Abstract
Cellular redox balance is vital in health and disease. In this Forum, we highlight the importance of reactive oxygen species (ROS) in the regulation of redox balance in different organ systems of the body and ROS contribution to the development of hypertension. The Forum examines interactions between oxidative and nitrosative stress in the brain, vasculature, and kidney, and redox effect on end-organ damage and hypertension. Furthermore, the Forum examines the role of immune cells in the modulation of hypertension. We also introduce a new role for endoplasmic reticulum stress in the induction of ROS and its possible contribution to the development of hypertension. Finally, we explore the clinical relevance of increased ROS in the setting of human hypertension. Antioxid. Redox Signal. 20, 69–73.
Introduction
In mammalian cells, aerobic respiration allows the reduction of molecular oxygen to water. During this process, molecular-free radical, protein, and lipid species of reactive oxygen species (ROS) are generated. These species are short-lived, and after completing their roles in routine cellular function/maintenance, they are scavenged by a series of antioxidant enzymes (13). Usually, the in vivo antioxidant defense is sufficient to metabolize these ROS. However, in conditions of persistent inflammation and oxidative stress, antioxidant molecules and enzymes can be depleted and/or inactivated, thereby impairing the overall antioxidant defense system (2). At elevated and/or uncontrolled concentrations, highly reactive species can interact with and cause damage to proteins, lipids, and DNA (2).
ROS are produced by numerous enzymes in many cell types, including endothelial, vascular smooth muscle, adventitial, neuronal, microglial, and various renal cells. The major ROS produced are the superoxide anion (·O2−), hydroxyl moiety (·OH), hypochlorite (·ClO−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH−). The superoxide anion can further combine with nitric oxide (NO), forming the reactive compound peroxynitrite (ONOO·) and generating a nitroso-redox imbalance. In addition, peroxynitrite oxidizes tetrahydrobiopterin, thereby leading to endothelial nitric oxide synthase (eNOS) uncoupling and diminished NO production. These ROS are generated as intermediate products in oxidative phosphorylation reactions and play a role in normal redox control of physiological signaling pathways. They also act as important second messengers and intracellular signaling molecules in cell growth, survival, and apoptosis. However, excessive ROS generation leads to oxidative stress, triggers cell dysfunction, lipid peroxidation, and DNA mutagenesis (12).
The major source of ROS generation, in the cardiovascular system, is NADPH oxidase. NADPH oxidase is composed of multiple subunits, which include two membrane-bound subunits gp91phox (also known as nox2, or the homologues nox1 and nox4) and p22phox, and the cytosolic subunits p47phox, p40phox, p67phox, and Rac1 (a small G-protein) (8). Consequent to stimulation, the cytosolic subunits migrate toward the plasma membrane-bound subunits and become enzymatically active on their binding, ultimately yielding superoxide by the 1 electron reduction of oxygen using NADPH as the electron donor: 2O2+NADPH→2O2−+NADP++ (8). Other enzymatic sources of ROS that are involved are xanthine oxidase, uncoupling of the mitochondrial respiratory chain, cytochrome p450, and uncoupling of eNOS. ROS are physiologically produced, but during diseased states, these ROS sources become the main culprits of sustained cellular and tissue damage, especially during the hypertensive state (6).
