Abstract
Aging is associated with a progressive deterioration in the structure and function of the pulmonary circulation. Remodeling of the pulmonary vasculature occurs from maturity to senescence that is characterized by an increase in pulmonary vascular stiffness, pulmonary vascular pressures, and pulmonary vascular resistance along with increased heterogeneity of alveolar ventilation and pulmonary perfusion and decreased pulmonary capillary blood volume and membrane diffusing capacity that is consistent with a reduction in alveolar-capillary surface area. In theory, the aforementioned age-related changes in the pulmonary circulation may conspire to make elderly individuals more susceptible to gas exchange abnormalities during exercise. However, despite the erosion in ventilatory reserve with aging, the healthy older adult appears able to maintain alveolar ventilation at a level that allows maintenance of arterial blood gases within normal limits, even during heavy exercise. This ability to maintain adequate gas exchange likely occurs because age-related reductions in the maximal metabolic demand of exercise occur at a rate equal to or greater than the rate of deterioration in ventilatory reserve. A more prominent aspect of aging is the loss of lung elastic recoil that is associated with a modest reduction in the expiratory boundary of the maximal flow-volume envelope. This in turn increases the severity of expiratory airflow limitation and induces dynamic lung hyperinflation during exercise. The consequences of this age-associated decrease in elastic recoil on the pulmonary circulation are speculative, but an age-associated decline in elastic recoil may influence pulmonary vascular resistance and cardiac output, in addition to its impact on the work and oxygen cost of breathing.
Keywords: Aging, pulmonary circulation, gas exchange, exercise
From maturity to senescence, there is a modest deterioration in the structure and function of the respiratory system, including the pulmonary circulation. These changes result in alterations in pulmonary vascular pressures, pulmonary vascular resistance, and gas exchange at rest, but these changes do not appear to limit exercise in the majority of healthy aged adults, as evidenced by the ability to maintain arterial blood gases within the range of normal values. However, healthy aging also includes changes in pulmonary mechanics that appear to alter the normal ventilatory and breathing pattern response to exercise, and these altered mechanics may contribute to exercise limitation in elderly subjects through an increase in the work and oxygen cost of breathing.
This review addresses the age-related structural and functional changes in the pulmonary circulation and assesses the impact of these changes on gas exchange and exercise responses in healthy elderly individuals. In addition, we briefly consider whether age-associated changes in the pulmonary circulation influence the normal response to exercise when combined with a maintained metabolic demand (i.e., trained, highly fit subjects) or cardiovascular with impairment (i.e., disease). Finally, we explore the concept that changes in pulmonary mechanics may limit exercise in healthy aged individuals, primarily through an effect on expiratory airflow-limitation and operational lung volumes rather than through an impact on the pulmonary circulation or gas exchange.
AGE-RELATED STRUCTURAL AND FUNCTIONAL CHANGES IN THE PULMONARY CIRCULATION
Aging results in structural and functional changes in the pulmonary circulation that affect pulmonary pressures, pulmonary hemodynamics, and gas exchange. Predicted equations for the most common of these changes along with the changes in gas exchange in relation to age are given in Table 1.
Table 1.
Predicted Equations for Pulmonary Vascular Pressures, Pulmonary Hemodynamics, and Gas Exchange Measurements in Relation to Age
| Variable | Predicted Equation | Subjects/Study |
|---|---|---|
| Ppa (rest) | 0.975(age)+13.038 | Males and females, 14 to 68 years (only)49,50 |
| Ppw (rest) | 0.0175(age)+9.3042 | Males and females, 14 to 68 years (only)49,50 |
| Ppa (exercise) | 10.02 +0.621(Q) | Males and females, young and old49,50 |
| Ppw (exercise) | 6.54 +0.335(Q) | Males and females, young and old49,50 |
| PVR (Ppa − Ppw, exercise) | 3.26 +0.359(Q) | Males and females, young and old49,50 |
| DLCO (rest) | 126 − 0.90(age) | Males and females, ≥69 years12 |
| 0.1646(ht) − 0.2290(age) +12.9 | Males only51 | |
| 0.1602(ht) − 0.1111(age) +2.2 | Females only51 | |
| DLNO (exercise) | 4.235(Q) +19.705(EILV) − 0.706(age) − 7.133 | Males and females, young and old26 |
| AWUV | 23.1 − 0.09(age) | Males and females, young and old19 |
| DAV(log) | 0.00232(age) +0.35 | Males and females, 18 to 71 years21 |
| DPP(log) | 0.00221(age) +0.32 | Males and females, 18 to 71 years21 |
| RDPP | 0.007(age) +0.009(ht) − 0.809 | Males and females, 21 to 76 years20 |
| Vc | 0.398(ht) − 0.286(age) +29.14 | Males only23 |
| 0.823(ht) − 0.240(age) − 52.41 | Females only23 | |
| AaDO2 | 0.080(age) +2.3 | Males and females, 18 to 71 years21 |
Ppa, pulmonary arterial pressure; Ppw, pulmonary wedge pressure; PVR, pulmonary vascular resistance; DLCO, lung diffusing capacity for carbon monoxide; DLNO, lung diffusing capacity for nitric oxide; AWUV, airspace wall surface area per unit volume of lung tissue; DAV, dispersion of alveolar ventilation; DPP, dispersion of pulmonary perfusion; RDPP, relative dispersion of pulmonary perfusion; Vc, pulmonary capillary blood volume; AaDO2, alveolar-to-arterial oxygen difference; age, age in yrs; Q, cardiac output; ht, height in cm.
Intrinsic Properties of the Pulmonary Vasculature
With aging (i.e., beyond age 30 to 35 years.), there is a gradual and variable decrease in the extensibility of the pulmonary artery and, to a lesser extent, the pulmonary vein, indicating that senescence is associated with an increase in the stiffness of the pulmonary vasculature.1–5 This increase in stiffness is likely caused by age-related remodeling of the pulmonary vasculature, with an increase in muscle content of the pulmonary artery3 and a modest increase in arterial3,4 and venous3 wall thickness commonly observed. In principle, an increase in the ratio of less extensible collagen fibers to more distensible elastin fibers in the pulmonary vessels with age may also, at least in part, contribute to the aforementioned increase in vascular stiffness. However, biochemical and histological estimations of collagen and elastin content of pulmonary vasculature strips obtained at necropsy have proven inconsistent, with some,1 but not all,2,3 showing that collagen content of the pulmonary artery increases with age. Similarly, elastin content of the pulmonary trunk has been shown to increase,2 decrease,1,3 or not change4 with advancing age. No such age-related changes in the intrinsic properties of the pulmonary vein have been reported.3 The inconsistency among these findings likely relates to differences in the biochemical and histological techniques used to assess the composition of the pulmonary vessels. Thus, while aging is associated with an increase in pulmonary vascular stiffness, the exact etiology of this change remains unclear.
