SUMMARY
Self-renewal and pluripotency of embryonic stem cells (ESCs) are established by multiple regulatory pathways operating at several levels. The roles of histone demethylases (HDMs) in these programs are incompletely defined. We conducted a functional RNAi screen for HDMs and identified five potential HDMs essential for mESC identity. In depth analyses demonstrate that the closely related HDMs, Jmjd2b and Jmjd2c, are necessary for self-renewal of ESCs and iPSC generation. Genome-wide occupancy studies reveal Jmjd2b unique, Jmjd2c unique, and Jmjd2b-Jmjd2c common target sites belong to functionally separable Core, Polycomb repressive complex (PRC) and Myc regulatory modules, respectively. Jmjd2b and Nanog act through an interconnected regulatory loop, whereas Jmjd2c assists PRC2 in transcriptional repression. Thus, two HDMs of the same subclass exhibit distinct and combinatorial functions in control of the ESC state. Such complexity of HDM function reveals a novel aspect of multilayered transcriptional control.
INTRODUCTION
Embryonic stem cells (ESCs) are capable of indefinite self-renewal and differentiation into all lineages. Somatic cell reprogramming to induced pluripotent stem cells (iPSCs) by defined factors has greatly improved prospects for cellular therapies (Takahashi and Yamanaka, 2006; Cherry and Daley, 2012). Although much has been learned, the components that establish and maintain ESC identity are incompletely defined.
ESC identity is maintained by activation of ESC-specific genes and repression of lineage-specific developmental genes. This balance of gene expression is maintained through cross talk between essential transcription factors (TFs) and chromatin regulators (Orkin and Hochedlinger, 2011; Young, 2011). Extensive studies of protein-protein and protein-DNA interactions have revealed distinct ESC regulatory modules, termed Core, Myc and Polycomb, that are essential for entire ESC regulatory network (Chen et al., 2008; Kim et al., 2010). The Core module is comprised of canonical ESC factors (Oct4, Sox2 and Nanog) and their associated partners, which positively regulate ESC-specific genes and repress lineage-specific genes (Kim et al., 2010; Young, 2011). The Myc module, consisting of cMyc and associated factors, is also transcriptionally active. However, the Myc module is functionally separable from the Core module and activated earlier than Core module during iPS generation at the partial iPS (piPS) stage (Sridharan et al., 2009; Soufi et al., 2012). The Polycomb repressive complex (PRC) module, comprised of PRC1 and PRC2 components function in repression of lineage-specific genes (Boyer et al., 2006; Kim et al., 2010; Margueron and Reinberg, 2011).
Various approaches have implicated role of chromatin regulators in self-renewal of ESCs (Fazzio et al., 2008; Hu et al., 2009; Kagey et al., 2010). Histone demethylases (HDMs) are histone-modifying enzymes, which have an opposing biochemical function to histone methyltransferase (HMTs). HDMs are required for normal development and implicated in pathologic states including cancers (Pedersen and Helin, 2010). HDMs are divided into two broad classes, FAD-dependent amine oxidases (Lsd1/ Kdm1), and Fe (II) & α-ketoglutarate (α-KG) dependent JmjC domain-containing HDMs (Mosammaparast and Shi, 2010). The JmjC domain-containing HDMs have a conserved catalytic triad (H, D/E, and H), which catalyses lysine demethylation of histones through oxidative reaction that requires Fe(II) and α-ketoglutarate as co-factors. JmjC domain-containing HDMs are further sub-classified based on their sequence homology, domains and substrate specificity (Agger et al., 2008; Pedersen and Helin, 2010). Studies have proposed roles for Jmjd1a, Jmjd2c, and Jarid1b/Kdm5b in ESC self-renewal (Loh et al., 2007; Xie et al., 2011). However, roles of other HDMs in ESCs remain unknown.
Here we conducted a functional RNAi screen against all annotated HDMs, and identified five candidate HDMs essential for mESC identity. To gain mechanistic insight, we chose two closely related HDMs, Jmjd2b/Kdm4b and Jmjd2c/Kdm4c, belonging to the same sub-class (HDMs for H3K9me2/me3 and H3K36me2/me3) for in-depth analysis. In addition to their requirement in ESCs, both HDMs are required for efficient somatic cell reprogramming. Although depletion of either HDM generates a similar differentiation phenotype, chromatin occupancy studies reveal both unique and common target sites. Jmjd2b unique, Jmjd2b-Jmjd2c common and Jmjd2c unique targets partition to the Core, Myc and Polycomb repressive complex (PRC) regulatory modules of the overall ESC network, respectively. Specifically, we show that Jmjd2b and Nanog act through an interconnected regulatory loop, whereas Jmjd2c assists PRC2 in full repression at poised and repressed target genes. The dedicated and combinatorial relationships between these two related HDMs reveal an unsuspected level of complexity in how HDMs participate in transcriptional control.
RESULTS
Functional RNAi screens reveal candidate HDMs for mESCs identity
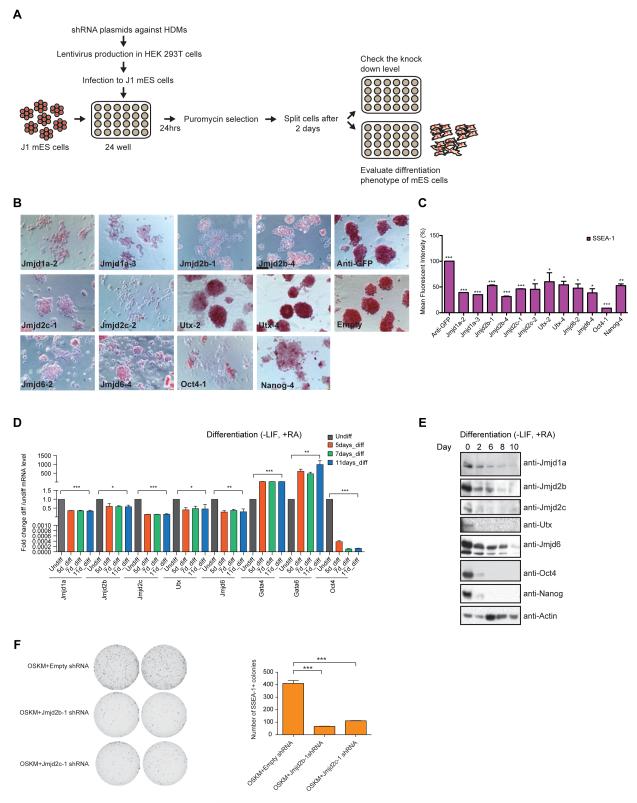
Most HDMs are expressed in mESCs (Figure S1A). To identify HDMs required for maintenance of the ESC state, we performed a functional RNAi screen. We used 5 different shRNA lentiviral constructs to knockdown (KD) each of 20 HDMs in mESCs. The screen was scored in terms of alterations of “ESC growth phenotype” and “colony morphology” (Table S1, Figure 1A, S1B). Normally, ESCs grow as spherical three-dimensional colonies. Upon depletion of a number of the candidate HDMs, cells exhibited flattened morphology and grew as a monolayer with reduced cell-cell contacts (Figure S1B), which we term a “differentiation” phenotype. A spectrum of differentiation phenotypes from mild to severe was observed (Table S1 & S1B). For each candidate HDM, we scored the phenotype from at least the two best individual shRNAs. Two secondary screens were performed for validation. All three screens reproduced the original phenotypes (Table S1).
Figure 1. Functional RNAi screens reveal candidate HDMs for mESCs identity.
(A) Schematic diagram representing outline of the RNAi screen.
(B) Alkaline Phosphatase staining of mESCs upon knockdown of candidate HDMs. Scale bar, 20μm.
(C) SSEA-1 staining of mESCs upon knockdown of candidate HDMs. SSEA-1 positive cells were quantified through FACS and percentage of Mean Fluorescent Intensity (% MFI) of candidate HDMs is represented. Data are represented as mean +/− SEM; n=3; p-values were calculated using t-test; ***p<0.0001, **p<0.001, *p<0.01.
(D) Real time PCR (RT-PCR) analyses of candidate HDMs at different time points during ESCs differentiation. mRNA expression was normalized by Actin, and expression levels are shown in “differentiated ESCs” relative to “undifferentiated ESCs” for each gene. Data are represented as mean +/− SEM; n=3; p-values were calculated using t-test; ***p<0.0001, **p<0.001, *p<0.01.
(E) Western blot analyses of candidate HDMs at different time points during ESCs differentiation.
(F) SSEA-1 staining of iPSCs colonies from Empty (control), Jmjd2b and Jmjd2c knockdown cells. Data are represented as mean +/− SEM; n=3; p-values were calculated using t-test; ***p <0.0001. See also Figure S1.
