Abstract
OBJECTIVE
Synthetic glucocorticoid administration to women threatening preterm delivery increases neonatal survival. However, mounting evidence shows that fetal exposure to glucocorticoid levels higher than appropriate for current maturation adversely programs offspring development. We examined fetal synthetic glucocorticoid multigenerational metabolic effects on F1 and F2 female offspring.
STUDY DESIGN
At 0.7 gestation, pregnant F0 ewes received 4 injections of dexamethasone (2 mg, approximately 60 ug.kg−1 day−1 12 hours apart) or saline (control). F1 female offspring were bred to produce F2 female offspring. Postpubertal pancreatic β-cell function was tested in F1 and F2 by intravenous glucose tolerance test.
RESULTS
F1 and F2 ewe lambs showed reduced birthweight and morphometrics, and similar increased fasting glucose and decreased intravenous glucose tolerance test β-cell response.
CONCLUSION
This is the first demonstration of multigenerational programming of later life β-cell response by clinically relevant doses of synthetic glucocorticoid indicating the need for study of long-term effects of fetal exposure to synthetic glucocorticoid.
Keywords: antenatal synthetic glucocorticoids, growth restriction, insulin and glucose metabolism, mutigenerational developmental programming, sheep
The ground-breaking demonstration by Liggins of glucocorticoid (GC) induced acceleration of fetal lung development in sheep led to a clinical trial showing decreased morbidity and mortality in premature infants whose mothers received synthetic GC.1,2 It is now routine practice to treat women threatening preterm delivery before 34 weeks’ gestation with GC.3 Although treatment with synthetic GC confers great benefit by lowering neonatal mortality and morbidity, evidence is mounting that in addition to the major benefits of accelerated lung maturation, fetal exposure to GC levels higher than those appropriate for the current stage of fetal maturation produces intrauterine growth restriction (IUGR) in several species, sheep,4,5 nonhuman primates,6 and humans7–9 altering the developmental trajectory of many fetal organ systems with potential for adverse developmental programming effects in later life. These similarities across species suggest common underlying mechanisms producing adverse outcomes. Animal studies demonstrate that delayed offspring endocrine, renal, and metabolic effects emerge later in life with the potential to predispose to chronic disease.10–14 The nature and extent of these long-term effects needs to be understood.
Fetal programming can be defined as—a response to a specific challenge during a critical fetal time window that alters development with persistent effects on offspring phenotype. Increases in fetal and maternal GC have been demonstrated to occur in response to many challenges that result in fetal programming.15,16 In addition, offspring outcomes resulting from challenges such as poor maternal nutrition can be prevented by inhibiting GC changes occurring in response to the challenge.17 As a result, many investigators consider exposure to excessive endogenous GC levels at critical fetal developmental periods to be a common causative factor of many, though not necessarily all, fetal programming responses to challenges during development, eg, maternal stress and poor nutrition. One leader in the field writes, “similarities in phenotype associated with dietary and glucocorticoid exposures have led to suggestions that they may act through a shared mechanism …”18
The only multigenerational study on effects of fetal exposure to GC was conducted in rodents—an altricial species with a very different profile of fetal development to precocial species such as humans and sheep. The paradigm was very different from the human clinical treatment regimen and involved exposure continuously for 6 days—approximating 30% of the whole of gestation. In addition, that study involved male offpring.13 To enable translation of any fetal programming effects of synthetic GC on insulin and glucose metabolism after human fetal exposure, it is necessary to conduct studies in precocial species with dosing protocols that produce exposures that equal the duration, dose, and critical developmental window of exposure experienced during human synthetic GC therapy in preterm labor.
We hypothesized that exposure of female fetal lambs (F1 generation) to synthetic GC administered to their mothers (F0 generation) at 27 weeks’ human gestation equivalent would have effects in F1 and F2 female offspring on metabolic phenotype as reflected by birthweight, morphometric measurements, postnatal growth patterns, and glucose and insulin dynamics. To test this hypothesis, we administered dexamethasone (DEX) to pregnant ewes at 103 and 104 days’ gestation (term 150 days) and determined F1 and F2 female offspring outcomes.