Pulmonary Vascular Pressures and Hemodynamics
The age-dependent structural changes in the pulmonary vasculature and the associated increase in pulmonary vascular stiffness serve to modify resting pulmonary vascular pressures and hemodynamics in older individuals. Several investigators have shown that with advanced age there is a modest increase in pulmonary arterial pressure (Ppa) and pulmonary wedge pressure (Ppw), with the effect becoming more pronounced after 45 years.6–10 For example, Ghali et al10 reported that mean Ppa at rest was 16.7 ± 4.6 cm H2O, 17.9 ± 6.4 cm H2O, and 20.6 ± 8.0 cm H2O in subjects aged < 45 years, 45 to 64 years, and ≥ 65 years, respectively. In addition, Kovacs et al8 reported that resting mean Ppa and Ppw were not different in healthy young (<30 years old) compared with healthy middle-aged (30 to 50 years old) subjects, but both pressures increased significantly beyond 50 years. The absolute age-associated increase in pulmonary arterial pressure is typically smaller than the accompanying age-associated pressure increase in the systemic circulation. When expressed as a percent increase, however, the age-related increase in systolic blood pressure is strikingly similar in the pulmonary as compared with the systemic circulation.9 Pulmonary vascular resistance [PVR =(Ppa−Ppw)÷ blood flow], also increases progressively with healthy aging.6,7,10 Similar to the age-associated increases in Ppa, the absolute increase in vascular resistance is greater in the systemic circulation than in the pulmonary circulation. However, the increase in PVR relative to the increase in systemic vascular resistance has been shown to increase with age, suggesting that the pulmonary circulation is more susceptible to age-related changes than the systemic circulation. While the age-associated remodeling of the pulmonary vasculature and the subsequent increase in pulmonary arterial stiffness are major contributors to the increases in resting pulmonary pressures and vascular resistance, an increase in pressure downstream of the pulmonary circulation caused by a decrease in left ventricular compliance secondary to left ventricular stiffening also likely has a role.11
Pulmonary Gas Exchange
There is a consensus in the literature that starting from early in adult life there is a progressive decline in pulmonary gas exchange with aging, as evidenced by a reduction in lung diffusing capacity for carbon monoxide (DLCO).12–15 Although some have shown the age-related decrease in DLCO to be linear,15 others have reported that DLCO decreases in a nonlinear fashion with advancing age due to a sharper decrease in pulmonary capillary blood volume beyond 50 years14 (see Pulmonary Capillary Blood Volume and Membrane Diffusing Capacity). Figure 1 shows cross-sectional data on over 100 healthy subjects collected in our laboratory and demonstrates the negative relationship between DLCO and age. Although there is some variability between subjects, linear regression analysis shows that DLCO decreases by ~0.15 mL·min−1·mm Hg−1·year−1 beyond age 20 to 25 years. These data are in agreement with the previous finding that DLCO is reduced by 0.2 to 0.32 mL·min−1·mm Hg−1·year−1 in males and 0.06 to 0.18 mL·min−1·mm Hg−1·year−1 in females.16 Likely contributors to the impairment in gas exchange with aging are a reduction in pulmonary capillary blood volume,14,17 a decrease in the number of capillaries perfusing the lungs,18 and a decrease in alveolar surface area17,19 with a subsequent reduction in membrane diffusing capacity14,17 and an increase in the heterogeneity of pulmonary perfusion20 and ventilation.21
Figure 1.
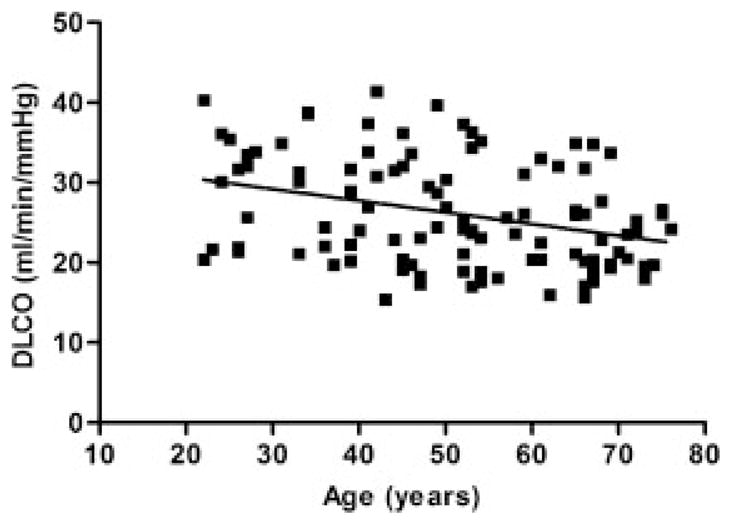
Cross-sectional data obtained in our laboratory showing the age-associated change in diffusion capacity of the lung for carbon monoxide (DLCO) in 105 healthy non-smokers. Linear regression beyond the age of 20 results in an annual decrease of 0.15 mL/min/mmHg/year [y =−0.1451 (age) +33.543].
ALVEOLAR-CAPILLARY SURFACE AREA
The healthy aged lung is characterized by a homogeneous enlargement of the alveolar airspaces with a subsequent fall in the total number of alveoli18,19,22 and a significant decrease in capillary density.18 Using a simple microscopic morphometric technique, Butler and Kleinerman18 assessed the relative alveolar-capillary density and the mean alveolar diameter in 15 healthy lungs obtained at autopsy. The ratio of alveolar-capillary density to alveolar diameter is a measure of the available capillary surface area to alveolar volume, and, as such, is an index of the maximal structural potential for gas exchange. The authors observed an increase in alveolar diameter and an equivocal decrease in alveolar-capillary density in the aged lungs. More recently, in a sample of 35 healthy nonsmokers, Gillooly and Lamb19 found a significant negative relationship between area per unit volume of lung tissue and age, implying a reduction in alveolar wall surface area and an increase in air space size. In combination, these findings suggest that there is a gradual reduction in the ratio of alveolar-capillary density to alveolar diameter in the aged lung that likely limits the potential for gas exchange and contributes at least in part to the diminished DLCO commonly observed in healthy older persons.