Our functional RNAi screen identified five candidate HDMs, namely Jmjd1a (Kdm3a), Jmjd2b (Kdm4b), Jmjd2c (Kdm4c), Utx (Kdm6a) and Jmjd6. Among these, Jmjd1a (Kdm3a) and Jmjd2c (Kdm4c) were identified previously as having a role in self-renewal of mouse ESCs (Loh et al., 2007). Jmjd1a, Jmjd2b, Jmjd2c and Jmjd6 knockdown showed moderate to severe differentiation phenotypes, whereas knockdown of Utx showed a more subtle phenotype (Table S1, Figure S1B). Further analyses were performed for the candidate HDMs. Alkaline phosphatase (AP) staining typical of pluripotent ESCs was reduced upon knockdown of candidate HDMs compared to controls (Anti-GFP and Empty) (Figure 1B). Pluripotency marker SSEA-1 expression was also reduced significantly upon knockdown (Figure 1C). We validated each by Western blotting (Figure S1C), and noted general correlation of knockdown efficiency with the extent of differentiation. mRNA expression and protein levels of all five candidate HDMs were reduced upon ESC differentiation (Figure 1D-E). Additionally, these candidate HDMs were highly expressed in mESCs compared to mouse embryonic fibroblasts (MEFs) (Figure S1D), consistent with crucial roles in mESCs identity.
Both Jmjd2b/Kdm4b and Jmjd2c/Kdm4c are required for ESC identity and efficient somatic cell reprogramming/ iPSCs generation
We selected two closely related candidate HDMs of the same subclass, Jmjd2b and Jmjd2c, for in-depth study (Agger et al., 2008). As depletion of either leads to an apparently similar differentiation phenotype, we suspected that their comparison might uncover insights into their non-redundant roles and possibly their relationship to each other.
We first determined that Jmjd2b and Jmjd2c restore the normal ESC growth and colony morphology in Jmjd2b-deficient and Jmjd2c-deficient ESCs (Figure S1E). Indeed, full length Jmjd2b and Jmjd2c restored the normal ESC growth phenotype, ESC colony morphology and also SSEA-1 expression (Figure S1F-G). However, catalytic domain (JmjC) containing Jmjd2b and Jmjd2c (1-333aa), and catalytic mutants (JmjC domain-containing catalytic triad (H, D/E, and H)) of Jmjd2b and Jmjd2c achieved only partial rescue (Figure S1F-G), suggesting that full length of Jmjd2b and Jmjd2c, as well as their catalytic residues, are required for self-renewal of mESCs.
Next, we tested the role of Jmjd2b and Jmjd2c in somatic reprogramming. Depletion of Jmjd2b and Jmjd2c showed reduced numbers of iPSCs colonies compared to control knockdown, as scored by colony morphology and SSEA-1 positive colonies (Figure 1F), which is reminiscent of the effects of knockdown of all H3K9 demethylases (Chen et al., 2012). Taken as a whole, the functional assay demonstrates that Jmjd2b and Jmjd2c are required for efficient somatic reprogramming induced by Oct4-Sox2-Kl4-Myc and establishment of the ESC-like state.
Jmjd2b/Kdm4b and Jmjd2c/Kdm4c are required for self-renewal of mESCs
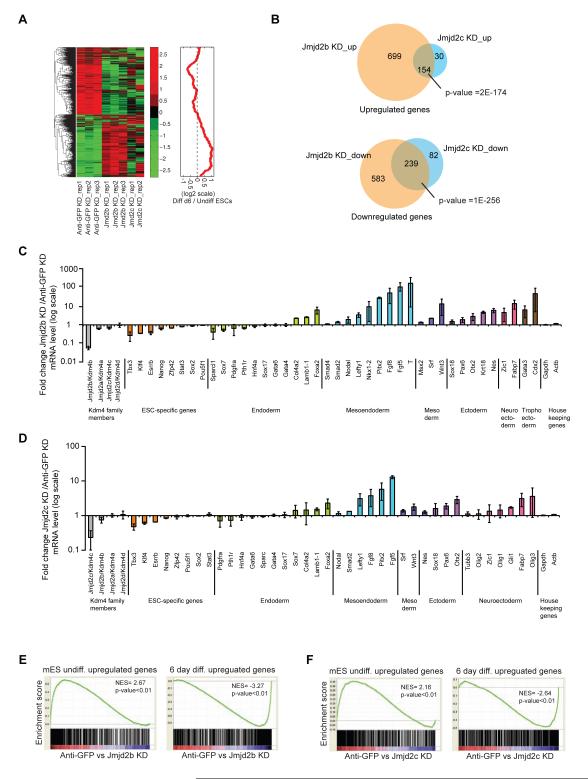
We next conducted gene expression profiling upon depletion of Jmjd2b and Jmjd2c, compared to control (Anti-GFP) knockdown. Gene expression profiling revealed that 1787 genes were differentially expressed by > 2-fold upon depletion of Jmjd2b and Jmjd2c (Figure 2A, left panel & Table S3). The gene cluster downregulated upon knockdown displayed higher expression in undifferentiated ESCs compared to differentiated ESCs; conversely, the gene cluster upregulated upon knockdown showed reduced expression in undifferentiated ESCs (Figure 2A, right panel & S2A).
Figure 2. Jmjd2b/Kdm4b and Jmjd2c/Kdm4c are essential for mESCs identity.
(A) Hierarchical clustering of gene expression profiles from Anti-GFP (control), Jmjd2b (Jmjd2b-1, 4 shRNAs) and Jmjd2c (Jmjd2c-1, 2 shRNAs) depleted cells. Average fold change from two different shRNAs was calculated. Heat map represents Z-score of expression values, while the red curve shows the relative expression changes of these cluster genes between undifferentiated and differentiated ES cells smoothed by a moving window of size 50.
(B) Venn diagrams represent overlapping up or down-regulated genes between Jmjd2b and Jmjd2c depleted cells.
(C & D) Gene expression analyses for ESC-specific genes and lineage-specific differentiation genes from Jmjd2b and Jmjd2c depleted cells. Gapdh and Actin used as internal control. Data shown are averaged from three biological replicates. Data are represented as mean +/− SEM; n=3; p-values were calculated using ANOVA; p-value <0.01.
(E & F) GSEA of differentially expressed gene set of Jmjd2b and Jmjd2c depleted cells. NES= normalized enrichment score, p= nominal p value. See also Figure S2.
Expression of individual ESC-specific and lineage-specific genes from Jmjd2b and Jmjd2c depleted cells revealed reduced ESC-specific gene expression and enhanced expression of differentiation genes (Figure 2C-D & S2B-C). We observed modest downregulation of ESC-specific genes, including Nanog, Esrrb, Klf4 and Tbx3, in Jmjd2b or Jmjd2c depleted cells. In addition, specific knockdown of Jmjd2b and Jmjd2c did not affect expression of other HDMs of the same sub-class. We observed upregulation of several lineage-specific genes upon depletion of Jmjd2b or Jmjd2c, such as Brachyury (T), Pitx2, Fgf8, Wnt, Fgf5 for mesoendoderm/ mesoderm, Otx2, Nestin, Pax6, Fabp7, Zic1 for ectoderm/ neuroectoderm, and Cdx2 for trophoectoderm (Figure 2C-D & S2B-C).
Gene ontology (GO) and Ingenuity pathway analyses (IPA) of the upregulated genes revealed significant enrichment for several developmental processes and related signaling pathways, whereas, the downregulated genes correlated with several metabolic pathways, including glycolysis and gluconeogenesis (Table S5, Figure S2D-E). Furthermore, gene set enrichment analyses (GSEA) demonstrated that Jmjd2b and Jmjd2c depletion significantly repressed the “undifferentiated” ESC state and enhanced the “differentiation” state (Table S4, Figure 2E-F & S2F), including changes in several developmental signaling pathway gene sets (Figure S2G). In these analyses we observed a similar overall pattern of global gene expression upon depletion of Jmjd2b or Jmjd2c. However, the total number of differentially expressed genes differed between Jmjd2b and Jmjd2c knockdown cells (Figure 2B), raising the possibility that Jmjd2b and Jmjd2c function through common and distinct mechanisms in self-renewal of ESCs.