Materials and Methods
Care and use of animals
All procedures were approved by the University of Wyoming Animal Care and Use Committee. Twenty-two F0 nulliparous Rambouillet X Columbia crossbred ewes (approximately 16 months of age) were used to produce the F1 and F2 ewe lambs studied. F0 ewes were bred to a single ram in 1 of 2 consecutive years. After natural mating, ewes were fed in accordance with National Research Council (NRC) maintenance19 requirements. On days 103 and 104 of gestation, ewes averaged 73.6 ± 4.0 kg (mean ± SEM) body weight and were randomly assigned to 1 of 2 treatment groups: DEX ewes (n = 10) received 4 injections of 2 mg of DEX (intramuscular [im]; Vedco, St. Joseph, MO) 12 hours apart, a dose equal to approximately 60 µg.kg−1:day−1. Control ewes (n = 12) received equivalent volumes of saline im. Ewes were allowed to lamb naturally. At lambing, F1 newborn body weight and morphometrics were recorded on all female lambs. There were 8 control twins (2 sets were both females) and 4 control singles. In the DEX lambs, there were 7 twins (3 female twin sets) and 3 singles. After lambing, all F0 ewes were given free choice access to alfalfa hay. Before 2 weeks of age, F1 female lambs were tail-docked as per Federation of Animal Science Societies’ recommendations.20 Two control and 3 DEX female F1 lambs all from above mentioned twin births died of natural causes before 3 months of age and their data was excluded from this study. The study was conducted on n = 10 DEX and 12 control F1 offspring. F1 ewe lambs were given free access to a standard commercially available creep feed (Lamb Creep B30 w/Bovatec; Ranch-way Feeds, Ft. Collins, CO) from birth to weaning at 4 months of age and placed in an outdoor housing facility with shelter and ad libitum water. These F1 ewe lambs after weaning were maintained in accordance with NRC requirements for replacement ewes.19 Diet consisted of alfalfa and corn to meet NRC requirements with ad libitum access to a trace mineral salt block. Diets were adjusted up for weight gain every month. Lamb body weights and ponderal index (lamb body weight, kg/[lamb height/m3]) were recorded at postnatal day 7, 14,28, and then monthly until 12 months of age.
F1 ewe lambs received maintenance requirements until either 16 or 28 months of age depending on the year in which they were born. F1 ewes weighed 65.1 ± 3.5 (n = 12) and 64.7 ± 3.7 kg (n = 10) control and DEX, respectively, when naturally mated to a single ram with mating date noted using a marking ram harness. Numbers of lambs born per F1 ewe were recorded as well as their birthweight and morphometrics. All F2 ewe lambs (n = 9, DEX and 8, control) were fed in a similar fashion as their F1 mothers and postnatal body weights were recorded at the same times. There were 3 control F2 twin sets (all male-female sets) and 5 control F2 singles. In the DEX F2 group, there were 4 twin sets (1 female twin set and 3 male-female twin sets) and 4 singles.
Investigators were blinded to treatment groups. For the study of F1 females, a random subsample (chosen by random number draw) of 6 per treatment group of female F1 offspring at 2.5 or 3.5 year of age (3 per age and birth type [twin or single] in each treatment group). For the F2 study, 6 females whose grandmothers received DEX and 6 whose grandmothers received saline randomly selected at 6 months of age (3 per birth type in each treatment group) were chosen for study. Acatheter (Abbocath, 16 ga; Abbott Laboratories, North Chicago, IL) was placed aseptically into the jugular vein 24 hours before the start of the intravenous glucose tolerance test (IVGTT) blood sampling. Catheters were sutured to the skin to secure them and an extension set (Seneca Medical, Tiffin, OH) attached for undisturbed infusion and sampling. The neck and shoulder area were covered with netting (Surgilast Tubular elastic dressing retainer, Derma Science Inc, Princeton, NJ) to prevent catheter damage. F1 ewes and F2 lambs were maintained in neighboring individual pens with free access to water. No feed was provided for ~18 hours before and during the IVGTT, which has been described in detail.21 Jugular blood samples (~6 mL) were obtained into chilled tubes (heparin plus NaF; 2.5 mg/mL; Sigma-Aldrich, St. Louis, MO) at −15, and 0 minutes relative to a 0.25 g/kg intravenous bolus infusion of 50% dextrose solution (Vedco, St, Joseph, MO) over 5 seconds. Blood samples were collected at 2, 5, 10, 15, 20, 30, 45, 60, 90, and 120 minutes after dextrose infusion. All blood samples were immediately placed on ice, centrifuged at 2500 × g and plasma was collected and stored at − 80°C. F1 and F2 offspring were given free access to pelleted alfalfa after the IVGTT and throughout the rest of the challenges.
Biochemical and hormone assays
Glucose was measured colorimetrically in triplicate (Liquid Glucose Hexokinase Reagent; Pointe Scientific, Inc, Canton, MI) as previously described.21 Mean intraassay coefficient of variation (CV) was 1.2% and interassay CV was 2.8%. Insulin was measured in duplicate by commercial radioimmunoassay (RIA) (Coat-A-Count Insulin RIA; Siemens Medical Solutions Diagnostics, Los Angeles, CA) with intra- and interassay CV of 8.9% and 13.2%, respectively, and a sensitivity of 2.6 µIU/mL.21
Statistical analysis
All data were normally distributed and were not transformed. Data are presented as least square means ± SEM, and differences considered significant at P ≤ .05, with a tendency set at P ≤ .10. Graphpad Prism (GraphPad Software Inc, La Jolla, CA) was used to calculate the area under the curve (AUC) for plasma glucose and insulin response curves during the IVGTT. Baseline concentrations of glucose and insulin in all samples before infusion were averaged to give baseline concentrations. An insulin to glucose ratio was calculated from the baseline values and using the AUC from the IVGTT. Plasma glucose and insulin during the IVGTT were analyzed as repeated measures using MIXED procedure of SAS (SAS Institute Inc, Cary, NC) with treatment and time, and their interaction in the model. AUC and fasting concentrations of glucose and insulin, birthweights and morphometrics, gestation lengths, number of lambs per ewe, and the baseline and AUC insulin to glucose ratios were analyzed using the GLM procedure of SAS with treatment in the model. Body weights of both F1 and F2 offspring both before 2 months of age and from 3 to 12 months of age were analyzed as repeated measures using MIXED procedure of SAS with treatment, date, and their interaction in the model. F1 ewe age (1 or 2 years) was initially included in all F1 ewe models but was found to be nonsignificant (P < .46) and was therefore removed from the models. Birth type (twin vs single) was initially included in all models but was found to be nonsignificant (P < .16) and was therefore removed. Data are provided as mean ± SEM throughout.