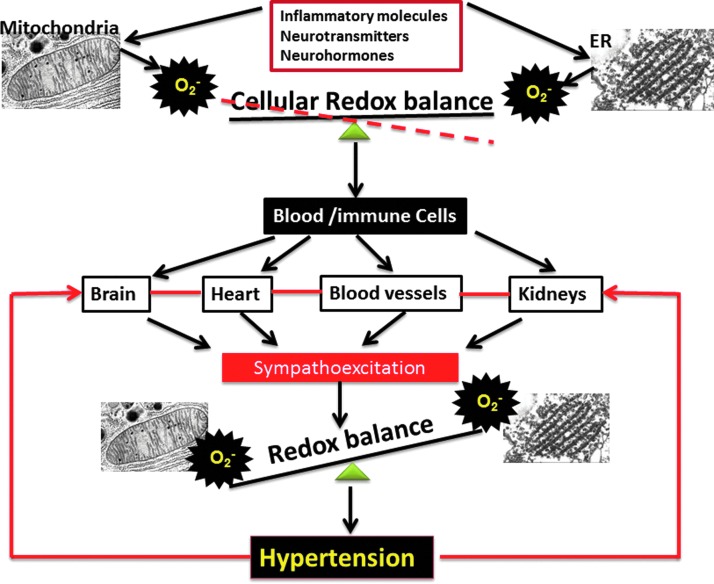
A growing body of evidence indicates that production of ROS and activation of oxidation-reduction dependent signaling cascades leads to oxidative stress and is centrally and critically involved in cardiovascular events (6). ROS and oxidative stress play an important role in diabetes mellitus, atherosclerosis, hyperlipidemia, heart failure, ischemic heart disease, and hypertension. Oxidative stress is defined as the imbalanced redox state in which pro-oxidants overwhelm intrinsic antioxidant systems, resulting in an increased production of ROS. During normal physiological conditions, ROS are produced in a controlled manner at low concentrations and function as signaling molecules or normal by-products of cellular reactions. Conversely, under pathological conditions, cell dysfunction, cell growth, monocyte invasion, lipid peroxidation, inflammation, and increased deposition of extracellular matrix proteins are important factors in vascular, renal, and cardiac damage during the pathogenesis of hypertension (12). A schematic of the proposed mechanism is shown in Figure 1: Inflammatory molecules, neurohormones, neurotransmitters, increased shear stress, and so on can induce cellular oxidative stress via mitochondrial and endoplasmic reticulum (ER), resulting in the spill-over of this increased oxidative stress into the circulating blood. This increased oxidative stress environment in the blood might polarize the cells to attain antigenic characteristics and migrate into the brain, heart, blood vessels, and kidney, thereby contributing to the exaggerated sympathetic activity. The increased sympathetic activity further increases tissue oxidative stress and redox imbalance, resulting in hypertension. In uncontrolled hypertension, this vicious cycle of redox imbalance, cellular migration, and enhanced sympathetic activity might lead to end-organ damage, resulting in stroke, heart failure, and renal dysfunction. The purpose of this Forum is to bring together the thought process of leading scientists in the field of hypertension toward better understanding the mechanisms of redox balance in hypertension.
FIG. 1.
Inflammatory molecules, neurohormones, neurotransmitters, increased shear stress, and so on can induce cellular oxidative stress via mitochondrial and endoplasmic reticulum, resulting in the spill-over of this increased oxidative stress into the circulating blood. This increased oxidative stress environment in the blood might polarize the cells to attain antigenic characteristics and migrate into the brain, heart, blood vessels, and kidney, thereby contributing to the exaggerated sympathetic activity. The increased sympathetic activity further increases tissue oxidative stress and redox imbalance, resulting in hypertension. In uncontrolled hypertension, this vicious cycle of redox imbalance, cellular migration, and enhanced sympathetic activity might lead to end-organ damage, resulting in stroke, heart failure, and renal dysfunction. ER, endoplasmic reticulum. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Hypertension and the Brain
Hypertension is a multifaceted disorder involving not only peripheral factors and organ systems, but also complex signaling mechanisms throughout the central nervous system (CNS). Over the past decade, multiple studies have confirmed a role for central neurogenic mechanisms in the maintenance and management of blood pressure and volume homeostasis through the vasculature, heart, and kidneys. This includes, but is not limited to, modulation of sympathetic tone, water and salt balance, and system-wide regulation of circulatory hormonal release. The nuclei involved in hypertension are situated throughout the forebrain, midbrain, and hindbrain regions, each with specific and overlapping roles in cardiovascular homeostasis and response. These nuclei receive inputs from afferent signals via baro-, chemo-, and osmoreceptors located throughout the body, as well as neural inputs from the circumventricular organs (CVOs), specialized regions of the brain that lack a fully developed blood brain barrier and enable the brain to detect blood-borne signaling hormones and blood osmolality levels. The CVOs, along with other cardio-regulatory regions of the brain, are implicated in the maintenance of many experimentally observed forms of hypertension (7).