Differential distribution of genome-wide targets of Jmjd2b/Kdm4b and Jmjd2c/Kdm4c in mESCs
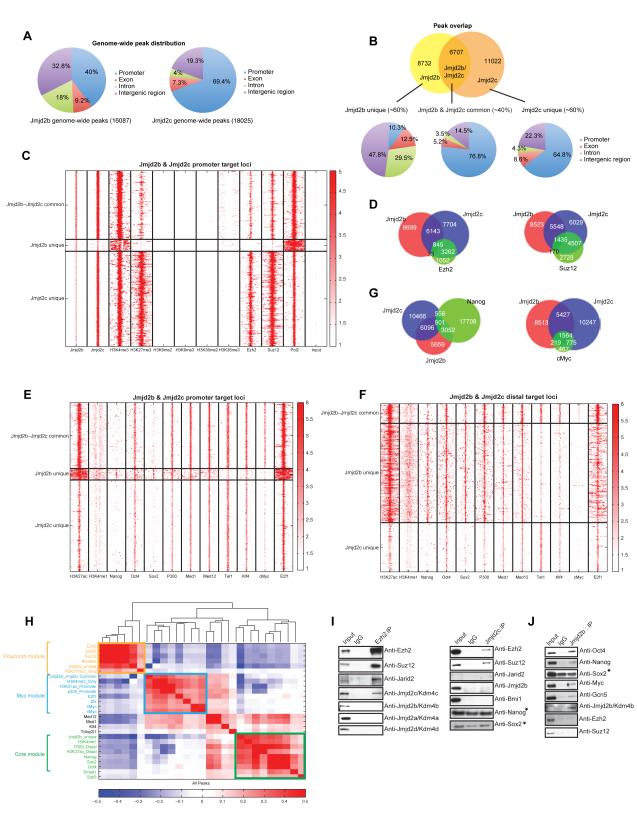
To dissect mechanisms by which Jmjd2b and Jmjd2c function, we determined their genome-wide DNA chromatin occupancy using ChIP sequencing. Due to the lack of suitable antibodies, we generated biotinylated-tagged versions of Jmjd2b and Jmjd2c in mESCs (Figure S3A-B), and performed in vivo biotinylation mediated ChIP-seq (Bio-ChIP-seq) (Kim et al., 2008; 2009; 2010). 16807 and 18025 significantly enriched binding peaks were identified for Jmjd2b and Jmjd2c, respectively (Table S6). Despite their close relationship, we observed differential genome-wide distribution of Jmjd2b and Jmjd2c peaks. Jmjd2b peaks are distributed to the promoter (~40%), intergenic (~32%) and gene body regions (~28%), whereas Jmjd2c mostly occupies promoter regions (~70%) compared to ~20% binding at intergenic and ~11% at gene body regions (Figure 3A). Jmjd2b and Jmjd2c share ~40% common peaks/targets (Figure 3B). We further classified targets as “Jmjd2b-Jmjd2c common”, “Jmjd2b unique” and “Jmjd2c unique” targets (Figure 3B). Interestingly, the majority of “Jmjd2b-Jmjd2c common” and “Jmjd2c unique” targets are distributed at promoter regions (~77% and ~65% respectively), whereas “Jmjd2b unique” targets predominantly localized to gene body regions (~42%), intergenic regions (~48%), and only ~10% to promoter regions (Figure 3B).
Figure 3. Genome-wide mapping of Jmjd2b/Kdm4b and Jmjd2c/Kdm4c in mESCs by ChIP-seq.
(A) Pie charts show genome-wide peak distribution of Jmjd2b and Jmjd2c.
(B) Venn diagram showing the number of Jmjd2b and Jmjd2c overlapped and unique peaks. Pie charts represent distribution of Jmjd2b unique, Jmjd2c unique and Jmjd2b-Jmjd2c common peaks.
(C & E) Heat maps show the ChIP intensities of selected histone marks, TFs, chromatin regulators including components of PRC2 and RNA Pol II at the ±5kb regions around peak summit of Jmjd2b unique, Jmjd2c unique and Jmjd2b-Jmjd2c common “promoter” target loci.
(D & G) Venn diagram shows the peak overlaps between Jmjd2b, Jmjd2c and the other factors- Ezh2, Suz12, Nanog and cMyc.
(F) Heat map shows the ChIP intensities of histone marks and TFs at the ±5kb regions around peak summit of Jmjd2b unique, Jmjd2c unique and Jmjd2b-Jmjd2c common target loci at distal regions.
(H) Correlation map of binding loci shows the degree of co-occupancy between selected histone modifications, TFs, chromatin regulators and three peak sets defined by combination of Jmjd2b and Jmjd2c as described in (B). Here the color scale represents the Pearson correlation coefficient and the clustering tree is derived from hierarchical clustering.
(I) Co-immunoprecipitation using antibodies against Ezh2, Jmjd2c and IgG. Endogenous Ezh2 and Jmjd2c were immunoprecipitated from nuclear extracts of mESCs and their interacting partners were analyzed by western blot using antibodies as shown right of the panel. (*) Indicate unspecific interactions.
(J) Jmjd2b antibody was used to immunoprecipitate endogenous Jmjd2b from nuclear extracts of mESCs, and its interacting partners were analyzed by western blot using antibodies. (*) Indicate unspecific interactions. See also Figure S3.
Next, we compared occupancy of Jmjd2b and Jmjd2c with four metagenes, groups of genes with correlated expression (high, moderate, low and very low) in mESCs. Further analyses showed the majority of “Jmjd2b-Jmjd2c common” targets and “Jmjd2b unique” targets are occupied at promoters and distal regions, respectively, and correspond to highly expressed genes, whereas the majority of “Jmjd2c unique” targets are occupied at the promoters of moderately, as well as lowly, expressed genes (Figure S3C). These initial observations reveal distinct distributions and functions of Jmjd2b and Jmjd2c in mESCs.
Jmjd2b/Kdm4b unique, Jmjd2c/Kdm4c unique and Jmjd2b/Kdm4b-Jmjd2c/Kdm4c common target regions belong to different regulatory modules of mESCs
To explore the significance of Jmjd2b-Jmjd2c common, Jmjd2b unique and Jmjd2c unique targets, we correlated occupancy maps with histone marks (H3K4me1/3, H3K9me2/me3, H3K27me3, H3K36me2/me3, and H3K27ac), components of PRC2 complex (Ezh2 and Suz12), ESC related transcription factors (TFs) (Oct4, Nanog, Sox2 and Klf4), associated transcription factors and chromatin regulators (cMyc, E2F1, p300, Med1 and Med12). As the majority of the Jmjd2b unique targets mapped to “non-promoter” regions (Figure 3B), we generated ChIP-seq intensity heat maps around the summit of all three-peak sets, which were further classified into “promoter” and “distal” regions. We found the three target classes associated with different histone marks and transcription factors. Jmjd2c unique targets strongly overlapped with bivalent marks (both H3K4me3 and H3K27me3 marks), and also with components of the PRC2 complex (Ezh2 and Suz12) at promoter and distal target loci (Figure 3C & S3E). ~80% of Ezh2 and Suz12 targets overlapped with Jmjd2c targets genome-wide (Figure 3D). Additionally, H3K36me3 and RNA Pol II occupancy were reduced at Jmjd2c unique promoter and distal target loci (Figure 3C & S3E), suggesting that the majority of Jmjd2c unique targets are poised or repressed. In contrast, Jmjd2b-Jmjd2c common and Jmjd2b unique targets showed reduced occupancy of H3K27me3, Ezh2 and Suz12, and gain of H3K4me3, H3K36me3 and RNA Pol II occupancy both at promoters and distal target loci (Figure 3C & S3E), indicating these targets are transcriptionally active. Notably, we found that binding of ESC-Core transcription factors, Nanog and Sox2, but not Oct4, was strongly biased towards Jmjd2b unique targets rather than the other two peak sets at both promoter and distal regions (Figure 3E-F). This was further supported by peak overlap analysis (Figure 3G). Nonetheless, H3K27ac, H3K4me1, p300, Mediators (Med1, Med12) and Klf4 binding sites overlapped with both Jmjd2b unique and Jmjd2b-Jmjd2c common targets, and targets of cMyc and its associated factor E2f1 showed significant overlap with Jmjd2b-Jmjd2c common target genes at promoter and distal target loci (Figure 3E-F & 3G).
For a global view of Jmjd2b unique, Jmjd2c unique and Jmjd2b-Jmjd2c common targets along with transcription factors and histone marks, we collated genome-wide targets. Hierarchical clustering tree and heat map representation of occupancy correlation revealed three distinct ESC regulatory modules – Polycomb, Myc and Core, as described previously (Kim et al., 2010). Jmjd2c unique targets segregated to the Polycomb (PRC) module, whereas Jmjd2b unique and Jmjd2b-Jmjd2c common target genes correlated to Core and Myc modules, respectively, which were interconnected by targets of mediators, Med1 and Med12 (Figure 3H).
