.6.1478. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Dinelle K, Kornelson R, Sossi V, Winstanley CA. Irrational choice under uncertainty correlates with lower striatal D(2/3) receptor binding in rats. J Neurosci. 2012;32(44):15450–15457. doi: 10.1523/JNEUROSCI.0626-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476(4):348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, McGinnis MY. Physical provocation of pubertal anabolic-androgenic steroid-exposed male rats elicits aggression towards females. Horm Behav. 2006;50(3):410–416. doi: 10.1016/j.yhbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34(1):50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Logge W, Johnston I. Single high-dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacol Biochem Behav. 2010;97(2):333–339. doi: 10.1016/j.pbb.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav Neurosci. 2003;117(5):904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- Farrell SF, McGinnis MY. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm Behav. 2004;46(2):193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers Center for Drug Evaluation and Research. [Accessed July 11, 2013];2005 http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf.
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009;29(16):5251–5259. doi: 10.1523/JNEUROSCI.0315-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum K. Sun-Sentinel; Deerfield Beach, FL: 1988. [Accessed November 20, 2013]. Mood changes focus of defense testimony. http://articles.sun-sentinel.com/1988-06-08/news/8802030649_1_insanity-defense-horace-williams-violent-behavior. [Google Scholar]
- Grimes JM, Melloni RH. Serotonin modulates offensive attack in adolescent anabolic steroid-treated hamsters. Pharmacol Biochem Behav. 2002;73(3):713–721. doi: 10.1016/s0091-3057(02)00880-8. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34(8):1353–1362. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalis A, Mojzisch A, Schweizer TS, Kaiser S. Weakness of will, akrasia, and the neuropsychiatry of decision making: an interdisciplinary perspective. Cog Affective Behav Neurosci. 2008;8(4):402–17. doi: 10.3758/CABN.8.4.402. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG., Jr Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009;104(12):1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr Illicit anabolic-androgenic steroid use. Horm Behav. 2010;58(1):111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DL, Pope HG. Anabolic-androgenic steroid-induced mental status changes. NIDA Res Monogr. 1990;102:215–223. [PubMed] [Google Scholar]
- Kindlundh AM, Lindblom J, Nyberg F. Chronic administration with nandrolone decanoate induces alterations in the gene-transcript content of dopamine D(1)- and D(2)-receptors in the rat brain. Brain Res. 2003;979(1-2):37–42. doi: 10.1016/s0006-8993(03)02843-9. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Kindlundh AM, Nyberg F, Bergstrom L, Wikberg JE. Anabolic androgenic steroid nandrolone decanoate reduces hypothalamic proopiomelanocortin mRNA levels. Brain Res. 2003;986(1-2):139–147. doi: 10.1016/s0006-8993(03)03223-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Tsang S, Wong TM. Testosterone is required for delayed cardioprotection and enhanced heat shock protein 70 expression induced by preconditioning. Endocrinology. 2006;147(10):4569–4577. doi: 10.1210/en.2006-0297. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacol. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY. Anabolic-androgenic steroids and aggression: studies using animal models. Ann N Y Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Breuer ME, Possidente B. Physical provocation potentiates aggression in male rats receiving anabolic-androgenic steroids. Horm Behav. 2002;41(1):101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- Midgley SJ, Heather N, Davies JB. Levels of aggression among a group of anabolic-androgenic steroid users. Med Sci Law. 2001;41(4):309–314. doi: 10.1177/002580240104100407. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160(3):290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Mitchell SH. Acute ethanol administration and reinforcer magnitude reduction both reduce responding and increase response latency in a go/no-go task. Alcohol Clin Exp Res. 2012;36(10):1803–1810. doi: 10.1111/j.1530-0277.2012.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS, Toli PN. Psychiatric and hostility factors related to use of anabolic steroids in monozygotic twins. Eur Psychiatry. 2006;21(8):563–569. doi: 10.1016/j.eurpsy.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience. 2007;145(1):225–231. doi: 10.1016/j.neuroscience.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am J Addict. 2013 doi: 10.1111/j.1521-0391.2013.12118. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51(1):28–31. [PubMed] [Google Scholar]
- Pope HG, Kouri EM, Powell KF, Campbell C, Katz DL. Anabolic-androgenic steroid use among 133 prisoners. Compr Psychiatry. 1996;37(5):322–327. doi: 10.1016/s0010-440x(96)90013-9. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Andreu JM. Aggression, and some related psychological constructs (anger, hostility, and impulsivity); some comments from a research project. Neurosci Biobehav Rev. 2006;30(3):276–291. doi: 10.1016/j.neubiorev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9(9):1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Sato SM, Johansen J, Jordan CL, Wood RI. Membrane, not nuclear, androgen receptor mediates androgen reinforcement. Psychoneuroendocrinol. 2010;35(7):1063–1073. doi: 10.1016/j.psyneuen.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte HM, Hall MJ, Boyer M. Domestic violence associated with anabolic steroid abuse. Am J Psychiatry. 1993;150(2):348. doi: 10.1176/ajp.150.2.348a. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision-making. Neuropsychopharmacology. 2009;34(10):2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, Banuelos C, Vokes CM, Taylor AB, Haberman RP, Bizon JL, Setlow B. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31(48):17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Modeling risky decision-making in rodents. Methods Mol Biol. 2012;829:165–175. doi: 10.1007/978-1-61779-458-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31(23):8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Chiu YC, Floresco SB. Differential effects of dopaminergic manipulations on risky choice. Psychopharmacol. 2010;211(2):209–221. doi: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–697. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Summers J. Steroids 101. Anabolics.com, Inc; Aurora, CO.: 2003. [Google Scholar]
- Tsutsui KT, Wood RI, Craft RM. Anabolic-androgenic steroid effects on nociception and morphine antinociception in male rats. Pharmacol Biochem Behav. 2011;99(3):500–508. doi: 10.1016/j.pbb.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA. [Accessed 05/02/13];2012 http://www.wada-ama.org/Documents/Resources/Testing-Figures/WADA-2011-Laboratory-Testing-Figures.pdf.
- Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164(4):1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24(20):4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16(1):106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- Wood RI, Armstrong A, Fridkin V, Shah V, Najafi A, Jakowec M. 'Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase. Physiol Behav. 2013;110-111:6–12. doi: 10.1016/j.physbeh.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Stanton SJ. Testosterone and sport: current perspectives. Horm Behav. 2012;61(1):147–155. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. J Psychopharmacol. 2011;25(7):934–943. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]