Abstract
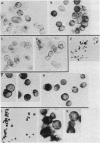
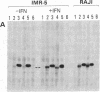
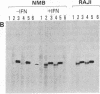
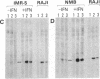
Previous work showed that each of four human neuronal cell lines expresses less than or equal to 0.5% of the HLA-A,B,C and beta 2-microglobulin seen in glial, lymphoid, and other cell types, and there is a corresponding weak expression in neuroblastoma tumor and adult brain. Here, we probe the genetic basis of this weak expression. For each of three neuroblastoma cell lines, we show that HLA-A,B,C and beta 2-microglobulin can be induced by interferon and that the induction occurs within every cell of the population. Class II (Ia) molecules are not detected. Microscopic assay and radioimmunoassay of intact cells suggest that the induced antigen appears at the cell surface as well as within each cell. Immunoblot analysis confirms that the induced proteins have the structure of class I molecules. Thus, the normal weak HLA and beta 2-microglobulin expression of these cell lines appears to reflect a regulatory control rather than a primary genetic lesion. According to current theory, lack of HLA-A,B,C should protect transformed, infected, or damaged neurons--but also neurons in neural transplants--from T-cell-mediated immunosurveillance. The possibility that neuronal HLA-A,B,C expression may be under regulatory control is of importance in this context.
Full text
PDF
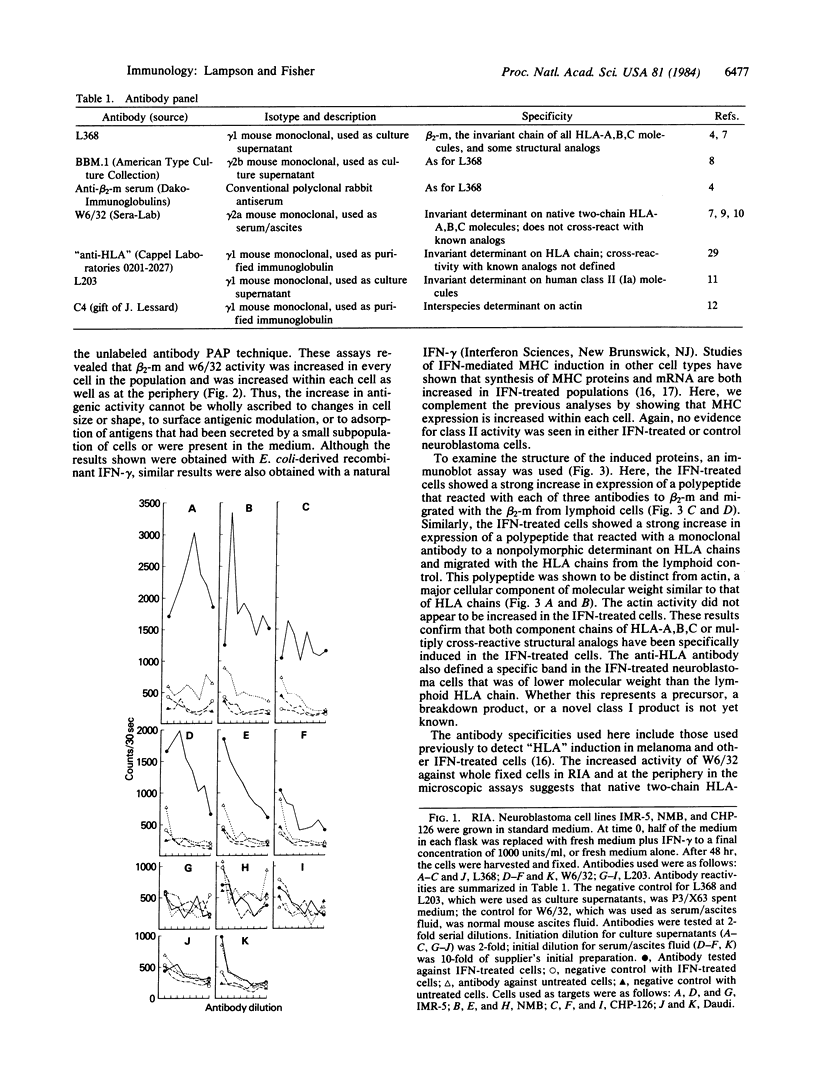
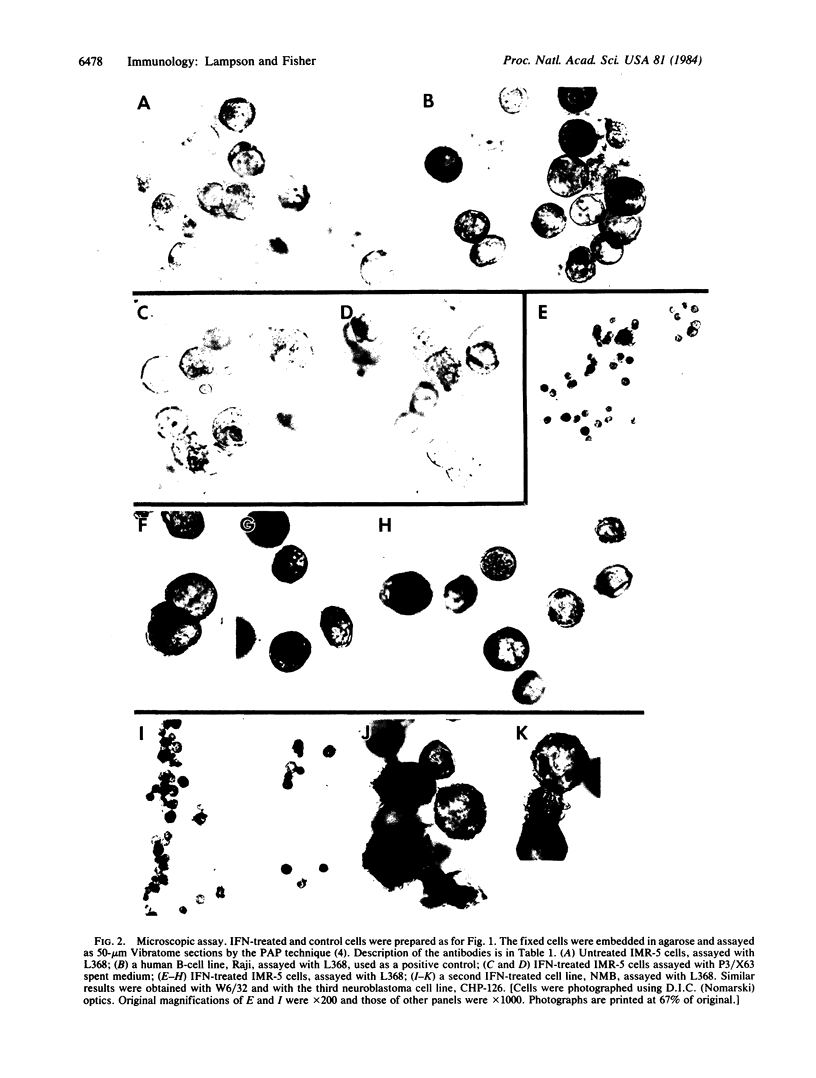


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce-Gomez B., Jones E. A., Barnstable C. J., Solomon E., Bodmer W. F. The genetic control of HLA-A and B antigens in somatic cell hybrids: requirement for beta2 microglobulin. Tissue Antigens. 1978 Feb;11(2):96–112. doi: 10.1111/j.1399-0039.1978.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Ball E. D., Guyre P. M., Glynn J. M., Rigby W. F., Fanger M. W. Modulation of class I HLA antigens on HL-60 promyelocytic leukemia cells by serum-free medium: re-induction by gamma-IFN and 1,25-dihydroxyvitamin D3 (calcitriol). J Immunol. 1984 May;132(5):2424–2428. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Bourgeade M. F., Creasey A. A., Merigan T. C. Interferon increases HLA synthesis in melanoma cells: interferon-resistant and -sensitive cell lines. Proc Natl Acad Sci U S A. 1982 May;79(10):3265–3269. doi: 10.1073/pnas.79.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F. Evolutionary significance of the HL-A system. Nature. 