To determine if genes targeted by Jmjd2b and Jmjd2c correlate to different functions as predicted by the correlation of common targets with the distinct modules, we performed GO analysis. Jmjd2b-Jmjd2c common target genes were highly enriched for transcriptional regulation, DNA binding and cell cycle related terms, whereas Jmjd2b and Jmjd2c unique targets related to nucleosome assembly and several development processes, respectively (Figure S3F). In addition, IPA analyses demonstrated that Jmjd2b-Jmjd2c common target genes were closely related to DNA damage, many cancer-related pathways, such as p53, PI3K-AKT and ESC pluripotency (Figure S3G), consistent with the Myc module (Kim et al., 2010). Meanwhile, Jmjd2c unique target genes were mainly associated with several neuronal signaling pathways (Figure S3G), compatible with association of the PRC2 module with development. Taken together, these data provide evidence that Jmjd2b and Jmjd2c act individually and combinatorially through different regulatory cassettes in mESCs.
Jmjd2b/Kdm4b and Jmjd2c/Kdm4c interact with key components of different regulatory modules
Nuclear factors that exhibit common targets often physically associate (Kim et al., 2010). Therefore, we examined physical interactions between Jmjd2b and Jmjd2c with core components of each regulatory module. Co-immunoprecipitation (Co-IP) with Ezh2 antibody revealed an association of Jmjd2c and components of PRC2 complex (Ezh2, Suz12, Jarid2) (Figure 3I). In contrast, other Kdm4 family members, Jmjd2a, Jmjd2b and Jmjd2d, did not co-immunoprecipitate with Ezh2. Conversely, Co-IP with Jmjd2c antibody confirmed interaction between Jmjd2c and members of the PRC2 complex, but not with key components (Nanog and Sox2) of other regulatory modules (Figure 3I). The interaction between Jmjd2c and PRC2 components is specific, as Jmjd2c does not interact detectably with the PRC1 subunit Bmi1. Jmjd2c do not interact with Jmjd2b (Figure 3I). We further examined whether stability of the PRC2 complex is affected upon depletion of Jmjd2c, and the converse. The level of Ezh2 protein was unaffected upon depletion of Jmjd2c; moreover, the level of Jmjd2c protein was unaltered upon Ezh2 depletion (Figure S3H). Finally, Co-IP of Jmjd2b revealed association with Oct4, Nanog and cMyc, but not with Ezh2 and Suz12 of the PRC2 complex (Figure 3J). These data strengthen the functional relationship between Jmjd2b and Jmjd2c with their central components of different ESC regulatory modules.
To explore molecular changes upon depletion of Jmjd2b and Jmjd2c, we examined global histone marks, including H3K9me2/me3 and H3K36me2/me3, the putative substrates of the Jmjd2b and Jmjd2c demethylases (Cloos et al., 2006; Agger et al., 2008). We did not observe significant global changes, including H3K9me2/me3 and H3K36me2/me3 (Figure S3I-J). To examine potential redundant function of Jmjd2b and Jmjd2c for these histone modifications, knock-down of both Jmjd2b and Jmjd2c was performed (Figure S3K). An increased level of global H3K9me3 was observed (Figure S3L). This finding implies redundant or overlapping roles among the members of the same sub-class.
Jmjd2b/Kdm4b and Nanog act through an interconnected regulatory loop
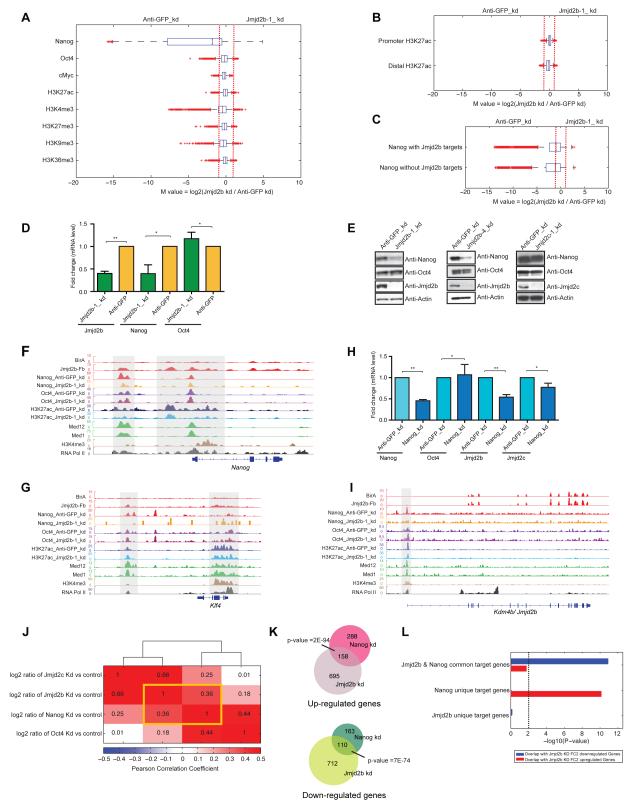
To interrogate potential molecular mechanisms of Jmjd2b action, we generated ChIP-seq datasets of Nanog, Oct4 and cMyc, as well as histone marks H3K4me3, H3K27me3, H3K36me3, H3K9me3 and H3K27ac from both control (Anti-GFP) and Jmjd2b depleted ESCs. We calculated the log2 ratio (M-value) of ChIP-seq intensities between two cell types using MAnorm, a quantitative comparison algorithm (Shao et al., 2012). The vast majority of Nanog binding peaks were reduced > 2-fold upon Jmjd2b knockdown (corresponding to M value <−1) (Figure 4A & Table S7). As Jmjd2b unique targets predominantly occupy distal regions, we assessed H3K27ac occupancy at distal regions from control and Jmjd2b depleted cells. Global occupancy of H3K27ac was unchanged at both promoter and distal regions upon depletion of Jmjd2b (Figure 4B). Further analyses showed that global Nanog occupancy was not dependent on Jmjd2b occupancy (Figure 4C), although global Nanog occupancy was reduced significantly upon depletion of Jmjd2b, suggesting other mechanisms by which Jmjd2b regulates Nanog binding.
Figure 4. Jmjd2b/Kdm4b and Nanog act through an interconnected regulatory loop.
(A) Box plots of the log2 fold changes of binding intensities between Anti-GFP (control) and Jmjd2b KD ESCs for Nanog, Oct4, cMyc, H3K27ac, H3K4me3, H3K27me3, H3K36me3 and H3K9me3. Red crosses correspond to outliers.
(B) Box plot of the log2 fold changes of H3K27ac marks at its promoter and distal targets from Anti-GFP (control) and Jmjd2b KD cells.
(C) Box plot of the log2 fold changes of Nanog binding intensities with or without Jmjd2b occupancy in Anti-GFP (control) and Jmjd2b KD cells.
(D) mRNA expression levels of Jmjd2b, Nanog and Oct4 from Anti-GFP (control) and Jmjd2b KD cells. Transcript levels were normalized using Gapdh. Data are represented as mean +/− SEM, n=3, p-values were calculated using t-test; **p <0.001, *p <0.01.
(E) Western blot analyses of Nanog and Oct4, from Jmjd2b (using two individual shRNAs), Jmjd2c and Anti-GFP (control) depleted cells.
(F-G) Genomic tracks of ChIP intensities of several factors and histone marks binding at Nanog and Klf4 loci.
(H) mRNA expression levels of Jmjd2b, Jmjd2c, Oct4 and Nanog from Nanog KD and Anti-GFP KD (control) cells. Transcript levels were normalized using Gapdh. Data are represented as mean +/− SEM, n=3, p-values were calculated using t-test; **p <0.001, *p <0.01.
(I) Genomic tracks of ChIP intensities of several factors and histone marks binding at Kdm4b/Jmjd2b locus. Jmjd2b shows no enrichment of its binding at its own locus.
(J) Clustering patterns of global gene expression changes by log2 ratio in Jmjd2b, Jmjd2c, Nanog and Oct4 depleted cells relative to their corresponding controls. Color and numbers represent the Pearson correlation coefficient.
(K) Venn diagram of overlapped up or down-regulated genes in Jmjd2b and Nanog knockdown cells.
(L) P-values represent enrichment of overlap between up or downregulated genes in Jmjd2b knockdown cells compared to Anti-GFP (control) knockdown cells and target genes occupied by Jmjd2b and Nanog both or either in mESCs (see Supplementary Information for the definition of Jmjd2b and Nanog ChIP target genes). Height of bars represents -log10 transformed P-value, derived from right-tailed Fisher’s exact test, and dotted line corresponds to P-value of 0.01, above which overlap will be considered as significantly enriched. See also Figure S4.