PULMONARY CAPILLARY BLOOD VOLUME AND MEMBRANE DIFFUSING CAPACITY
The reduction in alveolar–capillary interface along with the stiffening of the pulmonary vasculature serves to decrease pulmonary capillary blood volume (Vc) with aging.14,17,23 The age-associated reduction in Vc, however, appears to be small, with the fall approximating 2 to 5 mL per decade. For example, Chang et al17 reported that Vc was 72 ± 11 mL in 16 young men (mean 28 years) and 58 ± 10 mL in 13 older men (mean 60 years). Georges et al14 demonstrated that Vc did not change significantly until after 50 years in 70 healthy nonsmoking adults aged 18 to 78 years. These reductions in Vc, in conjunction with the aforementioned amelioration in alveolar-capillary surface area secondary to age-related remodeling, result in a reduction in the diffusing capacity of the alveolar-capillary membrane.14,17 This, in turn, is a probable contributor to the reduction in DLCO with advancing age, particularly in the later years of life (>50 years).
HETEROGENEITY OF ALVEOLAR VENTILATION AND PERFUSION
The inequality of alveolar ventilation to perfusion (VA/Qc) has been shown to increase with age in healthy individuals, with a subsequent reduction in resting partial pressure of arterial oxygen.21 The age-associated increase in VA/Qc inequality has been attributed primarily to an increase in the heterogeneity of alveolar ventilation. More recent evidence, however, has suggested that the heterogeneity of pulmonary perfusion may also increase with age.20 The age-associated loss of lung elastic recoil combined with a reduction in the number of elastic attachments of supporting alveoli causes smaller airways to close at higher lung volumes in older versus younger subjects. As a result, some airways may be narrowed or closed during normal tidal breathing in elderly individuals, which would be expected to increase the heterogeneity of VA in the older lung. In addition, it is likely that the age-associated decline in elastic recoil is not consistent throughout the lung and, as a consequence, regions of the aged lung may be more or less compliant than others, further promoting a nonuniform distribution of ventilation. Recent work by Levin and colleagues20 has shown that advancing age is also associated with an increase in the heterogeneity of pulmonary perfusion. Using magnetic resonance imaging, the authors demonstrated that the spatial heterogeneity of pulmonary blood flow was positively related to age, increasing ~0.1·decade−1 until age 50 to 59 years. This age-related disturbance in the distribution of pulmonary perfusion is likely related to the reduction in the extensibility and recruitment potential of the pulmonary vasculature secondary to age-associated remodeling. It is probable that the reduction in DLCO with age is, in part, a consequence of the increase in the heterogeneity of both alveolar ventilation and pulmonary perfusion, both of which are probable contributors to the increased inequality of VA/Qc with age.
IMPACT OF AGE-ASSOCIATED CHANGES ON THE RESPONSE TO EXERCISE
Figure 2 summarizes the age-associated changes in the pulmonary vasculature and gas exchange at rest and their effect on the response to exercise in the healthy older adult.
Figure 2.
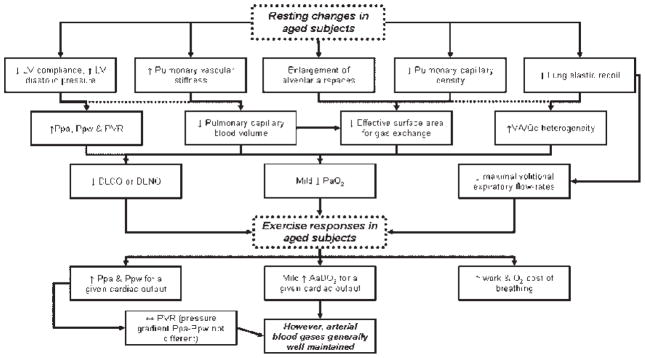
Overview of the age-associated changes in the pulmonary vasculature and gas exchange at rest, and their effect on the response to exercise in the healthy older adult.
Pulmonary Vasculature
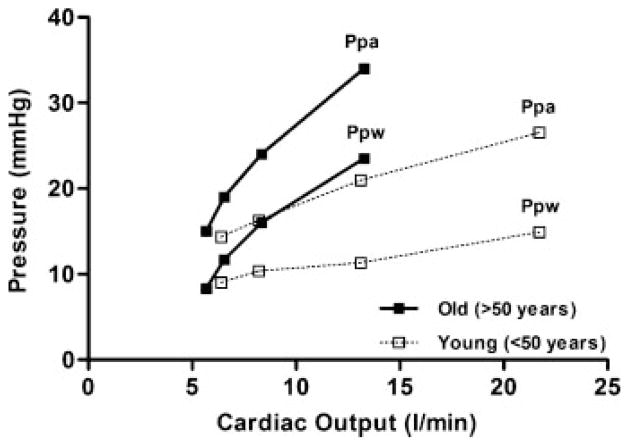
To increase blood flow through the lung the vascular driving pressure from the pulmonary artery to the left atrium must be increased. Accordingly, exercise is associated with an increase in Ppa and Ppw in both young and older subjects.6–8 The increases in pulmonary pressures with exercise in older subjects, however, are generally out of proportion to those determined at a similar VO2 and cardiac output (Q) in younger subjects, even during submaximal exercise (Fig. 3).6–8,24 For example, Kovacs et al8 reported that Ppa increased by ~60% from 13.8 mm Hg at rest to 21.8 mm Hg during mild exercise (VO2 = 1362 mL·min−1, Q =15.6 L·min−1) in subjects aged <50 years. By comparison, in subjects aged >50 years, Ppa increased by ~120% from 14.9 mm Hg at rest to 32.4 mm Hg during exercise that required a similar metabolic demand (VO2 = 1464 mL·min−1, Q =13.1 L·min−1). Similarly, Ppw increased from rest to exercise by 125 to 150% in older subjects compared with 25 to 35% in younger adults.8 In agreement, Reeves et al24 demonstrated that Ppa and Ppw increased linearly with increasing Q in older (61 to 83 years) and younger (16 to 40 years) adults. The increase in Ppa and Ppw was, however, greater in the older compared with the younger subjects. Using a pulmonary hemodynamic model, the authors estimated the extensibility (α) of the pulmonary vasculature during exercise as the fractional diameter change of the pulmonary vessels relative to the change in pressure and demonstrated that the increase in pulmonary pressures during exercise were related to greater distension of the pulmonary vasculature during exercise in both old and young subjects. That is, increased pulmonary pressures during exercise dilate the lung circulation in old as well as younger adults. However, although older adults become more hypertensive compared with their younger counterparts at a given VO2 and Q, the estimated values of α were smaller in older adults compared with their younger counterparts (α =0.020 vs 0.015). This is likely a consequence of the increase in vascular stiffness secondary to remodeling of the pulmonary vasculature commonly observed in aging lungs, as discussed previously. In theory, the increased pulmonary pressure response to exercise and the diminished ability to distend the pulmonary vasculature in older subjects may serve to increase resistance to blood flow through the pulmonary circulation during exercise, thus influencing Q and potentially pulmonary gas exchange. However, the difference between Ppa and Ppw (i.e., the pressure gradient) is remarkably similar between older and younger subjects. Accordingly, pulmonary vascular resistance during exercise is typically not different in older compared with younger adults. In addition, recent evidence has shown that acute increases in both Ppa and Ppw do not widen the alveolar–arterial O2 difference during exercise in young, healthy adults.25 Moreover, although older adults become more hypertensive compared with their younger counterparts at a given VO2 and Q, younger adults are typically able to achieve much higher metabolic work rates and therefore reach Ppa and Ppw values similar to those achieved in the older adult at lower workloads. Thus it is unlikely that changes in the pulmonary vasculature with age act to significantly limit Q or alter pulmonary gas exchange during exercise in healthy older subjects.