1972 May 19;237(5351):139–passim. doi: 10.1038/237139a0. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. HLA structure and function: a contemporary view. Tissue Antigens. 1981 Jan;17(1):9–20. doi: 10.1111/j.1399-0039.1981.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Bodmer W. F., Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979 Jul;9(7):536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Calvet M. C., Gresser I. Interferon enhances the excitability of cultured neurones. Nature. 1979 Apr 5;278(5704):558–560. doi: 10.1038/278558a0. [DOI] [PubMed] [Google Scholar]
- Cook A. W., Carter W. A., Nidzgorski F., Akhtar L. Human brain tumor--derived cell lines: growth rate reduced by human fibroblast interferon. Science. 1983 Feb 18;219(4586):881–883. doi: 10.1126/science.6401866. [DOI] [PubMed] [Google Scholar]
- Cotner T., Mashimo H., Kung P. C., Goldstein G., Strominger J. L. Human T cell surface antigens bearing a structural relationship to HLA antigens. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3858–3862. doi: 10.1073/pnas.78.6.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J., Contu L. Is the MHC a general self-recognition system playing a major unifying role in an organism? Hum Immunol. 1980 Jul;1(1):5–17. doi: 10.1016/0198-8859(80)90004-x. [DOI] [PubMed] [Google Scholar]
- Dausset J. The major histocompatibility complex in man. Science. 1981 Sep 25;213(4515):1469–1474. doi: 10.1126/science.6792704. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Monos D. S., Cooper H. L. Rapid turnover of HLA proteins in quiescent lymphocytes: proposed connection with immunologic surveillance. J Immunol. 1983 Jul;131(1):341–346. [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Rosa F., Fellous M., Dron M., Tovey M., Revel M. Presence of an abnormal beta 2-microglobulin mRNA in Daudi cells: induction by interferon. Immunogenetics. 1983;17(2):125–131. doi: 10.1007/BF00364752. [DOI] [PubMed] [Google Scholar]
- Tsukamoto L. F., Price R. W. Interferon protects neurons in culture infected with vesicular stomatitis and herpes simplex viruses. J Neurol Sci. 1982 Oct;56(1):115–128. doi: 10.1016/0022-510x(82)90066-1. [DOI] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Williams K. A., Hart D. N., Fabre J. W., Morris P. J. Distribution and quantitation of HLA-ABC and DR (Ia) antigens on human kidney and other tissues. Transplantation. 1980 Apr;29(4):274–279. doi: 10.1097/00007890-198004000-00002. [DOI] [PubMed] [Google Scholar]