To investigate how Jmjd2b impinges on the Nanog regulatory pathway, we examined expression of Nanog upon Jmjd2b knockdown, and observed significant reduction of Nanog, but not Oct4, expression, (Figure 4D-E). Subsequently, we examined binding of Jmjd2b, Jmjd2c, Nanog, Oct4, Mediators (Med1 & Med12) and H3K27ac specifically at the Nanog locus, and observed loss of Nanog binding selectively at its enhancer locus upon Jmjd2b knockdown (left shadow region, Figure 4F). Similarly, reduction or loss of Nanog binding was also found at regulatory regions of several of its downstream target genes, including both ESC specific (Klf4 and Tbx3) and lineage specific genes (Lefty1 and Otx2) upon knockdown of Jmjd2b (Figure 4G & S4A-C). Next, we determined whether Nanog regulates Jmjd2b expression; surprisingly, we found that expression of Jmjd2b was also reduced upon depletion of Nanog (Figure 4H), and occupancy of Nanog was reduced at the Jmjd2b locus in Jmjd2b knockdown cells (Figure 4I), suggesting that Jmjd2b and Nanog control each other through a regulatory loop.
Furthermore, we compared global gene expression changes upon knockdown of Jmjd2b, Jmjd2c, Oct4 and Nanog. We observed the highest correlation of global gene expression changes between Jmjd2b and Nanog knockdown samples (Figure 4J). Genes differentially expressed upon knockdown of Nanog significantly overlapped with differentially expressed genes following Jmjd2b depletion (Figure 4K), indicating that their regulatory pathways are tightly interconnected. Further analyses revealed that the combined binding targets of Jmjd2b and Nanog highly correlated with Jmjd2b depleted downregulated genes, whereas Nanog-only binding targets were correlated with Jmjd2b depleted upregulated genes (Figure 4L). These findings are consistent with the prior observation that Nanog acts combinatorially in target gene activation, but binds alone or with few other proteins at repressed or non-expressed targets (Kim et al., 2008). Taken together, our data argue that Jmjd2b and Nanog act through highly interconnected regulatory pathways.
To test this further, we performed rescue experiments with full length Jmjd2b, the catalytic domain (JmjC) of Jmjd2b (1-333aa), a catalytic mutant (HTE>ATA) of Jmjd2b and full length Nanog in Jmjd2b-deficient ESCs (Figure S1F & S4L-M). Full length Jmjd2b fully rescued Nanog expression, and vice versa, in Jmjd2b-deficient ESCs, whereas the catalytic domain (JmjC) containing Jmjd2b (1-333aa) and the catalytic mutant (HTE>ATA) of Jmjd2b only partially rescued Nanog expression (Figure S4L-M), which is correlated with the restored ESC growth phenotype and SSEA-1 expression (Figure S1F).
Our findings fail to replicate a prior report that Jmjd2c binds at the Nanog promoter and regulates Nanog expression through H3K9me3 levels (Loh et al., 2007). We observed Jmjd2b occupancy at the Nanog locus, but did not detect a change in H3K9me3 at this locus upon depletion of Jmjd2b (Figure S4D). Moreover, we observed significant reduction of Nanog expression and occupancy at target genes (Nanog, Klf4, Otx2) upon depletion of Jmjd2b, but not upon depletion of Jmjd2c (Figure S4E), suggesting Jmjd2b-Nanog interconnected regulation.
Jmjd2c/Kdm4c assists PRC2 in repression in mESCs
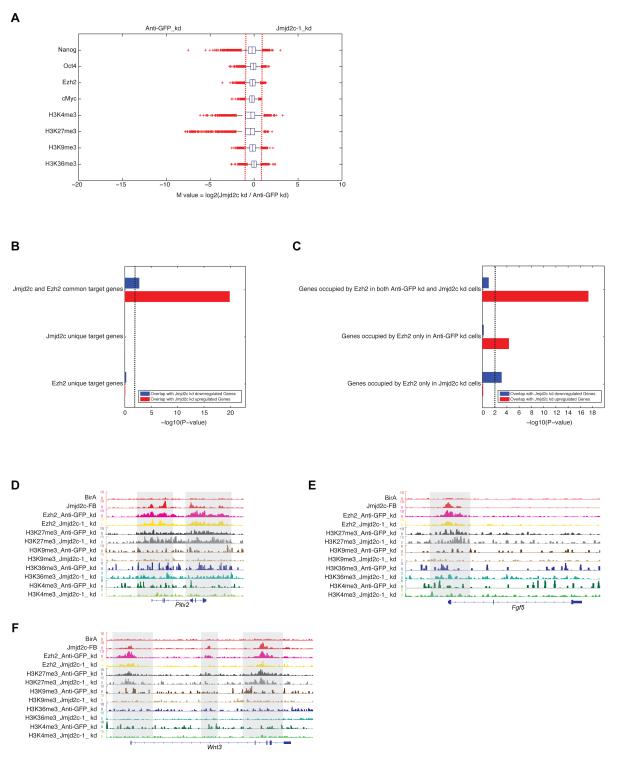
Jmjd2c associates with the PRC2 complex, and targets of Jmjd2c in chromatin correlate to the PRC2 regulatory module. To gain insight into these relationships, we performed ChIP-seq of Oct4, Nanog, Ezh2, cMyc, H3K4me3, H3K27me3, H3K36me3 and H3K9me3 in both control (Anti-GFP) and Jmjd2c knockdown mESCs. Quantitative comparison indicated that none of these factors demonstrated a significant global change of binding intensity (Figure 5A & Table S7), suggesting that the predominant effect of Jmjd2c depletion is restricted to a subset of genes.
Figure 5. Jmjd2c/Kdm4c assists PRC2 in repression in mESCs.
(A) Box plots of the log2 fold change of binding intensities between Anti-GFP (control) and Jmjd2c depleted cells for Nanog, Oct4, Ezh2, cMyc, H3K4me3, H3K27me3, H3K36me3 and H3K9me3. Red crosses correspond to outliers.
(B) P-values represent enrichment of overlap between upregulated or downregulated genes from Jmjd2c knockdown cells compared to Anti-GFP (control) knockdown cells and target genes occupied by either Jmjd2c or Ezh2 or both in mESCs at “promoters”. Height of bars represents -log10 transformed P-value derived from right-tailed Fisher’s exact test, and dotted line corresponds to P-value of 0.01.
(C) P-values represent enrichment of overlap between upregulated or downregulated genes from Jmjd2c knockdown cells compared to Anti-GFP (control) knockdown cells and the promoter target genes of Ezh2 in these two cell types. P-value represents same as (B).
(D-F) Genomic tracks of ChIP-seq intensities of Jmjd2c, Ezh2 and histones marks in both Anti-GFP (control) and Jmjd2c KD cells at selected upregulated gene loci upon Jmjd2c depletion (Pitx2, Fgf5 and Wnt3). See also Figure S5.
Genes that displayed increased expression upon Jmjd2c knockdown were comprised principally of developmentally regulated genes (Figure S2D-E). Since this class is highly enriched in bivalent marks (and the presence of Ezh2) in ESCs, we compared the binding of Ezh2/H3K27me3 and Jmjd2c at the “promoter” region of these genes. We observed highly significant co-occupancy of Jmjd2c and Ezh2 at these Jmjd2c depleted upregulated genes (Figure 5B). Yet, neither Jmjd2c nor Ezh2 alone significantly occupy these upregulated genes, suggesting that Jmjd2c and Ezh2 act combinatorially in gene repression and that Jmjd2c exert a direct gene regulatory influence through occupancy of these promoters (Figure 5B). Moreover, Jmjd2c knockdown did not significantly alter occupancy of Ezh2/H3K27me3 at Jmjd2c depleted upregulated genes (Figure 5C and S5A), indicating that Ezh2 alone is insufficient to repress these upregulated genes and requires Jmjd2c for full repression. Further study of several developmental genes, Pitx2, Fgf5, Wnt3, T and Olig3 that were strongly upregulated in Jmjd2c knockdown cells, showed that indeed these gene loci are occupied by both Jmjd2c and Ezh2. However, the binding of Ezh2 and the presence of H3K27me3 were largely “invariant” at these loci upon depletion of Jmjd2c (Figure 5D-F & S5B-C). Thus, although recruitment of PRC2/Ezh2 and H3K27me3 deposition are independent of Jmjd2c, full repression associated with PRC2 at these targets requires Jmjd2c.