Figure 3.
Pulmonary arterial pressure (Ppa) and pulmonary wedge pressure (Ppw) at rest and in response to exercise in young (<50 years of age, open squares) and old (>50 years of age, closed squares). Based on data obtained from Emirgil et al,6 Kovacs et al,8 and Reeves et al.24
Pulmonary Gas Exchange
Theoretically, the age-associated reductions in pulmonary capillary blood volume and pulmonary membrane diffusing capacity consistent with a reduction in alveolar–capillary surface area, along with the increase in the heterogeneity of alveolar ventilation and perfusion, could limit the adaptations available to maintain adequate pulmonary gas exchange homeostasis during exercise in older subjects. However, as will be demonstrated, despite these age-related changes in the pulmonary circulation at rest, gas exchange is impaired in only a minority of older adults during heavy exercise.
LUNG DIFFUSION
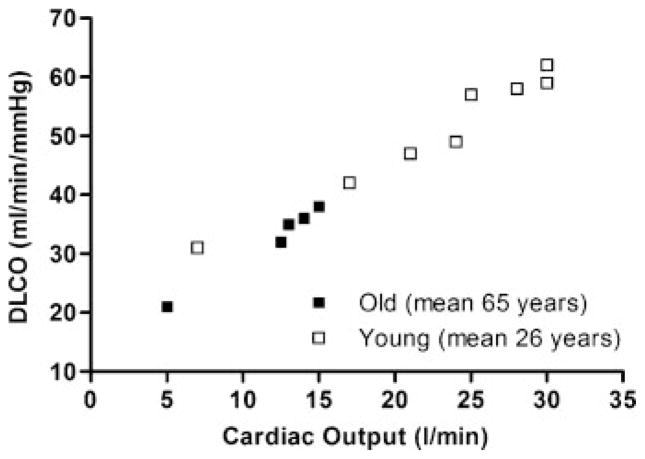
There is a paucity of data regarding the effect of aging on lung diffusion capacity during exercise. In a limited number of healthy subjects (n =12) with a broad age range (23 to 79 years old), Tamhane et al26 showed that the diffusion capacity of the lungs for nitric oxide (DLNO) increases linearly from rest to exercise with increasing cardiac output regardless of age. In our laboratory, we have measured DLCO in young, highly fit subjects and in a small number (n =9) of older adults at rest and in response to progressive exercise. The relationship of DLCO to cardiac output for both younger and older adults is shown in Fig. 4. As described earlier, the diffusion capacity of the older lung is lower at rest compared with its younger counterpart. In agreement with Tamhane et al,26 DLCO increases linearly with increasing exercise intensity in the older as well as the younger adults, even though the older adults are at a significantly reduced metabolic demand. These findings imply that despite stiffening of the pulmonary vasculature, reductions in pulmonary capillary blood volume and membrane diffusing capacity, and greater imbalance between alveolar ventilation and pulmonary perfusion with age, these changes are relatively mild and are not sufficient to affect the recruitment of effective alveolar–capillary surface area for gas exchange during exercise.
Figure 4.