Mounting evidence indicates that the brain plays a major role in the pathogenesis of hypertension and that neurogenic mechanisms are dominant in more than 40% of essential hypertensive patients (5), more specifically, that the sympathetic nervous system (SNS) plays a major role in the pathogenesis of hypertension. When acutely and chronically activated, the SNS can become involved in 24-h blood pressure patterns and the sustained progression of hypertension, ultimately resulting in metabolic abnormalities, end-organ damage, and even death (7). Over the past several years, new evidence has also emerged that clearly demonstrates brain ROS involvement in blood pressure regulation. ROS serve as signaling molecules within neurons of cardiovascular regulatory centers and in the regulation of the SNS (15). ROS and oxidative stress components are linked to sympathetic modulation during both normal physiological and pathophysiological functions, indicating the relevance of oxidant/anti-oxidant balance within the CNS. In this Forum, Chan and Chan (3) examine the interaction between ROS and NO in the brain stem and its effect on hypertension. The authors examine activation of the different NOS isoforms in brain stem neurons and study how NOS interaction with ROS contributes to hypertensive response. They decipher how the uncoupling of NOS affects baroreceptor sensitivity in hypertension. The authors also bring in the neurotransmitter modulation within the brain stem neurons, including nucleus tractus solatorius, rostro ventro lateral medulla neurons, and its effect on baroreceptor sensitivity in neurogenic hypertension. These concepts have opened up a novel field of study for hypertension signaling pathways and reveal possible new therapeutic approaches that are centered on redox mechanisms in the brain.
Kidney Redox Mechanisms in Hypertension
While the heart is considered the central component of the cardiovascular system, the kidney is integral to maintaining systemic homeostasis with regard to blood volume and pressure, as well as modulating ion concentrations and waste elimination. Current anti-hypertensive treatments are fairly effective in reducing the severity of hypertensive renal disease, but hypertension-induced kidney disease is still a significant cause of morbidity and mortality in hypertensive patients. The disease process remains progressive, thereby underscoring the need for additional therapeutic options.
In the renal cortex, the renal medulla, and the renal vasculature, Nox enzymes and mitochondria are major producers of superoxide. Within the renal cortex, tubuloglomerular feedback (TGF) and increased sodium delivery, along with mechanical factors, induce the Nox enzymes in the macula densa to produce superoxide. Superoxide can directly enhance the TGF response by constriction of afferent arterioles, and indirectly, by scavenging NO in the macula densa while increasing adenosine production, ultimately leading to prolonged vascular constriction. Araujo and Wilcox (1) thoroughly review how increased oxidative stress in various segments of the nephron affects renovascular hemodynamics. The authors examine how the interaction between superoxide and antioxidant enzymes modulates renal function. More importantly, the authors explain how this interaction varies in different models of hypertension and how it affects renal hemodynamics. In addition, they provide a detailed explanation of different antioxidant systems affecting renal blood flow, renovascular resistance, and, ultimately, affecting blood pressure, in different models of hypertension. Finally, a detailed overview on the nervous system role in modulating redox balance in the kidney and its effect on renal hemodynamics and blood pressure regulation is being examined.