Results
Pregnancy and growth
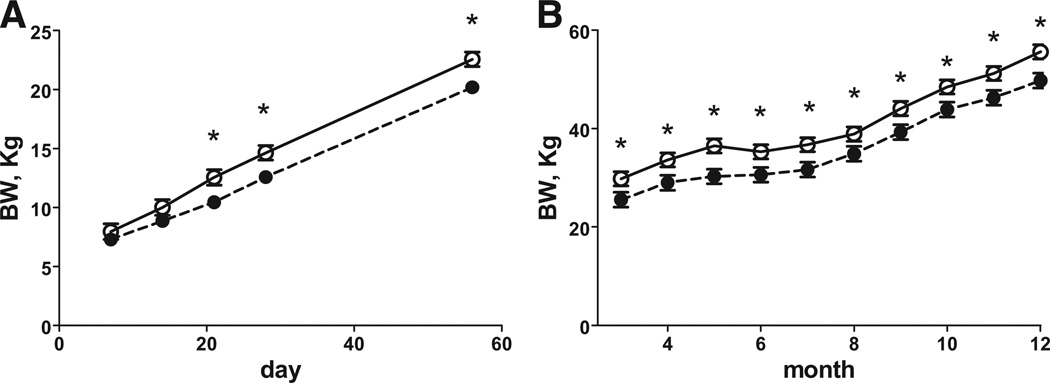
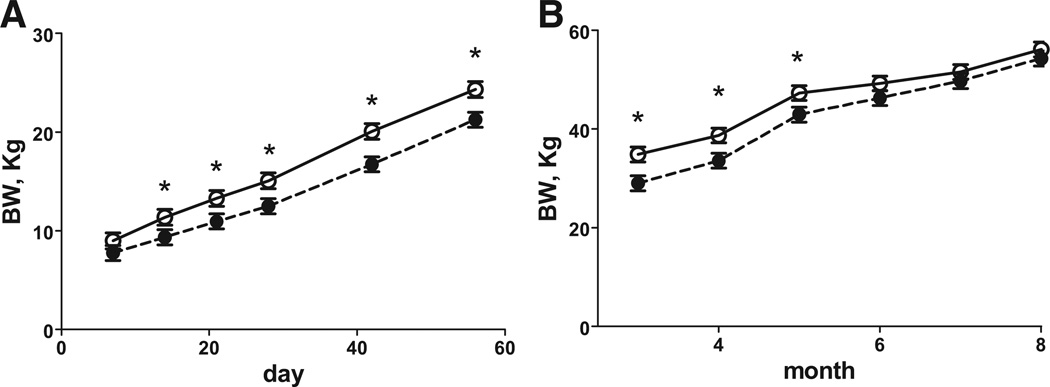
Gestation length was 3 days shorter in the DEXF0 ewes compared with the control F0 ewes (P < .05; 152.3 ± 0.5 vs 155.4 ± 0.5, respectively). The duration of pregnancy in DEX F1 offspring tended to be shorter than their corresponding controls by almost 2 days, though this difference did not quite reach significance (147.6 ± 0.7 vs 149.3 ± 0.06, respectively, [P < .06]). There was no difference in the number of lambs delivered by each F0 ewe (1.67 ± 0.14 vs 1.70 ± 0.15) or F1 ewe (1.16 ± 0.13 vs 1.40 ± 0.14, control and DEX, respectively). Birthweights and morphometrics for F1 and F2 offspring are shown in the Table. Female F1 offspring of DEX treated ewes had reduced birthweights, crown rump lengths, and thoracic circumference compared with control F1 female newborn lambs. Interestingly, DEX F2 newborn lambs also had reduced birthweight, crown rump length, thoracic, and, additionally, abdominal circumference was reduced compared with F2 control newborn lambs (Table). Ponderal index in both DEX F1 and F2 offspring compared with their corresponding control offspring (Table). From day 7 to 56 of age (Figure 1, A) there was a treatment * day interaction, with body weight being less in F1 DEX than F1 control lambs at days 21 to 56. From 3 to 12 months of age (Figure 1, B) DEX F1 lambs weighed less than control F1lambs. Growth patterns of theF2female lambs are shown in Figure 2. From days 7 to 56 of age (Figure 2, A), DEX F2 lambs weighed less than control F2 lambs. There was a treatment * month interaction with DEX F2 female lambs having reduced body weights from 3 to 5 months of age (Figure 2, B), then returning to a similar body weight from 6 to 8 months of age. Body weights of DEX F1 ewes at catheterization for the IVGTT were less (P < .05) than control F1 ewes (F1 65.1 ± 2.5 vs 72.2 ± 2.5 kg, respectively). Body weights of DEX F2 lambs at catheterization were less (P < .05) than control F2 lambs (44.8 ± 2.4 vs 51.2 ± 2.4 kg, respectively).
