Abstract
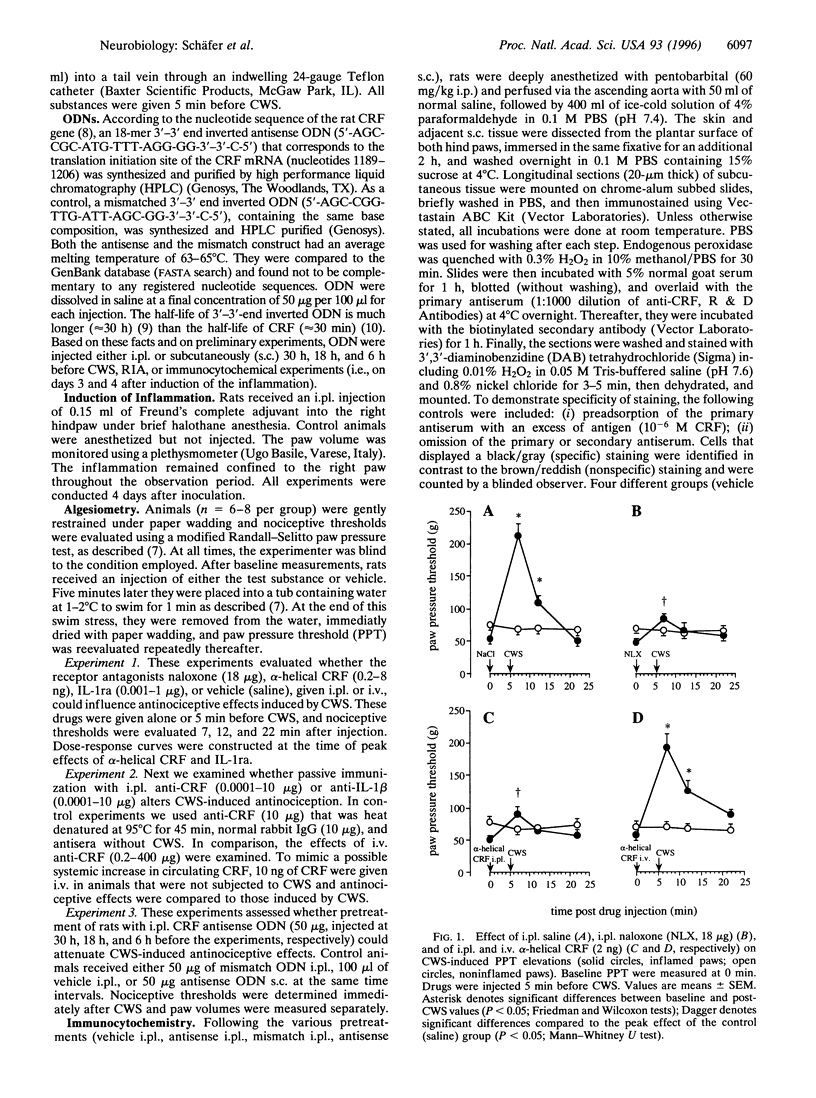
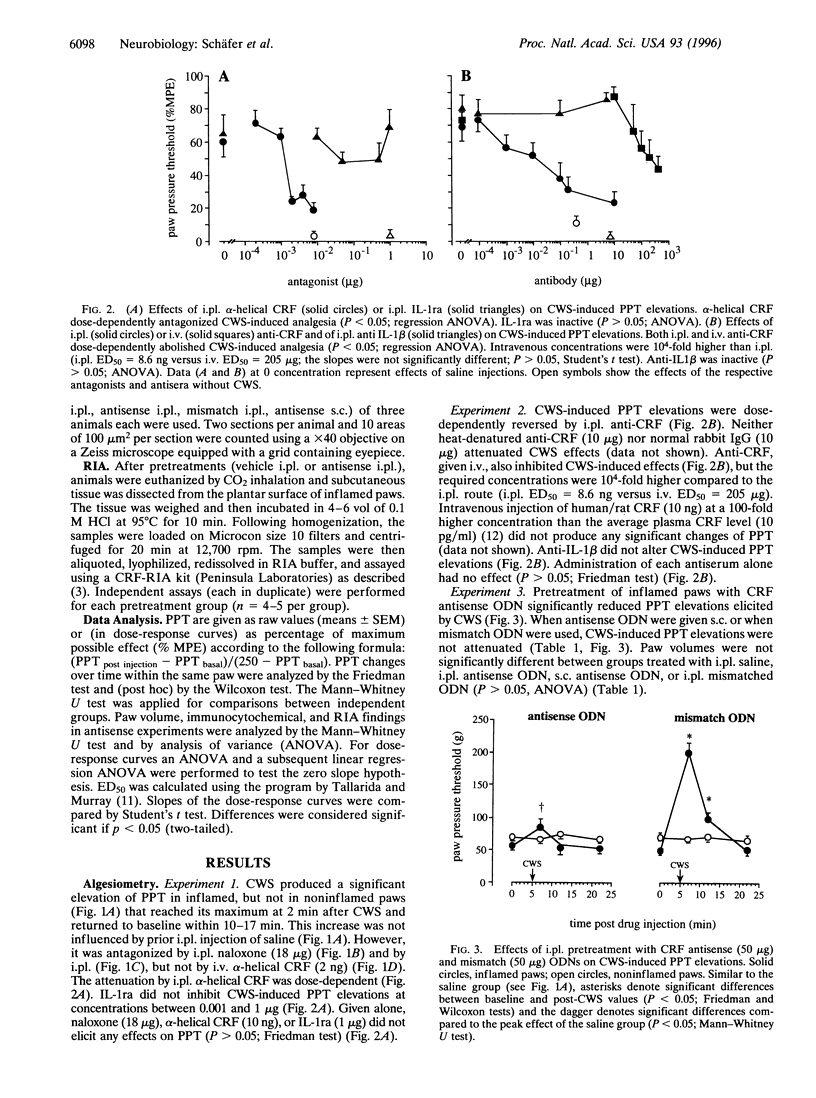
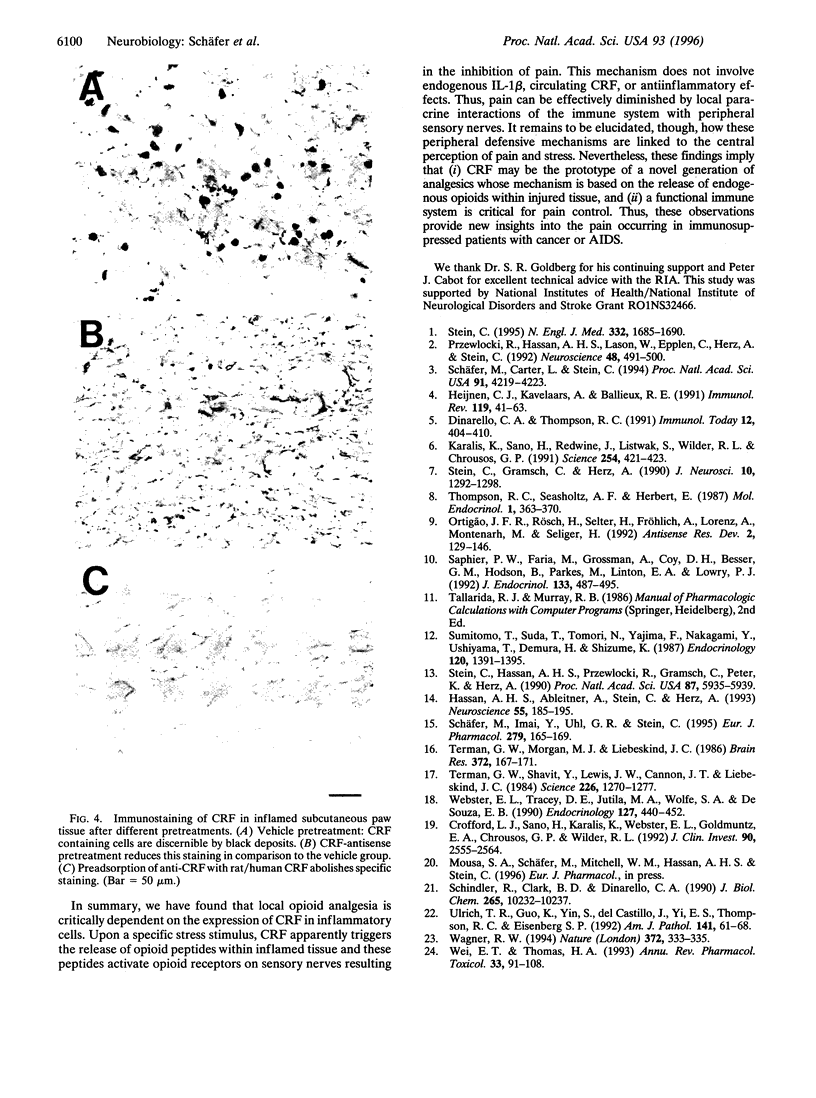
Immune cell-derived opioid peptides can activate opioid receptors on peripheral sensory nerves to inhibit inflammatory pain. The intrinsic mechanisms triggering this neuroimmune interaction are unknown. This study investigates the involvement of endogenous corticotropin-releasing factor (CRF) and interleukin-1beta (IL-1). A specific stress paradigm, cold water swim (CWS), produces potent opioid receptor-specific antinociception in inflamed paws of rats. This effect is dose-dependently attenuated by intraplantar but not by intravenous alpha-helical CRF. IL-1 receptor antagonist is ineffective. Similarly, local injection of antiserum against CRF, but not to IL-1, dose-dependently reverses this effect. Intravenous anti-CRF is only inhibitory at 10(4)-fold higher concentrations and intravenous CRF does not produce analgesia. Pretreatment of inflamed paws with an 18-mer 3'-3'-end inverted CRF-antisense oligodeoxynucleotide abolishes CWS-induced antinociception. The same treatment significantly reduces the amount of CRF extracted from inflamed paws and the number of CRF-immunostained cells without affecting gross inflammatory signs. A mismatch oligodeoxynucleotide alters neither the CWS effect nor CRF immunoreactivity. These findings identify locally expressed CRF as the predominant agent to trigger opioid release within inflamed tissue. Endogenous IL-1, circulating CRF or antiinflammatory effects, are not involved. Thus, an intact immune system plays an essential role in pain control, which is important for the understanding of pain in immunosuppressed patients with cancer or AIDS.