Neuroendocrine Immune Interactions in Hypertension
Immune cells play a major role in maintaining homeostasis, health, and well-being of humans and animals. The products of immune cells are cytokines, which mediate and control immune and inflammatory responses. Complex interactions between cytokines, inflammation, and adaptive responses are required to maintain equilibrium in the system. Similar to the stress response, the inflammatory reaction is crucial for the survival of the organism and is regulated by the stimulus and the length of time that the stimulus is present in the system. During a full-fledged systemic inflammatory reaction, there is induction of the acute phase reaction, sickness syndrome, pain program, and the stress response, all of which are mediated through the hypothalamo-pituitary adrenal (HPA) axis and the sympathetic system. Autoimmunity, chronic infections, and sepsis are characterized by a dysregulation in the balance between pro and anti-inflammatory cytokines, modulated by T helper (Th) 1 versus Th2. In addition, recent evidence also indicates the involvement of pro-inflammatory cytokines in the pathogenesis of hypertension. In this Forum, Crowley (4) emphasizes the importance of immune cells in modulating the hypertensive response. The author provides a thorough overview of the synergistic and bidirectional communication between oxidative stress and immune cells in the development of hypertension, and also explains the contribution of adaptive and innate immune cells to the development of hypertension, and how this system can act locally within each major organ of the body that contributes to the development of hypertension. The author also addresses the intracellular molecular event that facilitates the bidirectional communication within the cells which contributes to the development of hypertension. Finally, the author brings out the clinical relevance of c-reactive proteins in the bidirectional communication between oxidative stress and inflammation.
Nonconventional Cardiovascular Effects and Hypertension
Central to the function of the cardiovascular system is the heart. Indeed, it was more than 50 years ago when researchers hypothesized that structural and morphological changes within the vasculature would further increase the total systemic resistance which occurs in hypertension and contribute to a worsening of the disease. Consequently, these vascular changes would elicit alterations in the heart, causing remodeling and hypertrophy and continuing to perpetuate the vicious cycle of the hypertensive state. Numerous extensive reviews and studies have detailed the role of ROS in vascular changes and dysfunction in hypertension (7). Although the role of vascular endothelial growth factor (VEGF) has been extensively studied in the setting of angiogenesis, its effect on inducing endothelin-1 (ET-1) to modulate ROS is relatively new.
VEGF, an important survival factor for endothelial cells in the process of normal physiological or abnormal angiogenesis, induces the expression of antiapoptotic proteins in the endothelial cells. It is also the major initiator of angiogenesis in cancer and diabetic retinopathy, where it is up-regulated by oncogenic expression and different types of growth factors. Hence, inhibition of angiogenesis using human monoclonal antibodies to VEGF, or with VEGF receptor tyrosine kinase inhibitors, is one of the established treatments for various forms of cancer. However, inhibition of VEGF-mediated signaling is frequently associated with the development of severe forms of hypertension. In this Forum, Lankhorst et al. (9) examine the clinical relevance and the latest mechanism for hypertension that is induced by VEGF inhibition during cancer therapy. The current thought is that hypertension induced by angiogenesis inhibition is mediated by the depletion of NO. However, there is a conflicting report on the NO inhibition theory, as the experimental evidence was not well supported. More recently, the ET-1 axis has emerged as a major cause for the abnormal increase in blood pressure during anti-angiogenic treatment of cancer. The authors examine molecular mechanism by which VEGF modulates the ET-1 axis via oxidative stress to drive the development of hypertension in cancer therapy, and discuss methods to counter-act this response.
ER Stress, a Novel Player in Hypertension
Recent evidence indicates that ER stress plays an important pathophysiological role in a number of chronic diseases, including cardiovascular disease. The ER, the cellular organelle responsible for protein synthesis, folding, and trafficking, is also recognized as a primary sensor of cellular stress. Any conditions that challenge normal ER function, such as increased protein synthesis, alterations in cellular redox status, or disturbances in intracellular Ca2+ levels, may induce ER stress and lead to activation of a conserved set of intracellular signaling cascades called the unfolded protein response (UPR). The UPR facilitates adaptation to acute cellular perturbations and reestablishes ER homeostasis (10). However, long-term UPR activation can disrupt cellular function and lead to chronic disease. In this Forum, Santos and colleagues (14) elegantly review the contribution of ER stress in the development of hypertension. They specifically examine the involvement of Nox-mediated ROS signaling and ER stress in the peripheral vasculature and the heart during the development of hypertension.