Jmjd2c/Kdm4c and PRC2 block transcription at PRC2 target genes
Since the H3K27me3 mark is largely unchanged at upregulated genes upon Jmjd2c depletion (Figure 5C-F & S5A), we speculated that Jmjd2c might affect other aspects of PRC2-mediated repression. Recent studies indicate that bivalent genes bound by PRC2 exhibit RNA Pol II pausing at proximal promoters with reduction of productive transcriptional elongation (Min et al., 2011), thereby providing another mechanism of repression. Through Co-IP we observed that both Ezh2 and Jmjd2c interact with pausing factors (NelfA and Spt5), as well as with components of the core transcription machinery involved in initiation and elongation, including unphosphorylated RNA Pol II (RNAP) (initiation), RNA Pol II Ser-5p (RNAP-S5p) (initiation), RNA Pol II Ser-2p (RNAP-S2p) (elongation), Cdk9 (initiation), Ctr9 (component of Paf1c elongation complex), and cMyc (initiation, involves in pause release) (Peterlin and Price, 2006; Rahl et al., 2010; Nechaev and Adelman, 2011) (Figure S6A). Furthermore, full length Jmjd2c, as well as its catalytic domain (1-333aa), interacted with Ezh2, RNAP and Cdk9 (Figure S6B).
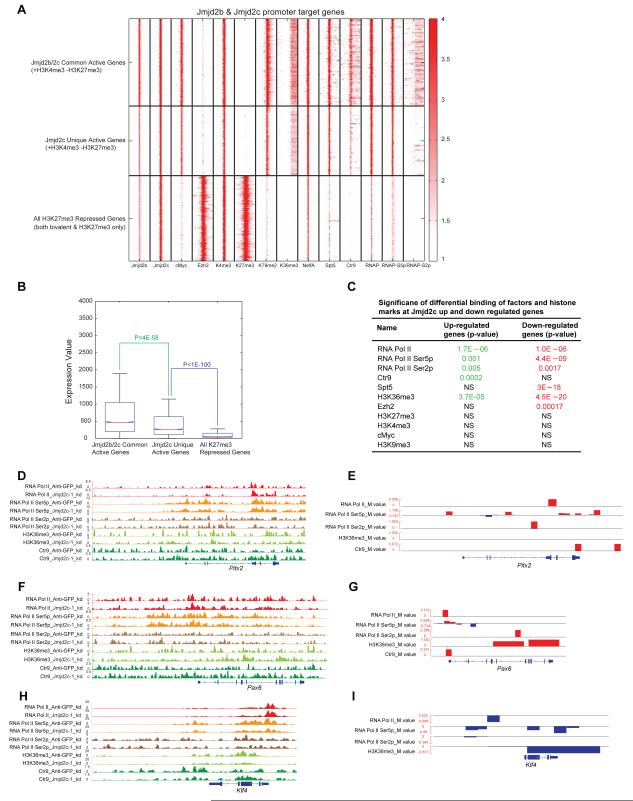
To gain further insights into the mechanistic role of Jmjd2c and Ezh2 in RNA Pol II pausing, we examined three sets of genes defined by promoter occupancy of Jmjd2b, Jmjd2c, H3K4me3 and H3K27me3: Jmjd2b-Jmjd2c-common active genes (H3K4me3-only), Jmjd2c unique active genes (H3K4me3-only) and all H3K27me3 repressed genes (including both bivalent and H3K27me3-only genes, most of which are PRC2 target genes and overlapped with Jmjd2c target genes) (Figure S3D & 6A). Jmjd2b-Jmjd2c common active genes showed strong binding of transcription initiation factors, including RNAP, RNAP-S5p, NelfA, Spt5 and cMyc at TSS, as well as strong occupancy of factors associated with elongation, including RNAP-S2p, Ctr9, Spt5, H3K36me3 and H3K79me2 at gene bodies (Figure 6A). Binding of these transcription initiation and elongation factors at Jmjd2c unique active genes was reduced compared to Jmjd2b-Jmjd2c common active genes. In addition, repressed genes bound by Jmjd2c and PRC2 (H3K27me3) showed dramatic loss of binding of these factors at TSS and gene bodies (Figure 6A), indicating that they are indeed poised/repressed in mESCs. Furthermore, comparing the expression levels among these three sets of genes confirmed that they exhibit significantly different levels of expression, which are themselves correlated with occupancy of transcription initiation and elongation factors (Figure 6B). Specifically, we observed that combined PRC2/Jmjd2c target genes are occupied with RNAP, RNAP-S5p, NelfA and Spt5 at the promoter regions, but not with elongation factors (RNAP-S2p, Ctr9, H3K36me3 and H3K79me2) at gene bodies, suggesting these poised/repressed genes are probably paused with RNA Pol II (Figure 6A) (Nechaev and Adelman, 2011). We observed that cMyc occupancy directly correlated with transcription of two sets of active genes, but not with PRC2 target genes (Figure 6A), consistent with a role for cMyc in active transcription through RNA Pol II pause release at actively transcribed genes in mESCs (Rahl et al., 2010). These data point to a role for Jmjd2c in transcriptional repression, most likely through blocking of RNA Pol II recruitment and/or pausing of RNA Pol II at Jmjd2c occupied PRC2 target genes.
Figure 6. Jmjd2c/Kdm4c and PRC2 block transcription at PRC2 target genes.
(A) Heat map shows the ChIP-seq densities of selected factors, histone marks, and a different modified form of RNA Pol II (RNAP, RNAP-S5p and RNAP-S2p) at the ±5kb regions around TSS of promoters of 1) Jmjd2b-Jmjd2c common active genes 2) Jmjd2c-unique active genes and 3) all H3K27me3 target genes.
(B) Box plot of gene expression values of Jmjd2b-Jmjd2c common active genes, Jmjd2c unique active genes, and all H3K27me3 target genes.
(C) Significance of binding changes of profiled factors at differentially expressed genes in Jmjd2c KD cells relative to Anti-GFP (control) KD cells. P-values were calculated by using K-S test to compare the global distribution of log2 fold changes of ChIP intensities (M-values inferred by MAnorm program) of each factor at its peak loci associated with up or down regulated genes relative to those with non-differentially expressed genes as control. Significant P-values (<0.01) are marked in red or green; otherwise they are labeled as non-significant (NS).
(D, F & H) Genomic tracks represent ChIP intensities of several transcription initiation and elongation factors binding in Jmjd2c KD and Anti-GFP (control) KD cells at Jmjd2c depleted up (Pitx2, Pax6) and down (Klf4) regulated gene loci.
(E, G & I) Genomic tracks represent log2 fold change of binding intensities at each peak loci between selected pairs of ChIP-seq data sets shown in (D, F & H), which is referred by M-values derived by MAnorm model. More description, please see figure legends of S6F & H. See also Figure S6.
Next, we examined occupancy of selected factors and histone marks involved in transcription initiation and elongation in Jmjd2c and control (Anti-GFP) knockdown cells. Global occupancy of these factors and histone marks did not differ significantly between these cells (Figure 5A, S6C & Table S7). We then used a “novel quantitative comparison” method to discover factors whose binding is altered significantly at Jmjd2c depleted up and downregulated genes (see supplementary information for detail method). Jmjd2c depleted upregulated genes reveal significantly higher binding of RNAP, RNAP-S5p, RNAP-S2p, Ctr9 and H3K36me3 mark (a mark associated with transcription elongation), whereas Jmjd2c depleted downregulated genes showed reduced occupancy of these factors and histone marks (Figure 6C & S6D). Moreover, we observed increased and decreased occupancy of transcription initiation and elongation factors at specific upregulated and downregulated gene loci, respectively, upon Jmjd2c depletion (Figure 6D-I & S6E-H), indicating correlation between occupancy of the transcription machinery and gene expression. We demonstrated that Jmjd2c and Ezh2 co-occupy significantly at Jmjd2c depleted upregulated genes compared to downregulated genes (Figure 5B), and these upregulated genes display increased binding of transcription machinery factors and histone marks upon depletion of Jmjd2c, suggesting that Jmjd2c directly regulates these upregulated genes. On the other hand, no correlation was observed between Jmjd2c occupancy and Jmjd2c depleted downregulated genes (Figure 5B), indicative of indirect regulation of these genes through Jmjd2c. Taken together, these data indicate that Jmjd2c is required in ESCs for full repression by PRC2 at poised/repressed developmental genes.