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crofford L. J., Sano H., Karalis K., Webster E. L., Goldmuntz E. A., Chrousos G. P., Wilder R. L. Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J Clin Invest. 1992 Dec;90(6):2555–2564. doi: 10.1172/JCI116150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Thompson R. C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991 Nov;12(11):404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Hassan A. H., Ableitner A., Stein C., Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993 Jul;55(1):185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- Heijnen C. J., Kavelaars A., Ballieux R. E. Beta-endorphin: cytokine and neuropeptide. Immunol Rev. 1991 Feb;119:41–63. doi: 10.1111/j.1600-065x.1991.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Karalis K., Sano H., Redwine J., Listwak S., Wilder R. L., Chrousos G. P. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991 Oct 18;254(5030):421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Ortigão J. F., Rösch H., Selter H., Fröhlich A., Lorenz A., Montenarh M., Seliger H. Antisense effect of oligodeoxynucleotides with inverted terminal internucleotidic linkages: a minimal modification protecting against nucleolytic degradation. Antisense Res Dev. 1992 Summer;2(2):129–146. doi: 10.1089/ard.1992.2.129. [DOI] [PubMed] [Google Scholar]
- Przewłocki R., Hassan A. H., Lason W., Epplen C., Herz A., Stein C. Gene expression and localization of opioid peptides in immune cells of inflamed tissue: functional role in antinociception. Neuroscience. 1992;48(2):491–500. doi: 10.1016/0306-4522(92)90509-z. [DOI] [PubMed] [Google Scholar]