Diffusion capacity of the lung for carbon monoxide (DLCO) obtained by rebreathing at rest and during exercise relative to cardiac output in young highly trained adults (open circles, n =8) and older, healthy adults of average fitness (closed squares, n =7). DLCO is reduced at rest in older adults but increases in a linear fashion with progressive exercise similar to that in young adults. Reprinted by permission of Oxford University Press.52
ALVEOLAR TO ARTERIAL GAS EXCHANGE
Despite the resting changes in the aging lung and pulmonary circulation, arterial blood-gas homeostasis is generally well maintained during exercise in older adults. Figure 5 demonstrates the average changes in arterial oxygen partial pressure (PaO2) with progressive exercise in 70-year-old subjects relative to average but fit and well-trained young adults. In older subjects, the alveolar to arterial oxygen difference (AaDO2) begins to widen at 40 to 50% of VO2max and continues to widen until it is approximately threefold greater than resting values at maximal exercise. This increase in AaDO2 with exercise in the older adult is partially dependent on a reduction in PaO2 and is strikingly similar to that observed in the young adult with average fitness. To date, however, there is only limited evidence that alveolar to arterial gas exchange is impaired in older subjects, as evidenced by the fact that exercise-induced arterial hypoxemia (EIAH) is rare in this population. For example, in a small group (n =19) of extremely fit (VO2max ~200% of predicted) older subjects studied in our laboratory, the mean PaO2 remained within 5 mm Hg of resting values during maximal exercise. Moreover, Préfaut et al27 showed that arterial blood gas concentrations did not change with progressive exercise in 10 aged (mean age 68 years) subjects of average fitness. However, there was a large degree of variability in the PaO2 response to exercise in the well-trained elderly subjects previously tested in our laboratory, with approximately one third demonstrating a ≥10 mm Hg drop in PaO2 from rest during exercise at intensities requiring a VO2 of 40 mL·kg·−1min−1. Similarly, all eight “master” athletes studied by Préfaut et al27 demonstrated EIAH during exercise of a similar intensity. Moreover, McClaran et al28 observed significant EIAH (arterial O2 saturation ≤ 92%), in six of 18 highly fit (VO2max ~200% of predicted) aged subjects during maximal exercise requiring a VO2 of 36 to 51 mL·kg·−1min−1. That significant EIAH occurs in some highly fit, elderly subjects suggests that an effect of healthy aging on pulmonary gas exchange that is not due to the prevalence of its occurrence, which is small, but was detected because hypoxemia occurred at a metabolic demand where hypoxemia of a similar magnitude would not be present in healthy young adults.29 EIAH in younger adults has been attributed primarily to an increase in VA/Qc mismatching and a diffusion limitation for oxygen, with a small contribution from physiological shunts. It can be postulated that the small reduction in pulmonary capillary blood volume with age could increase the transit time of blood through the pulmonary capillaries at a lower cardiac output in older compared with younger adults. This reduction in capillary blood volume, in conjunction with the increase in alveolar ventilation and perfusion heterogeneity observed in older adults, may contribute to the EIAH occasionally seen at lower metabolic demands in highly fit elderly subjects. However, it must be stressed that little or no EIAH is shown in elderly subjects of average fitness. Thus it can be concluded that the reserve available to the respiratory system for gas exchange is in excess of the age-associated disturbances in the pulmonary circulation. Accordingly, pulmonary gas exchange is not limited during exercise in the majority of older subjects. The role of intrapulmonary shunts contributing to EIAH remains speculative. If this mechanism contributed significantly to arterial hypoxemia, one would think that it would be more prominent in aging, given the higher pulmonary vascular pressures typically observed at a given cardiac output in older compared with younger individuals.
Figure 5.
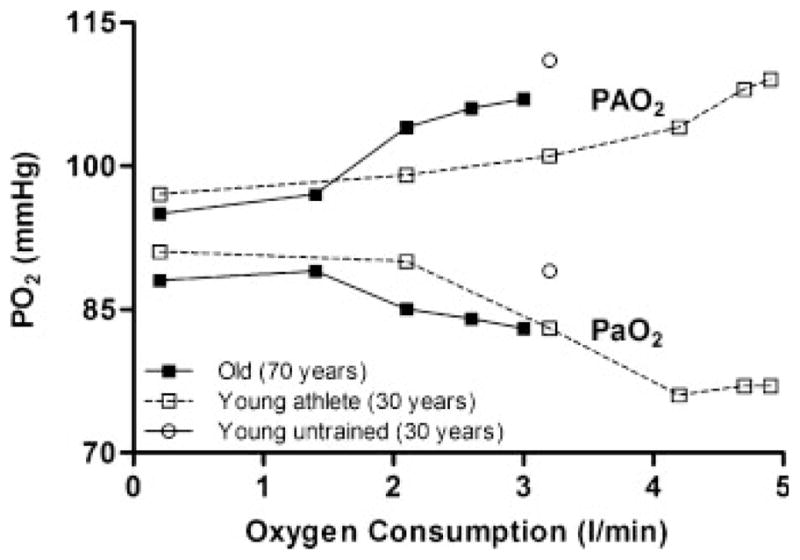
Alveolar (PAO2) and arterial (PaO2) oxygen tensions during progressive exercise in young, untrained adults (open diamonds, only peak exercise response shown); young, endurance-trained athletes (open circles); and older subjects of average fitness (closed circles). From Johnson.48
Scaling of Capacity to Demand
So far, we have suggested that exercise is generally not limited by inadequate pulmonary gas exchange in healthy older adults, likely because the age-associated decline in the maximal metabolic demand of exercise occurs at a rate equal to or greater than the changes in the pulmonary circulation. We now discuss whether maintenance of metabolic demand at exceedingly high levels (i.e., increased fitness through exercise training) or acceleration of the aging process (i.e., reduced capacities caused by disease) in combination with the normally occurring changes in the pulmonary circulation with aging alters the normal response to exercise.
CONDITIONING AND THE RESPONSE TO EXERCISE
After the second and, in some cases, the third decade of life, maximal aerobic capacity (VO2max) falls ~1% per year due to reductions in maximal heart rate, ejection fraction, and cardiac output plus a loss of skeletal muscle mass, with considerable evidence that up to 50% of the decline in VO2max is due to deconditioning rather than aging per se.30 In theory, maintenance of aerobic fitness level through conditioning may cause the demand for cardiac output to remain elevated thus causing pulmonary blood flow during exercise to be higher in highly fit older subjects compared with their younger counterparts. These changes, in the face of the age-related deterioration in the structure and function of the pulmonary circulation, may predispose highly fit aged individuals to gas exchange impairments. However, although some have shown that the diminution in VO2max is reduced by 30 to 50% with physical endurance training,31 others have reported that VO2max falls more sharply with age in extremely fit versus sedentary older subjects,28,32 with absolute VO2max similar between the two groups in the sixth decade of life.32 In addition, sustained exercise training has been shown to ameliorate the increase in left ventricular stiffness that occurs with aging11 and to preserve myocardial relaxation.33 Moreover, habitual exercise in previously sedentary individuals has been shown to reduce the stiffness of the large elastic arteries.34 While it is unclear whether conditioning has a similar effect on the pulmonary artery, any such reduction in stiffness in combination with the aforementioned increase in ventricular compliance would allow cardiac output and pulmonary blood flow to increase during exercise without large increases in pulmonary pressures and vascular resistance and would promote homogeneity of pulmonary perfusion, all of which would benefit pulmonary gas exchange. Thus, although in theory maintenance of metabolic demand despite a continued deterioration in the pulmonary circulation with aging may predispose highly fit elderly subjects to disturbances in pulmonary gas exchange, it appears that habitual physical activity has little or no effect on the normal reduction in the metabolic demand of exercise and minimizes the vascular and cardiac stiffening associated with aging. This notion is supported by the finding that EIAH is rarely observed in extremely fit older subjects.28
CARDIOVASCULAR IMPAIRMENT AND THE PULMONARY CIRCULATION DURING EXERCISE
Exercise intolerance is a feature of cardiac dysfunction, with the degree of intolerance providing an indication of disease severity in heart failure (HF) patients. Although HF stems from cardiac dysfunction, the factors that limit exercise tolerance remain speculative and are likely multi-factorial. Indeed, early studies did not demonstrate an association between resting measures of left ventricular function and exercise capacity.35 Venous hypertension is also a hallmark of HF along with variable increases in pulmonary vascular pressures and PVR during exercise.36,37 Moreover, HF patients may develop a reactive component to the PVR due to hypoxemia or sleep-disordered breathing, both of which are common in HF. In principle, an increase in PVR would impair blood flow through the pulmonary circulation, thereby limiting cardiac output and underperfusing the ventilated lung and, consequently, causing gas exchange to be impaired during exercise. Recent evidence has shown that the increase in PVR is associated with impairment in lung diffusing capacity, cardiac output, and VO2 during exercise in HF patients,36,37 suggesting that elevated pulmonary pressures and PVR may limit exercise tolerance through impairments in pulmonary blood flow and pulmonary gas exchange. However, Agostoni et al38 failed to demonstrate that lung diffusion impairment affected exercise capacity in HF patients. Hence, while it can be postulated that cardiovascular impairment may accelerate the aging process sufficiently to impair the function of the pulmonary circulation and limit pulmonary gas exchange and exercise tolerance in aged, diseased individuals, the exact role the pulmonary circulation plays in limiting exercise in HF remains unclear.