TABLE.
Birthweights and morphometrics for F1 and F2 offspring
| Variable V F1 offspring |
Control | DEX | P value |
|---|---|---|---|
| n | 12 | 10 | |
| Birthweight, kg | 5.59 ±0.17 | 5.03 ±0.18 | .04 |
| Crown rump length, cm | 57.6 ±1.0 | 53.9 ±1.0 | .02 |
| Ponderal index, kg/m3 | 29.25 ±1.00 | 32.12 ±1.06 | .04 |
| Left humerus length, cm | 15.1 ±0.4 | 14.0 ±0.5 | .09 |
| Right humerus length, cm | 15.0 ±0.5 | 13.7 ±0.5 | .06 |
| Biparietal distance, cm | 18.8 ±0.3 | 18.1 ±0.3 | .09 |
| Thoracic circumference, cm | 42.3 ± 0.4 | 40.7 ± 0.4 | .02 |
| Abdominal circumference, cm | 40.1 ± 0.7 | 39.9 ± 0.7 | .80 |
| F2 offspring | Saline | DEX | P value |
| n | 8 | 9 | |
| Birthweight, kg | 6.62 ± 0.29 | 5.72 ± 0.26 | .03 |
| Crown rump length, cm | 56.0 ±1.3 | 50.2 ±1.2 | .01 |
| Ponderal index, kg/m3 | 37.70 ±1.16 | 45.22 ±1.33 | .03 |
| Left humerus length, cm | 14.2 ±0.5 | 14.0 ± 0.4 | .68 |
| Right humerus length, cm | 14.4 ±0.5 | 14.2 ± 0.4 | .69 |
| Biparietal distance, cm | 15.4 ±0.5 | 15.0 ±0.5 | .52 |
| Thoracic circumference, cm | 44.3 ± 0.8 | 41.6 ±0.7 | .02 |
| Abdominal circumference, cm | 43.9 ± 0.8 | 40.1 ± 0.8 | .01 |
Birthweight and morphometrics of F1 female and F2 female offspring of F0 mothers who received 4 injections of 2 mg of dexamethasone 12 hr apart starting at d 103 and 104 of gestation (DEX) and F1 and F2 offspring whose mothers received a similar timed injection of saline (control). Values are means ± SEM.
FIGURE 1. Body weight of female F1 offspring.
Body weight of female F1 offspring of mothers who received 4 injections of 2 mg of DEX12 hours apart on d 103 and 104 of gestation (closed symbol, n = 10) and F1 offspring whose mothers received similar timed injections of saline (open symbol, n = 12). A, Postnatal day 7 to 56 and B, postnatal age 3 to 12 months. Values are means ± SEM. A, Trt P < .01, day P < .0001, Trt* day P < .001, and B, Trt P = .01, month P < .0001, Trt* month P = .75, day 7 to 56 and 3 to 12 months, respectively.
*Means differ P < .05.
FIGURE 2. Body weight of female F2 offspring.
Body weight of female F2 offspring whose grandmothers (F0) received 4 injections of 2 mg of DEX 12 hours apart on d 103 and 104 of gestation (closed symbol, n = 9) and F2 offspring whose grandmothers (F0) received similar timed control injections of saline (open symbol, n = 9). A, Postnatal day 7 to 56 postnatal and B, postnatal age 3 to 8 months. Values are means ± SEM. A, Trt P = .03, day P < .0001, Trt* day P = .16, and B, Trt P = .46, month P < .0001, Trt* month P = .04, day 7 to 56 and 3 to 8 months, respectively.
*Means differ P < .05.