DISCUSSION
Jmjd2b/Kdm4b and Jmjd2c/Kdm4c belong to distinct regulatory modules in mESCs
Depletion of either Jmjd2b or Jmjd2c leads to ostensibly similar differentiation phenotypes and comparable overall global gene expression patterns in ESCs (Figure 1B, S1B & 2). However, global chromatin occupancy studies of Jmjd2b and Jmjd2c demonstrate each occupies numerous unique and common targets and correlates with functionally distinct regulatory modules. Jmjd2b unique, Jmjd2b-Jmjd2c common and Jmjd2c unique targets partition to the Core, Myc and Polycomb repressive complex (PRC) regulatory modules of the overall ESC network, respectively (Figure 3H). Thus, these closely related HDMs sub-serve specialized targets, in part relating to their combinatorial partitioning. Metagene analyses provide further support that occupancy of Jmjd2b and Jmjd2c unique and common targets is correlated with the pattern of gene expression in mESCs (Figure S3C). Protein-protein interaction studies corroborate that Jmjd2b and Jmjd2c physically interact with distinct regulators, specifically Jmjd2b with factors of the Core pluripotency network and Jmjd2c with components of the PRC2 complex (Figure 3I-J). Therefore, we speculate that combinatorial binding patterns of HDMs across the genome will be critical for understanding regulatory programs in cell type specific transcriptional regulation.
Jmjd2b/Kdm4b, Jmjd2c/Kdm4c and histone modifications
Combinations of histone modifications play crucial role in gene regulation (Zhou et al., 2010). Jmjd2b and Jmjd2c are classified as H3K9me2/3 and H3K36me2/3 HDMs (Agger et al., 2008; Cloos et al., 2006). As H3K36me2/me3 and H3K9me2/3 are indicative of divergent transcriptional outcomes, it is possible that Jmjd2b and Jmjd2c function differently in a cell type specific and context dependent manner. A relatively low level of H3K9me3 correlates with the highly active and open ESCs and iPSCs genome (Meshorer and Misteli, 2006; Soufi et al., 2012). Recent studies also demonstrate that depletion of all H3K9 demethylases, including Jmjd2b and Jmjd2c, significantly inhibits reprogramming of pre-iPSCs to iPSCs. Conversely, depletion of the H3K9 methyltransferase Setdb1 leads to highly efficient reprogramming, indicating removal of H3K9me3 is essential for establishment of the ESC/iPSC state (Chen et al., 2012). In this study, we did not observe significant changes in “global” histone levels or occupancy, including H3K9me2/3 and H3K36me2/3 marks, upon depletion of Jmjd2b and Jmjd2c (Figure S3I-J, 4A & 5A). However, double knockdown of Jmjd2b and Jmjd2c led to an increased level of global H3K9me3 (Figure S3K-L), suggesting redundant function in ESCs. We also monitored effects of Jmjd2b and Jmjd2c depletion on histone marks (H3K4me3, H3K27me3, H3K9me3 and H3K36me3) at several “individual” loci. For Jmjd2b-Jmjd2c common active genes, we observed reduction of H3K4me3 and H3K36me3 occupancy at several active gene loci, such as Klf4, Tbx3 and Esrrb, upon depletion of either Jmjd2b or Jmjd2c (Figure S4F-K, 7 & S7A-C). In context of Jmjd2b-Nanog interconnected regulation, we did not observe any significant change of H3K9me3 at the Nanog locus and its downstream target genes upon depletion of Jmjd2b. However, we observed occupancy changes at several discrete loci for H3K4me3 (e.g. Nanog, Klf4, Tbx3 and Esrrb promoters) and H3K36me3 (e.g. Klf4, Tbx3 and Otx2 gene bodies) (Figure S4F-K & 7). Interestingly, we found some of the Jmjd2b-Jmjd2c common active genes are the downstream targets of Nanog, suggesting fraction of common targets are regulated through Jmjd2b-Nanog regulatory loop (Figure 4G, S4A-C, S7A-C & 7). On the other hand, with respect to the role of Jmjd2c in PRC2-mediated repression, we observed that the upregulated genes upon Jmjd2c depletion are significantly co-occupied by Jmjd2c and Ezh2, and Jmjd2c assists PRC2 in full repression at these poised/ repressed genes (e.g, Fgf5, Pitx2, Pax6) through transcription repression (Figure 5B). Moreover, these upregulated genes showed significantly increased binding of transcriptional machinery (RNAP, RNAP-S5p, RNAPS2p, Ctr9) and histone marks, especially H3K36me3 (an elongation mark), but not other histone marks upon depletion of Jmjd2c (Figure 6C-I, S6D-H, 7), indicative of a link between Jmjd2c-PRC2 and transcription elongation. This supports a previous observation, in which PCL-PRC2 recognizes H3K36me3, and silence active chromatin regions for maintenance of ESCs pluripotency (Cai et al., 2013).
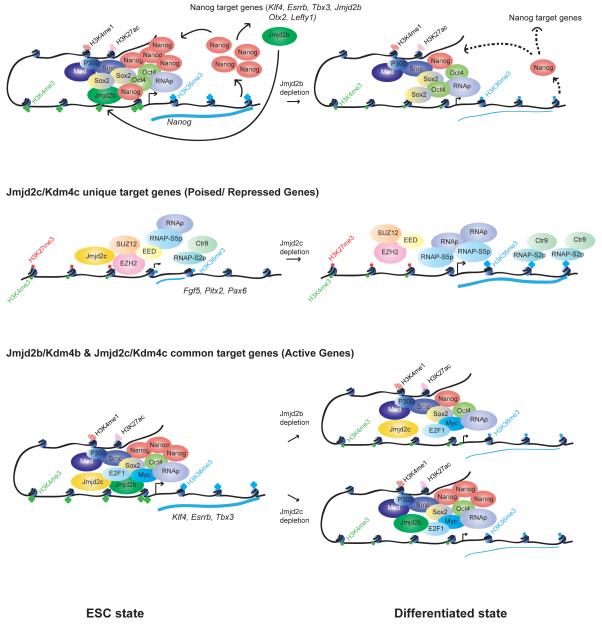
Figure 7. The working model of Jmjd2b/Kdm4b and Jmjd2c/Kdm4c in mESCs.
Jmjd2b is generally associated with active genes, whereas Jmjd2c is associated with poised/repressive genes. However, we found most of the active genes are occupied with both Jmjd2b and Jmjd2c. We propose a model, in which Jmjd2b and Jmjd2c work distinctly and combinatorially. (Blue thick and thin line corresponds to higher and lower level of transcription respectively. Black dotted line represents lower binding of Nanog to its target genes upon depletion of Jmjd2b). See also Figure S7.
Our study reveals unexpected outcomes of histone demethylase function of Jmjd2b and Jmjd2c, and its substrate specificity. However, rescue studies demonstrate catalytic activity of Jmjd2b and Jmjd2c is indeed required for self-renewal of ESCs. Moreover, our findings indicate a complex interplay between various histone modifications and/or Jmjd2b and Jmjd2c may have additional roles beyond histone targets. Recent studies have indicated that Jmjd2b is an integral part of the MLL2 complex that deposits the H3K4me3 mark, and H3K9me3 is a prerequisite of H3K4me3 deposition for ERα-activated gene transcription (Shi et al., 2011), suggesting crosstalk between histone modifications.
Jmjd2b/Kdm4b and Jmjd2c/Kdm4c act through distinct and combinatorial pathways in mESCs
As a core pluripotency factor Nanog functions together with other Core transcription factors (such as Oct4 and Sox2) to positively regulate their expression through auto-regulatory loops, as well as occupy and activate ESC specific genes and repress lineage specific genes to maintain the ESC state (Young, 2011). We demonstrate that Jmjd2b is involved in regulation of Nanog expression and occupancy of its downstream target genes. Moreover, Nanog regulates Jmjd2b expression that is important for maintenance of Nanog expression through a feedback loop (Figure 4A-I, 7).
In contrast, Jmjd2c physically interacts with components of PRC2 repressive complex, and its targets correlate with the PRC regulatory module (Figure 3). Furthermore, PRC2 requires Jmjd2c for full repressive function (Figure 5). Although the molecular details of PRC2-mediated gene repression remain incomplete, previous studies implicate several mechanisms in the control of transcriptional output including recruitment block of RNA Pol II, as well as promoter-proximal pausing of RNA Pol II at PRC2 target genes (Boyer et al., 2006; Lee et al., 2006; Min et al., 2011). Our data suggest that Jmjd2c may influence PRC2 activities for both RNA Pol II recruitment and pausing at PRC2/Jmjd2c targets (Figure 6A-C & S6A-D). High co-occupancy of Jmjd2c-Ezh2 at Jmjd2c depleted upregulated genes (Figure 5B) and increased distribution of RNAP, RNAP-S5p, RNAPS2p, Ctr9 and H3K36me3 at these Jmjd2c/PRC2 targeted upregulated genes in Jmjd2c depleted cells (Figure 6C-I, S6D-H) argue persuasively for the role of Jmjd2c in transcriptional repression. Therefore, Jmjd2c is required for the full repressive function of PRC2 at PRC2/Jmjd2c common target genes in ESCs.