- Saphier P. W., Faria M., Grossman A., Coy D. H., Besser G. M., Hodson B., Parkes M., Linton E. A., Lowry P. J. A comparison of the clearance of ovine and human corticotrophin-releasing hormone (CRH) in man and sheep: a possible role for CRH-binding protein. J Endocrinol. 1992 Jun;133(3):487–495. doi: 10.1677/joe.0.1330487. [DOI] [PubMed] [Google Scholar]
- Schindler R., Clark B. D., Dinarello C. A. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J Biol Chem. 1990 Jun 25;265(18):10232–10237. [PubMed] [Google Scholar]
- Schäfer M., Carter L., Stein C. Interleukin 1 beta and corticotropin-releasing factor inhibit pain by releasing opioids from immune cells in inflamed tissue. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4219–4223. doi: 10.1073/pnas.91.10.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M., Imai Y., Uhl G. R., Stein C. Inflammation enhances peripheral mu-opioid receptor-mediated analgesia, but not mu-opioid receptor transcription in dorsal root ganglia. Eur J Pharmacol. 1995 Jun 12;279(2-3):165–169. doi: 10.1016/0014-2999(95)00150-j. [DOI] [PubMed] [Google Scholar]
- Stein C., Gramsch C., Herz A. Intrinsic mechanisms of antinociception in inflammation: local opioid receptors and beta-endorphin. J Neurosci. 1990 Apr;10(4):1292–1298. doi: 10.1523/JNEUROSCI.10-04-01292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Przewłocki R., Gramsch C., Peter K., Herz A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995 Jun 22;332(25):1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Sumitomo T., Suda T., Tomori N., Yajima F., Nakagami Y., Ushiyama T., Demura H., Shizume K. Immunoreactive corticotropin-releasing factor in rat plasma. Endocrinology. 1987 Apr;120(4):1391–1396. doi: 10.1210/endo-120-4-1391. [DOI] [PubMed] [Google Scholar]