IVGTT
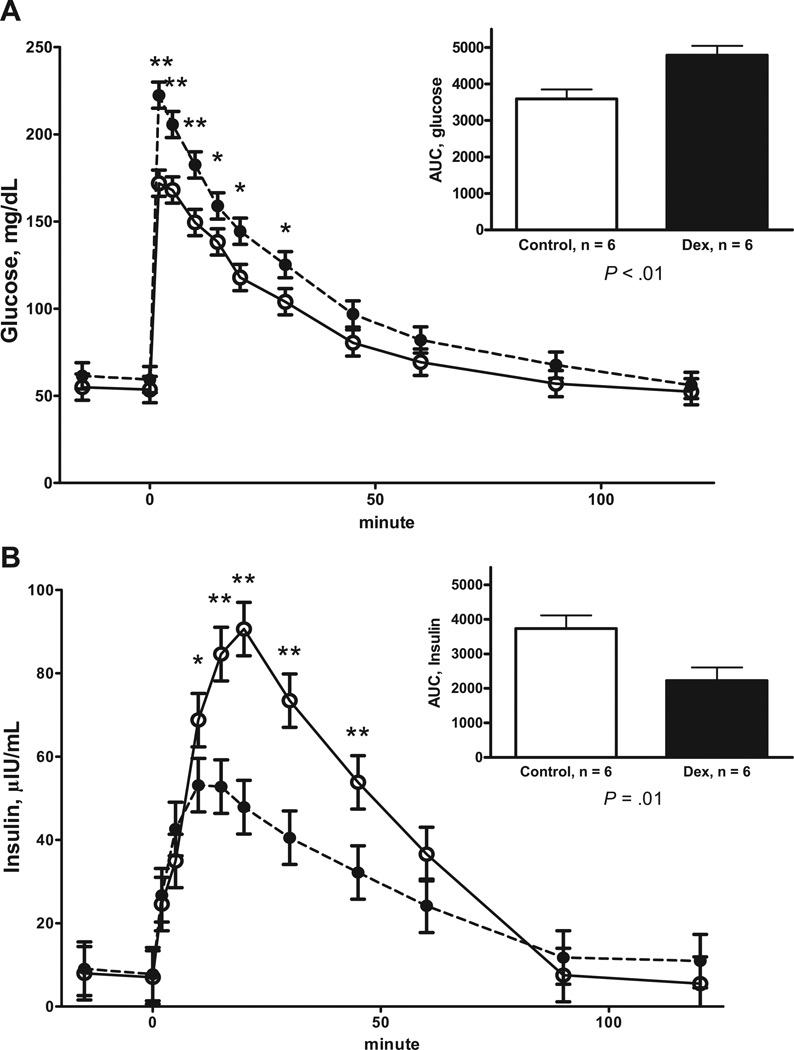
The plasma glucose and insulin concentrations before and during the IVGTT for F1 female offspring at 2.5 to 3.5 years of age are shown in Figure 3. Plasma glucose was elevated in DEX F1 offspring at baseline compared with control F1 offspring (62.0 ± 2.5 vs 54.0 ± 2.5 mg/dL, respectively; P = .03). However, there was no difference in fasting baseline plasma insulin between DEX and control F1 offspring (6.59 ± 1.16 vs 7.66 ± 1.16 µIU/mL, respectively). There was a tendency for the fasting baseline insulin to glucose ratio to be reduced in DEX F1 offspring compared with control F1 offspring (0.10 ± 0.02 vs 0.14 ± 0.02, respectively; P = .07). Plasma glucose concentrations were higher (Figure 3, A) and AUC for glucose higher in DEX F1 offspring compared with control F1 offspring during the IVGTT. There was also a treatment * time interaction (P < .01, Figure 3, B) for plasma insulin concentration during the IVGTT, with DEX F1 female offspring having reduced plasma insulin concentration at 10 to 45 minutes postglucose administration. As a result, DEX F1 offspring showed a reduced AUC for plasma insulin compared with control F1 offspring. The insulin to glucose ratio for the AUC during the IVGTT were reduced (P < .01) in DEX F1 offspring compared with control F1 offspring (0.48 ± 0.12 vs 1.07 ± 0.12, respectively).
FIGURE 3. Responses to an IVGTT.
A, Plasma glucose and B, plasma insulin responses to an IVGTT in 2.5 or 3.5-year-old F1 female offspring of mothers who received 4 injections of 2 mg of DEX 12 hours apart on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and F1 control offspring whose mothers received similar timed injections of saline (open symbol, n = 6). Area under the curve (AUC) is depicted in the inset Values are means ± SEM. A, Trt P < .0001, time P < .0001, Trt* time P = .09; and B, Trt P = .04, time P < .0001, Trt* time P < .01
*Means differ P < .05; **Means differ P < .01
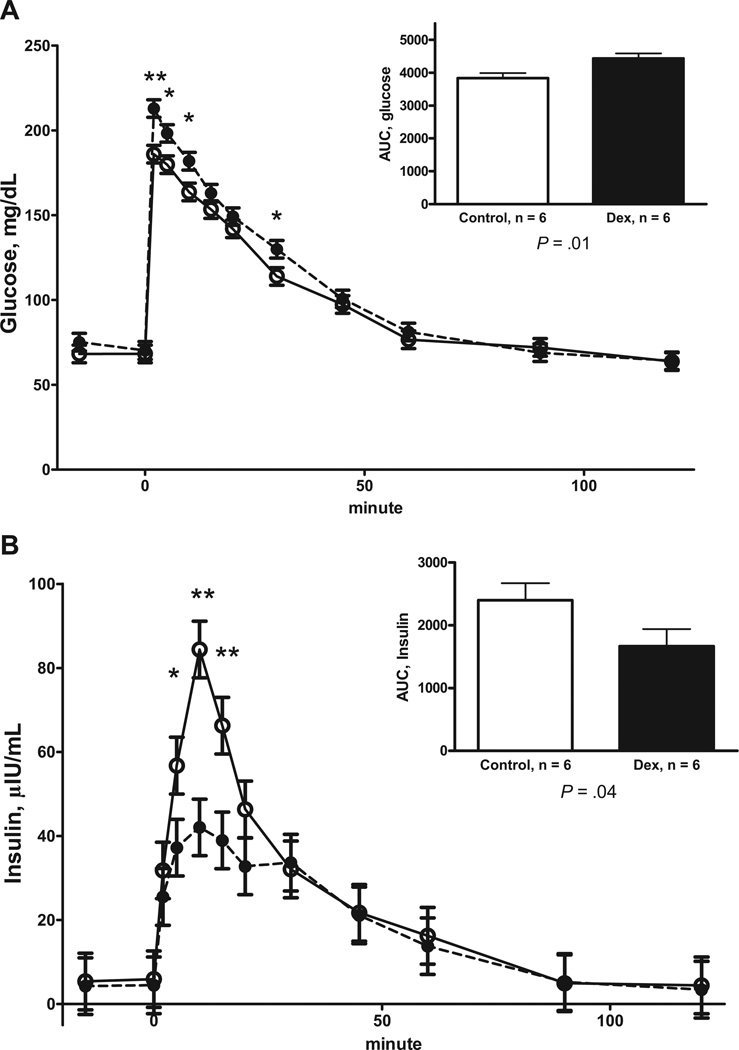
Similar to DEX F1 ewes, DEX F2 offspring had elevated fasting plasma glucose concentrations during the baseline sampling compared with control F2 offspring (74.9 ± 2.4 vs 68.3 ± 2.4 mg/dL, respectively; P = .04) when tested at 6 months of age. There was a tendency for fasting baseline plasma insulin to be reduced in DEX F2 offspring compared with control F2 offspring (4.35 ± 0.6vs5.75 ± 0.6 µIU/mL, respectively; P = .06). The insulin to glucose ratio for the fasted baseline samples were lower in the DEX F2 offspring compared with control F2 offspring (0.060 ± 0.09 vs 0.082 ± 0.009, respectively; P < .05). The plasma