Global Jmjd2b and Jmjd2c targets are differentially distributed. However, Jmjd2b and Jmjd2c share ~40% common targets (Figure 3B). These common targets are associated with active genes and belong to Myc modules (Figure 3). Jmjd2b and Jmjd2c do not physically interact with each other, but both Jmjd2b and Jmjd2c can physically interact with cMyc (Figure 3J & S6A). Nevertheless, occupancy of cMyc is not significantly changed globally or locally upon depletion of either Jmjd2b or Jmjd2c (Figure 4A, 5A & S7). Interestingly, we found some of the Jmjd2b-Jmjd2c common active genes (Klf4, Tbx3 and Esrrb) are downstream targets of Nanog, and its occupancy was reduced at these loci upon depletion of Jmjd2b, suggesting fraction of common targets are regulated through Jmjd2b-Nanog regulatory loop. We observed reduction of H3K4me3 and H3K36me3 at these common target genes upon depletion of either Jmjd2b or Jmjd2c (Figure S4 & S7) suggesting, indeed Jmjd2b and Jmjd2c combinatorially regulate these active gene loci.
We present a model in Figure 7 that summarizes our findings regarding how Jmj2b and Jmjd2c exert both distinct and combinatorial functions in ESCs identity (Figure 7). Our study suggests that exploration of combinatorial roles of HDMs with other chromatin regulators, transcription factors, and histone marks will elucidate multilayered regulatory networks in ESC biology.
EXPERIMENTAL PROCEDURES
ES Cell Lines and Culture
Mouse ES cell lines were maintained in ES medium as documented in supplemental information.
Lentiviral Production and RNAi screen
Individual shRNA constructs were used for lentivirus production. We used 5 different shRNA lentiviral constructs to knockdown (KD) each of 20 histone demethylases in mESCs. mESCs cells were seeded in 48-well plates and infected with individual shRNA lentivirus, and the screen was scored in terms of alterations of “ESC colony morphology”. Detailed protocol of lentiviral production and infection to mESCs is available in supplemental information.
Gene Expression Analysis
mRNA profiling of knock down samples were performed using Affymetrix mouse genome 430 2.0 Arrays. See Supplemental Experimental Procedures.
ChIP
Bio-ChIP and native antibody ChIP were performed as described elsewhere (Kim et al., 2008; Kim et al., 2009). Detailed procedures and list of antibodies are available in supplemental information.
ChIP sequencing and Library Generation
Purified ChIP DNA was used to prepare illumina multiplexed sequencing libraries. NEB next generation sequencing kit was used to prepare the libraries.
Chip-seq Data Analysis
Peaks were called using Model-based Analysis for ChIP-Seq (MACS), peak-finding algorithm to identify regions of ChIP-seq enrichment over background (Zhang et al., 2008). Detailed analyses are available in supplemental information.
Co-immunoprecipitation
Nuclear extracts were prepared from J1 mouse ES cells (Kim et al., 2010) and immunoprecipitated using specific antibodies,
Supplementary Material
HIGHLIGHTS.
-Jmjd2b and Jmjd2c are required for self-renewal of mESCs
-Jmjd2b and Jmjd2c are associated with different regulatory modules in mESCs
-Jmjd2b and Nanog act through an interconnected regulatory loop
-Jmjd2c assists PRC2 in full transcriptional repression
ACKNOWLEDGMENTS
We thank Dan Bauer, Jian Xu for critical reading of the manuscript, and David Hendrix for discussions. We also thank Marc Kerenyi for the reagents. We thank F. Abderazzaq and R. Rubio at CCCB sequencing facility at DFCI for Illumina HiSeq2000 Sequencing. This work was supported by funding from NIH Grant HLBI U01HL100001. S.H.O. is an Investigator of the Howard Hughes Medical Institute (HHMI). The authors declare no conflict of interest.
Footnotes
The cDNA microarray and ChIP-seq data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE43231.
SUPPLEMENTAL INFORMATION Supplemental information for this article includes seven figures, seven tables and experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Current Opinion in Genetics & Development. 2008;18:159–168. doi: 10.1016/j.gde.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Cai L, Rothbart SB, Lu R, Xu B, Chen W-Y, Tripathy A, Rockowitz S, Zheng D, Patel DJ, Allis CD, et al. An H3K36 Methylation-Engaging Tudor Motif of Polycomb-like Proteins Mediates PRC2 Complex Targeting. Molecular Cell. 2013;49:571–582. doi: 10.1016/j.molcel.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liu H, Liu J, Qi J, Wei B, Yang J, Liang H, Chen Y, Chen J, Wu Y, et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nature Genetics. 2012;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of External Signaling Pathways with the Core Transcriptional Network in Embryonic Stem Cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Cherry ABC, Daley GQ. Reprogramming Cellular Identity for Regenerative Medicine. Cell. 2012;148:1110–1122. doi: 10.1016/j.cell.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloos PAC, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. An RNAi Screen of Chromatin Proteins Identifies Tip60-p400 as a Regulator of Embryonic Stem Cell Identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Kim J, Xu Q, Leng Y, Orkin SH, Elledge SJ. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes & Development. 2009;23:837–848. doi: 10.1101/gad.1769609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cantor AB, Orkin SH, Wang J. Use of in vivo biotinylation to study protein–protein and protein–DNA interactions in mouse embryonic stem cells. Nat Protoc. 2009;4:506–517. doi: 10.1038/nprot.2009.23. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An Extended Transcriptional Network for Pluripotency of Embryonic Stem Cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH. A Myc Network Accounts for Similarities between Embryonic Stem and Cancer Cell Transcription Programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K-I, et al. Control of Developmental Regulators by Polycomb in Human Embryonic Stem Cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Zhang W, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes & Development. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlan TS, Gifford WD, Driscoll S, Lettieri K, Rowe HM, Bonanomi D, Firth A, Singer O, Trono D, Pfaff SL. Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 2012;487:57–63. doi: 10.1038/nature11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7:540–546. doi: 10.1038/nrm1938. [DOI] [PubMed] [Google Scholar]
- Min IM, Waterfall JJ, Core LJ, Munroe RJ, Schimenti J, Lis JT. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes & Development. 2011;25:742–754. doi: 10.1101/gad.2005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosammaparast N, Shi Y. Reversal of Histone Methylation: Biochemical and Molecular Mechanisms of Histone Demethylases. Annu. Rev. Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- Nechaev S, Adelman K. Pol II waiting in the starting gates: Regulating the transition from transcription initiation into productive elongation. Biochimica Et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2011;1809:34–45. doi: 10.1016/j.bbagrm.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH, Hochedlinger K. Chromatin Connections to Pluripotency and Cellular Reprogramming. Cell. 2011;145:835–850. doi: 10.1016/j.cell.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The Polycomb Group Protein Suz12 Is Required for Embryonic Stem Cell Differentiation. Molecular and Cellular Biology. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MT, Helin K. Histone demethylases in development and disease. Trends in Cell Biology. 2010;20:662–671. doi: 10.1016/j.tcb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the Elongation Phase of Transcription with P-TEFb. Molecular Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc Regulates Transcriptional Pause Release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Zhang Y, Yuan G-C, Orkin SH, Waxman DJ. MAnorm: a robust model for quantitative comparison of ChIP-Seq data sets. Genome Biology. 2012;13:R16. doi: 10.1186/gb-2012-13-3-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu Y, Hsu Y-J, Fujiwara Y, Kim J, Mao X, Yuan G-C, Orkin SH. EZH1 Mediates Methylation on Histone H3 Lysine 27 and Complements EZH2 in Maintaining Stem Cell Identity and Executing Pluripotency. Molecular Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and Impediments of the Pluripotency Reprogramming Factors’ Initial Engagement with the Genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, Zhou Q, Plath K. Role of the Murine Reprogramming Factors in the Induction of Pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes & Development. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Xie L, Pelz C, Wang W, Bashar A, Varlamova O, Shadle S, Impey S. KDM5B regulates embryonic stem cell self-renewal and represses cryptic intragenic transcription. The EMBO Journal. 2011;30:1473–1484. doi: 10.1038/emboj.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a Multisubunit Complex Containing RD, Cooperates with DSIF to RepressRNA Polymerase II Elongation. 1999:1–11. doi: 10.1016/s0092-8674(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Young RA. Control of the Embryonic Stem Cell State. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nature Publishing Group. 2010;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.