- Terman G. W., Morgan M. J., Liebeskind J. C. Opioid and non-opioid stress analgesia from cold water swim: importance of stress severity. Brain Res. 1986 Apr 30;372(1):167–171. doi: 10.1016/0006-8993(86)91472-1. [DOI] [PubMed] [Google Scholar]
- Terman G. W., Shavit Y., Lewis J. W., Cannon J. T., Liebeskind J. C. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984 Dec 14;226(4680):1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Seasholtz A. F., Herbert E. Rat corticotropin-releasing hormone gene: sequence and tissue-specific expression. Mol Endocrinol. 1987 May;1(5):363–370. doi: 10.1210/mend-1-5-363. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Guo K., Yin S., del Castillo J., Yi E. S., Thompson R. C., Eisenberg S. P. Endotoxin-induced cytokine gene expression in vivo. IV. Expression of interleukin-1 alpha/beta and interleukin-1 receptor antagonist mRNA during endotoxemia and during endotoxin-initiated local acute inflammation. Am J Pathol. 1992 Jul;141(1):61–68. [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Tracey D. E., Jutila M. A., Wolfe S. A., Jr, De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990 Jul;127(1):440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- Wei E. T., Thomas H. A. Anti-inflammatory peptide agonists. Annu Rev Pharmacol Toxicol. 1993;33:91–108. doi: 10.1146/annurev.pa.33.040193.000515. [DOI] [PubMed] [Google Scholar]