There is compelling evidence that alterations in the CNS ER environment lead to long-term changes in neural function through changes in molecules which are known to be involved in CNS-driven hypertension, including Ca2+, ROS, and transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein 1 (AP-1). Interestingly, cellular perturbations known to cause ER stress, including oxidative stress and alterations in intracellular Ca2+, are prevalent in hypertension.
Clinical Implications of ROS Activation
Accumulating evidence from animal studies indicates a major role for oxidative stress in the development of hypertension. However, in clinical medicine, the causal relationship between ROS and hypertension is yet to be established. Most human studies examining ROS are based on the associations between increased plasma or urinary markers of oxidative stress, including thiobarbituric acid-reactive substances or 8-epi-isoprostanes in hypertensive patients. In addition, there is reduced antioxidant capacity and decreased levels of antioxidant vitamins and enzymes in patients with hypertension. Thus, these studies linking hypertension and oxidative stress are more or less correlative rather than confirmatory. Coupled with these findings, a lack of blood pressure and cardiovascular effects from multicenter clinical trials using anti-oxidant therapy leads to a dilemma regarding the role of ROS in clinical human hypertension. This could be due to the fact that very few studies examine the molecular mechanism of ROS biology in human tissue or the lack of adequate methods to measure ROS in a clinical setting, and very few clinical trials are designed to evaluate the antioxidant therapy in hypertension. Montezano and Touyz (11) bring a clinical perspective to this Forum by reviewing the oxidative stress literature in human hypertension and its relevance to clinical medicine. The authors elegantly review the mechanism of abnormal elevation of ROS leading to aberrant signaling of redox-sensitive pathways in the cardiovascular system and possibly contributing to end-stage organ damage. In addition, the authors describe the molecular and cellular mechanism by which ROS influences the vascular system and its effect on blood pressure elevation. Finally, they examine the studies on Nox isoforms in the vasculature and their contribution to blood pressure regulation.
Conclusion
With recent enhanced capabilities for detecting and studying ROS in experimental models and patients, there has been a surge in the study of redox mechanisms in the pathogenesis of hypertension, both systemically and in specific organs, tissues, and cell sites. This Forum collects much of the current research on ROS signaling pathways in hypertension and explores the clinical implications of ROS in the development of hypertension. With detection techniques continuing to progress, and more advanced animal models becoming available, these signaling patterns will become more evident, and as such, support the advancement towards more beneficial and efficacious treatment strategies in the fight against hypertension.
Abbreviations Used
- CNS
central nervous system
- CVOs
circumventricular organs
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ET-1
endothelin-1
- NF-κB
nuclear factor-kappa B
- ROS
reactive oxygen species
- SNS
sympathetic nervous system
- Th
T helper
- TGF
tubuloglomerular feedback
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
References
- 1.Araujo M. and Wilcox CS. Oxidative stress in hypertension: Role of the kidney. Antioxid Redox Signal 20: 74–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Chan SHH. and Chan JYH. Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid Redox Signal 20: 146–163, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal 20: 102–120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esler M. and Kaye D. Is very high sympathetic tone in heart failure a result of keeping bad company? Hypertension 42: 870–872, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Griendling KK, Sorescu D, and Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Harrison DG. and Gongora MC. Oxidative stress and hypertension. Med Clin North Am 93: 621–635, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lankhorst S, Kappers MHW, van Esch JHM, Danser AHJ, van den Meiracker AH. Hypertension during vascular endothelial growth factor inhibition: Focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal 20: 135–145, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Malhotra JD. and Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montezano AC. and Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid Redox Signal 20: 164–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdoch CE, Zhang M, Cave AC, and Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 71: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Paravicini TM. and Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension. Diabetes Care 31: S170–S180, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Santos CXC, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV. and Lopes LR. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role of hypertension. Antioxid Redox Signal 20: 121–134, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman MC, Lazartigues E, Sharma RV, and Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]