AIRFLOW LIMITATION AND OPERATIONAL LUNG VOLUMES DURING EXERCISE
The modest changes that occur in the pulmonary circulation with healthy aging are paralleled by changes in pulmonary mechanics that appear to alter the ventilatory and breathing pattern response to exercise in aged subjects. The primary determinant of the change in pulmonary mechanics is the reduction in static elastic recoil pressure of the airways and lung parenchyma (0.1 to 0.2 cm H2O·year−1 beyond 20 years) that occurs as part of normal aging. This loss in elastic recoil is associated with a commensurate reduction in the expiratory boundary of the maximal flow-volume loop (MFVL) at both low, and to a lesser extent, high lung volumes in older adults.28,39 The initial [>70 to 75% of total lung capacity (TLC)] as well as the lower (<70 to 75% of TLC) portion of the expiratory limb of the MFVL is dependent at least in part on the recoil characteristics of the airways. Accordingly, loss of elastic recoil in the senile airways is associated with a parallel reduction in maximum volitional expiratory flow rates at 25%, 50%, and 75% of TLC.28 Thus the expiratory boundary of the MFVL appears more “scooped” in shape in older adults when compared with their younger counterparts (Fig. 6B).28,39,40
Figure 6.
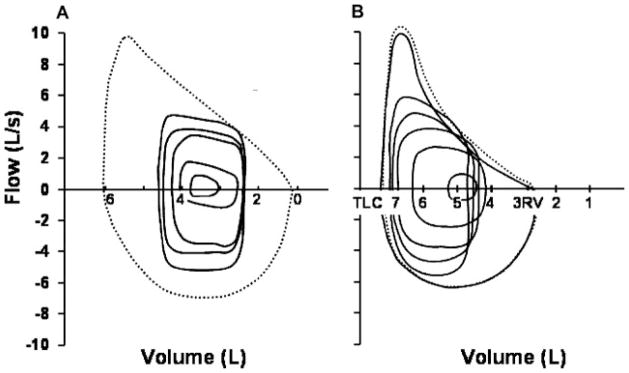
Ventilatory response to exercise in young, untrained adults (A) and older active adults (B) matched for exercise capacity. Tidal-breathing exercise flow-volume loops are plotted at increasing exercise intensity according to measured end-expiratory lung volume with the maximal volitional flow-volume loop. Additional mean gas exchange parameters are given for both groups. From Johnson.48
Regulation of Operational Lung Volumes
Figure 6 compares flow and volume responses to progressive exercise in younger and older adults. Shown are tidal breathing flow-volume loops at rest and during progressive exercise plotted within the MFVL in younger (Fig. 6A) and older (Fig. 6B) adults. At the onset of exercise in the young adult, tidal volume (VT) is increased through encroachment on both the inspiratory and the expiratory reserve volumes.41,42 The encroachment on the expiratory reserve volume is achieved by a reduction in end-expiratory lung volume (EELV) below the relaxation volume.41,42 This is thought to assist the diaphragm and other inspiratory muscles to produce force by improving their length–tension relationships, promoting lung volume expansion at the initiation of inspiration via passive recoil of the chest wall as well as allowing the tidal breath during exercise to stay on the linear portion of the pressure–volume relationship of the lung and chest wall, thus minimizing the elastic work and oxygen cost of breathing. The reduction in EELV typically progresses with exercise intensity and averages 0.5 to 1.0 L in normal young adults at peak exercise.43 However, during severe exercise (VE > 115 to 120 L·min−1) in highly fit young adults (VO2max > 65 to 70 mL·kg−1·min−1), expiratory flow rates occasionally increase to a level where the tidal flow-volume loop begins to encroach upon the MFVL, and significant expiratory flow limitation is then encountered (~50% of VT).41 In most subjects, EELV increases toward or even above resting values to move the entire VT away from the lower lung volume where flow limitation is occurring. In older subjects, EELV decreases in a similar fashion with the onset of exercise as in the younger adult.28,39 However, due to the age related reductions in maximal available expiratory flow rates, older subjects become significantly flow limited (25 to 30% of VT) during only moderate-intensity exercise (VE = 40 to 70 L·min−1, 40 to 50% of VO2max).28,39 Consequently, dynamic lung hyperinflation occurs at much lower exercise intensities in older compared with younger subjects. Thus, in the older adult, EILV encroaches to a greater extent on TLC than in the younger adult, and the potential benefits from an increase in inspiratory length likely become compromised.
Work and Cost of Breathing during Exercise
As noted earlier, the breathing pattern and strategy for low levels of exercise (VE < 40 L·min−1) is similar in older compared with younger subjects. Accordingly, the work and oxygen cost of breathing is also comparable. However, the increased severity of expiratory flow limitation experienced by older subjects as exercise progresses is associated with a rise in EELV and a substantial increase in the work of breathing. When expiratory flow becomes limited during exercise, there is a corresponding increase in expiratory pressures. In addition, EILV increases in line with the increase in EELV and may reach ≥85% of TLC during moderate-intensity exercise in older individuals.28,39 Such an increase in EILV impacts upon the inspiratory muscles by decreasing their length, limiting their capacity for maximum pressure generation, increasing the elastic load placed upon them, and requiring them to work at a higher percentage of their capacity for a given ventilation.40 These factors, in combination with the higher ventilatory response for a given work rate in older subjects, would greatly increase overall ventilatory work. Harms and colleagues44,45 have shown that the respiratory muscles may preferentially recruit blood flow at the expense of the limb locomotor muscles when the work of breathing is high. Accordingly, exercise limitation in older subjects may be more likely due to a redistribution of blood flow secondary to an enhanced work and oxygen cost of breathing rather than impairments in pulmonary gas exchange.