glucose and insulin concentrations for the F2 female offspring are shown in Figure 4. Plasma glucose concentrations were higher (Figure 4, A) in DEX F2 offspring at 6 months of age compared with control F2 offspring during the IVGTT. DEX F2 offspring showed a greater AUC for plasma glucose than control F2 offspring. There was a treatment * time interaction (Figure 4, B) for plasma insulin concentration during the IVGTT, with DEX F2 female offspring having reduced plasma insulin concentration at 5 to 15 minutes postdextrose infusion compared with control F2 offspring. This resulted in DEX F2 offspring having a reduced AUC for plasma insulin concentration compared with control F2 offspring. The insulin to glucose ratio for the AUC during the IVGTT was reduced in DEX F2 offspring compared with control F2 offspring (0.37 ± 0.06 vs 0.63 ± 0.06, respectively; P < .01).
FIGURE 4. Responses to an IVGTT.
A, Plasma glucose and B, plasma insulin responses to an IVGTT in 6-month-old F2 female offspring whose grandmothers (F0) received 4 injections of 2 mg of DEX12 hours apart on d 103 and 104 of gestation (DEX; closed symbol, n = 6) and control F2 offspring whose grandmothers (F0) received similar timed injections of saline (open symbol, n = 6). Area under the curve (AUC) is depicted in the inset. Values are means ± SEM. A, Trt P = .02, time P < .0001, Trt* time P = .06; and B, Trt P = 11, time P < .0001, Trt* time P = .02.
*Means differ P < .05; **Means differ P < .01
Comment
The incidence of preterm delivery has risen to over10% of pregnancies, accounting for 75% of all neonatal deaths.3,22 In most of these pregnancies, synthetic GC are administered to mothers to accelerate fetal lung maturation in accordance with National Institute of Health (NIH), exposing the fetus to GC levels in excess of those appropriate for the current stage of fetal maturation.3 One study by the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network in which the majority of patients received 4 or more courses, delivery at less than 32 weeks occurred in only 24% of synthetic GC treated pregnancies and at less than 37 weeks in 63%.22 Therefore, 76% of all synthetic GC exposed fetuses remained in utero for a significant period and over a third can be considered as delivering at term. Although treatment benefits in decreasing morbidity and mortality in premature infants is unquestioned, it is important to consider potential negative consequences in treated pregnancies where pregnancy continues to term or near term. These fetuses will have experienced a very different GC exposure than unexposed fetuses during normal development.