Could Airflow Limitation and Lung Hyperinflation Affect the Pulmonary Circulation?
Although somewhat speculative, it is possible that the increased expiratory pressures secondary to expiratory flow limitation in older adults may limit blood flow through the pulmonary circulation. Previous data from our laboratory have shown that an increase in abdominal and intrathoracic pressure in response to artificially induced expiratory flow limitation impairs the cardiac output response to dynamic steady-state exercise, presumably through a reduction in venous return from the locomotor limb muscles.46 In addition, lung inflation has been shown to reduce venous return via mechanical compression of the superior and inferior vena cava and cause a rise in PVR.47 In theory, a reduction in venous return in combination with an increase in PVR may conspire to limit pulmonary blood flow, thus compromising oxygen delivery to the exercising muscles. Lung hyperinflation, however, has also been associated with an improvement in cardiac output relative to expiratory flow limitation alone.46 Moreover, multivariate regression analysis in 12 exercising healthy subjects with a broad age range (23 to 79 years old) has demonstrated that lung diffusing capacity is positively related to increases in lung volume, suggesting that lung hyperinflation may in fact improve pulmonary gas exchange in the exercising human (see predicted equation for DLNO in Table 1).26 Based on the aforementioned considerations it is possible that improvements in cardiac function and pulmonary gas exchange with lung hyperinflation may offset any impairments associated with increased intrathoracic and abdominal pressures associated with expiratory air-flow limitation.
SUMMARY
In summary, several structural and functional changes occur in the pulmonary circulation with advancing age that, in theory, could impair pulmonary gas exchange during exercise in healthy, older adults. However, despite the erosion in ventilatory reserve with aging, alveolar ventilation is generally sufficient to maintain arterial oxygen and carbon dioxide levels within normal limits, even during heavy exercise. This is likely because age-related reductions in the maximal metabolic demand of exercise occur at a rate equal to or greater than the rate of deterioration in ventilatory reserve. Although exercise-induced arterial hypoxemia occurs in a small number of older subjects, the incidence does not appear to be enhanced in healthy, extremely fit adults but does occur at lower workloads than typically observed in the young athlete. Adverse changes in lung mechanics and the ventilatory response to exercise secondary to a loss of lung elastic recoil may have a more profound effect on exercise tolerance in older adults. Indeed, even during low-level exercise requiring ventilations of 40 to 70 L·min−1, older adults experience significant expiratory airflow limitation and large increases in the work of breathing, which could result in significant competition for blood flow between the locomotor muscles and the respiratory muscles.
Acknowledgments
Bryan J. Taylor is supported by a UK Fulbright Commission Distinguished Scholar Award. Work is funded by NIH Grant HL71478.
References
- 1.Harris P, Heath D, Apostolopoulos A. Extensibility of the human pulmonary trunk. Br Heart J. 1965;27:651–659. doi: 10.1136/hrt.27.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosoda Y, Kawano K, Yamasawa F, Ishii T, Shibata T, Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984;35:615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- 3.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax. 1978;33:335–344. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plank L, James J, Wagenvoort CA. Caliber and elastin content of the pulmonary trunk. Arch Pathol Lab Med. 1980;104:238–241. [PubMed] [Google Scholar]
- 5.Gozna ER, Marble AE, Shaw A, Holland JG. Age-related changes in the mechanics of the aorta and pulmonary artery of man. J Appl Physiol. 1974;36:407–411. doi: 10.1152/jappl.1974.36.4.407. [DOI] [PubMed] [Google Scholar]
- 6.Emirgil C, Sobol BJ, Campodonico S, Herbert WH, Mechkati R. Pulmonary circulation in the aged. J Appl Physiol. 1967;23:631–640. doi: 10.1152/jappl.1967.23.5.631. [DOI] [PubMed] [Google Scholar]
- 7.Ehrsam RE, Perruchoud A, Oberholzer M, Burkart F, Herzog H. Influence of age on pulmonary haemodynamics at rest and during supine exercise. Clin Sci (Lond) 1983;65:653–660. doi: 10.1042/cs0650653. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 9.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghali JK, Liao Y, Cooper RS, Cao G. Changes in pulmonary hemodynamics with aging in a predominantly hypertensive population. Am J Cardiol. 1992;70:367–370. doi: 10.1016/0002-9149(92)90621-5. [DOI] [PubMed] [Google Scholar]
- 11.Arbab-Zadeh A, Dijk E, Prasad A, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. doi: 10.1161/01.CIR.0000142863.71285.74. [DOI] [PubMed] [Google Scholar]
- 12.Guénard H, Marthan R. Pulmonary gas exchange in elderly subjects. Eur Respir J. 1996;9:2573–2577. doi: 10.1183/09031936.96.09122573. [DOI] [PubMed] [Google Scholar]
- 13.Stam H, Hrachovina V, Stijnen T, Versprille A. Diffusing capacity dependent on lung volume and age in normal subjects. J Appl Physiol. 1994;76:2356–2363. doi: 10.1152/jappl.1994.76.6.2356. [DOI] [PubMed] [Google Scholar]
- 14.Georges R, Saumon G, Loiseau A. The relationship of age to pulmonary membrane conductance and capillary blood volume. Am Rev Respir Dis. 1978;117:1069–1078. doi: 10.1164/arrd.1978.117.6.1069. [DOI] [PubMed] [Google Scholar]
- 15.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Murray J. Aging. In: Murray J, editor. The Normal Lung. Philadelphia, PA: WB Saunders; 1986. pp. 339–360. [Google Scholar]
- 17.Chang SC, Chang HI, Liu SY, Shiao GM, Perng RP. Effects of body position and age on membrane diffusing capacity and pulmonary capillary blood volume. Chest. 1992;102:139–142. doi: 10.1378/chest.102.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Butler CII, Kleinerman J. Capillary density: alveolar diameter, a morphometric approach to ventilation and perfusion. Am Rev Respir Dis. 1970;102:886–894. doi: 10.1164/arrd.1970.102.6.886. [DOI] [PubMed] [Google Scholar]