To our knowledge, this is the first report showing that maternal synthetic GC treatment results in fetal and postnatal growth restriction in both the F1 and F2 generations in a precocial species. A recent human literature review shows that exposure to synthetic GC contributes to birthweight reduction.23 Our model shows that both longitudinal fetal growth and overall weight are negatively affected by maternal DEX administration in both the F1 and F2 generation. Repeat late gestational synthetic GC administration to pregnant ewes results in decreased offspring body weight at 3 months and a tendency for reduced body weight at 6 months of age.24 There was, however, no difference in body weight at 1 year of age or older in F1 offspring from other reported studies but these studies did not use treatment regimens that resemble human clinical dosing in exposure timing, administration route, or dose.24–26
Our F1 female DEX offspring were lighter as adults (2.5–3.5 years of age) indicating permanent stunting. F2 female offspring of F0 DEXs mothers showed catch up growth by 7 and 8 months of age. Rapid catch up growth predisposes offspring to a variety of chronic diseases.27 The differences in birthweight and gestation length between the F1 and F2 generation could be due to differences in the Ram used (sire effects) and also a change in the developmental programming effects over the generation. Although late gestation maturation of other fetal organs in addition to the fetal lung is dependent on the level of maturation of the fetal pituitary adrenal axis28 in both humans and sheep, fetal cortisol also plays a central role in the initiation of parturition in sheep and thus, the early delivery in the F1 and F2 DEX exposed females may reflect altered offspring pituitary adrenal development. Both we and others have shown programming of the fetal pituitary adrenal axis by maternally administered synthetic GCs.11
Maternal (F0) DEX treatment produces fasting hyperglycemia, glucose intolerance, and an increased glucose-stimulated insulin secretion in adult (F1) offspring in male and female rats and sheep.13,24,29 Both F1 and F2 DEX offspring show altered fasting plasma insulin and glucose concentrations with hyperglycemia and hypoinsulinemia during an IVGTT indicating depressed islet function. The differences in fasting and IVGTT glucose and insulin between the F1 and F2 generations may reflect differences in diet amount and composition associated with the difference in the requirements of mature ewes compared with growing lambs.19 Maternal administration of multiple (4 doses of 0.5 mg/kg body weight of betamethasone) doses of synthetic GC in late gestation ewes results in hyperglycemia and insulinemia in yearling F1 offspring of both sexes and also as these offspring age.24,30 During an IVGTT, offspring from ewes given multiple treatments of synthetic GC have elevated glucose similar to ourF1lambs.24,30 However, insulin during an IVGTT in these other experiments was elevated in both young (6 months of age) and old (2 and 3 years of age) offspring in contrast to our experiment where insulin secretion decreased during the IVGTT.24,30 Interpretation of differences in the degree and rate of emergence of programmed changes needs to allow for breed differences, postnatal diets and other husbandry details in addition to the exact timing, nature and quantity of steroid administered.
It is important to consider potential mechanisms that result from the fetal GC exposure that alter pancreatic development and lead to later emergence of problems. Normal fetal pancreatic development is regulated by physiologic levels of GC exposure.31 The mechanism is thought to be that GC regulate fetal IGF2, which then regulates growth, pancreatic apoptosis, and remodeling essential to fetal pancreatic development.31–34 Importance of this mechanism is supported by observations in fetal rodents in which GC concentrations negatively correlate with pancreatic insulin content and β cell mass increases when fetal steroid production is reduced.35 Maternal treatment with 3 courses of synthetic GC in the ewe results in alterations in fetal β cell morphology, and changes in islet insulin and pancreatic duodenal homeobox-1 (Pdx-1) protein expression, which has been shown to be a marker of pancreatic development and plays a major role in glucose dependent regulation of insulin secretion.36,37 There is also evidence for GC effects on developing fetal skeletal muscle and adipose tissue that could result in development of insulin resistance.38,39
The mechanism(s) that programs outcomes of fetal insults either nutritional or GC across multiple generations are poorly understood. However, in a recent review paper, Matthews and Phillips40 propose 2 possible mechanisms that would allow for the multigenerational transmission of late gestational GC exposure: epigenetic modification that alters gene transcription and translation41 and changes in F1 ewes’ insulin and glucose, and/or pituitary adrenal metabolism that result in metabolic dysfunction when F1 ewes themselves become pregnant and alter in F2 phenotype development. Both mechanisms merit further study.
In conclusion, other research groups have shown that repeated maternal administration of synthetic GC results in fetal IUGR, reduced postnatal growth, altered insulin and glucose metabolism in F1 offspring. The multigenerational effects we demonstrate followed fetal exposure equivalent to 1 course of treatment at one-third the clinical dose. We, and others have drawn attention to the issue that this life-saving synthetic GC treatment has never been assessed for dose effectiveness and cost-benefit. Because pregnant women over a wide range of weights receive the same sGC dose, it is at least arguable that a dose, which is correct for a very heavy woman is supramaximal for a pregnant woman half or even one third her weight. We have shown that half the weight-adjusted clinical dose (85 µg.kg.−1:day−1 betamethasone phosphate) for 2 days given to pregnant sheep at the equivalent of 30 weeks human gestation has an equivalent effect to the full dose in improving fetal lung pressure volume curves 48 hours later.42 This report is the first demonstration in a precocial species, importantly the species in which the therapy to accelerate fetal lung maturation was first demonstrated, that these unwanted F1 generation side effects are carried over to F2 offspring. These findings further emphasize the need for carefully designed studies to establish cost-benefit of antenatal GC therapy.
Acknowledgments
This project was supported by the University of Wyoming National Institute of Health Grants INBRE P20-RR-16474-04 and HD 21350.
Footnotes
The authors report no conflict of interest.
Presented, in part, at the Society for the Study of Reproduction 44th annual meeting, Portland, OR, July 31-Aug. 4, 2011.