- 19.Gillooly M, Lamb D. Airspace size in lungs of lifelong non-smokers: effect of age and sex. Thorax. 1993;48:39–43. doi: 10.1136/thx.48.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin DL, Buxton RB, Spiess JP, Arai T, Balouch J, Hopkins SR. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging. J Appl Physiol. 2007;102:2064–2070. doi: 10.1152/japplphysiol.00512.2006. [DOI] [PubMed] [Google Scholar]
- 21.Cardús J, Burgos F, Diaz O, et al. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156(2 Pt 1):648–653. doi: 10.1164/ajrccm.156.2.9606016. [DOI] [PubMed] [Google Scholar]
- 22.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung: comparison with normal and emphysematous lungs, II: Functional aspects. Chest. 1992;101:800–809. doi: 10.1378/chest.101.3.800. [DOI] [PubMed] [Google Scholar]
- 23.Crapo RO, Morris AH, Gardner RM. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopathol Respir. 1982;18:893–899. [PubMed] [Google Scholar]
- 24.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–L425. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 25.Stickland MK, Welsh RC, Haykowsky MJ, et al. Effect of acute increases in pulmonary vascular pressures on exercise pulmonary gas exchange. J Appl Physiol. 2006;100:1910–1917. doi: 10.1152/japplphysiol.01484.2005. [DOI] [PubMed] [Google Scholar]
- 26.Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 27.Préfaut C, Anselme F, Caillaud C, Massé-Biron J. Exercise-induced hypoxemia in older athletes. J Appl Physiol. 1994;76:120–126. doi: 10.1152/jappl.1994.76.1.120. [DOI] [PubMed] [Google Scholar]
- 28.McClaran SR, Babcock MA, Pegelow DF, Reddan WG, Dempsey JA. Longitudinal effects of aging on lung function at rest and exercise in healthy active fit elderly adults. J Appl Physiol. 1995;78:1957–1968. doi: 10.1152/jappl.1995.78.5.1957. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol. 1984;355:161–175. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldspink DF. Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics. 2005;48:1334–1351. doi: 10.1080/00140130500101247. [DOI] [PubMed] [Google Scholar]
- 31.Hollmann W, Strüder HK, Tagarakis CV, King G. Physical activity and the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14:730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- 32.Pimentel AE, Gentile CL, Tanaka H, Seals DR, Gates PE. Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men. J Appl Physiol. 2003;94:2406–2413. doi: 10.1152/japplphysiol.00774.2002. [DOI] [PubMed] [Google Scholar]
- 33.Kivistö S, Perhonen M, Holmström M, Lauerma K. Assessment of the effect of endurance training on left ventricular relaxation with magnetic resonance imaging. Scand J Med Sci Sports. 2006;16:321–328. doi: 10.1111/j.1600-0838.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 34.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587(Pt 23):5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franciosa JA, Ziesche S, Wilen M. Functional capacity of patients with chronic left ventricular failure. Relationship of bicycle exercise performance to clinical and hemodynamic characterization. Am J Med. 1979;67:460–466. doi: 10.1016/0002-9343(79)90794-0. [DOI] [PubMed] [Google Scholar]
- 36.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 37.Smith AA, Cowburn PJ, Parker ME, et al. Impaired pulmonary diffusion during exercise in patients with chronic heart failure. Circulation. 1999;100:1406–1410. doi: 10.1161/01.cir.100.13.1406. [DOI] [PubMed] [Google Scholar]
- 38.Agostoni PG, Bussotti M, Palermo P, Guazzi M. Does lung diffusion impairment affect exercise capacity in patients with heart failure? Heart. 2002;88:453–459. doi: 10.1136/heart.88.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson BD, Reddan WG, Pegelow DF, Seow KC, Dempsey JA. Flow limitation and regulation of functional residual capacity during exercise in a physically active aging population. Am Rev Respir Dis. 1991;143(5 Pt 1):960–967. doi: 10.1164/ajrccm/143.5_Pt_1.960. [DOI] [PubMed] [Google Scholar]
- 40.Johnson BD, Reddan WG, Seow KC, Dempsey JA. Mechanical constraints on exercise hyperpnea in a fit aging population. Am Rev Respir Dis. 1991;143(5 Pt 1):968–977. doi: 10.1164/ajrccm/143.5_Pt_1.968. [DOI] [PubMed] [Google Scholar]
- 41.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73:874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 42.Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1988;64:135–146. doi: 10.1152/jappl.1988.64.1.135. [DOI] [PubMed] [Google Scholar]
- 43.Johnson BD, Seow KC, Pegelow DF, Dempsey JA. Adaptation of the inert gas FRC technique for use in heavy exercise. J Appl Physiol. 1990;68:802–809. doi: 10.1152/jappl.1990.68.2.802. [DOI] [PubMed] [Google Scholar]
- 44.Harms CA, Wetter TJ, McClaran SR, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 45.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 46.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96:1920–1927. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 47.Wallis TW, Robotham JL, Compean R, Kindred MK. Mechanical heart-lung interaction with positive end-expiratory pressure. J Appl Physiol. 1983;54:1039–1047. doi: 10.1152/jappl.1983.54.4.1039. [DOI] [PubMed] [Google Scholar]
- 48.Johnson BD. Respiratory system responses to exercise in aging. In: Weisman I, Zeballos J, editors. Clinical Exercise Testing. Basel: Karger; 2002. pp. 89–98. [Google Scholar]
- 49.Ekelund LG. Circulatory and respiratory adaptation during prolonged exercise of moderate intensity in the sitting position. Acta Physiol Scand. 1967;69:327–340. doi: 10.1111/j.1748-1716.1967.tb03529.x. [DOI] [PubMed] [Google Scholar]
- 50.Ekelund LG. Circulatory and respiratory adaptation during prolonged exercise. Acta Physiol Scand Suppl. 1967;292:1–38. [PubMed] [Google Scholar]
- 51.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state: predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 52.Johnson BD. Age-associated changes in pulmonary reserve. In: Evan J, Williams T, Beattie B, Wilcock G, editors. Oxford Textbook of Geriatric Medicine. 2. New York, NY: Oxford Univesity Press; 2000. pp. 484–497. [Google Scholar]