REFERENCES
- 1.Liggins GC. Preterm delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969;45:515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- 2.Liggins GC, Howie RN. A controlled trail of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in preterm infants. Pediatrics. 1972;50:515–525. [PubMed] [Google Scholar]
- 3.National Institutes of Health. Consensus development panel on the effects of corticosteroids for fetal maturation on perinatal outcomes: effects of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–885. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- 5.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med. 1997;8:81–87. doi: 10.1002/(SICI)1520-6661(199905/06)8:3<81::AID-MFM3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JWC, Mitzner W, Beck JC, et al. Long-term effects of betamethasone on fetal development. Am J Obstet Gynecol. 1981;141:1053–1064. doi: 10.1016/s0002-9378(16)32697-7. [DOI] [PubMed] [Google Scholar]
- 7.Banks BA, Cnaan A, Morgan MA, et al. Multiple courses of antenatal corticosteroids and outcome of premature neonates. Am J Obstet Gynecol. 1999;181:709–717. doi: 10.1016/s0002-9378(99)70517-x. [DOI] [PubMed] [Google Scholar]
- 8.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 9.Bloom SL, Sheffield JS, McIntire DD, Leveno KJ. Antenatal dexamethasone and decreased birth weight. Obstet Gynecol. 2001;97:485–490. doi: 10.1016/s0029-7844(00)01206-0. [DOI] [PubMed] [Google Scholar]
- 10.Sloboda DM, Moss TJ, Gurrin LC, Newnham J, Challis JRG. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 11.Sloboda DM, Newnham JP, Challis JRG. Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function of the ovine fetus at term. J Endocrinol. 2000;165:79–91. doi: 10.1677/joe.0.1650079. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Bi J, Valego N, et al. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2010;299:R793–R803. doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;228:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 14.Nyirenda MJ, Welberg LA, Seck JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- 15.Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588:1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zambrano E, Martinez-Samayoa PM, Bautista CJ, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 18.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. doi: 10.1159/000273066. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Nutrient requirements of sheep. Washington, DC: National Academy Press; 1985. [Google Scholar]
- 20.Federation of Animal Science Societies. Guide for the care and use of agricultural animals in agricultural research and teaching. 3rd ed. Savoy, IL: Fed Anim Sc Soc; 2010. [Google Scholar]
- 21.Ford SP, Hess BW, Schwope MM, et al. Maternal undernutrition during early gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85:1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- 22.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 23.Knan AA, Rodiguez A, Kaakinem M, Pouta A, Hartikainen AL, Jarvelin MR. Does in utero exposure to synthetic glucocorticoids influence birthweight, head circumference and birth length? A systematic review of current literature. Pediatric Perinat Epidemiol. 2011;25:20–36. doi: 10.1111/j.1365-3016.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- 24.Moss TJM, Sloboda DM, Gurrin LC, Harding R, Challis JRG, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–R970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- 25.De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrine Metab. 2007;293:E75–E82. doi: 10.1152/ajpendo.00689.2006. [DOI] [PubMed] [Google Scholar]
- 26.Moss TJM, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192:146–152. doi: 10.1016/j.ajog.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 27.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25:669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 28.Thomas AL, Krane EJ, Nathanielsz PW. Changes in the fetal thyroid axis after induction of premature parturition by low dose continuous intravascular cortisol infusion to the fetal sheep at 130 days of gestation. Endocrinol. 1978;103:17–23. doi: 10.1210/endo-103-1-17. [DOI] [PubMed] [Google Scholar]
- 29.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate caroxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloboda DM, Moss TJ, Li S, et al. Hepatic glucose regulation and metabolism in adult sheep: effects of prenatal betamethasone. Am J Physiol Endocrinol Metab. 2005;289:E721–E728. doi: 10.1152/ajpendo.00040.2005. [DOI] [PubMed] [Google Scholar]
- 31.Hill DJ. Fetal programming of the pancreatic β cells and the implications for postnatal diabetes. Semin Neonat. 1999;4:99–113. [Google Scholar]
- 32.Hill DJ, Duvillie B. Pancreatic development and adult diabetes. Pediatr Res. 2000;48:269–274. doi: 10.1203/00006450-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Hill DJ, Petrik J, Arany E. Growth factors and the regulation of fetal growth. Diabetes Care. 1998;21:60B–69B. [PubMed] [Google Scholar]
- 34.Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islets cells of the neonatal rat is associated with a reduced expression in insulin-like growth factors II that may act as a survival factor. Endocrinol. 1998;139:2994–3004. doi: 10.1210/endo.139.6.6042. [DOI] [PubMed] [Google Scholar]
- 35.Blondeau B, Lesage J, Czernichow P, Dupouy JP, Breant B. Glucocorticoids impair fetal bête-cell development in rats. Am J Physiol Endocrinol Metab. 2001;281:E592–E599. doi: 10.1152/ajpendo.2001.281.3.E592. [DOI] [PubMed] [Google Scholar]
- 36.Sloboda DM, Newnham JP, Challis JRG. Society for Gynecological Investigation; 2002. Los Angeles CA: 2002. Betamethasone administration and fetal pancreatic development; p. 290A. [Google Scholar]
- 37.Han SI, Yasuda K, Kataoka K. ATF2 interacts with Beta-cell-enriched transcription factors MafA Pdx1 and Beta2, and activated insulin gene transcription. J Biol Chem. 2011;286:10449–10456. doi: 10.1074/jbc.M110.209510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cleasby ME, Kelly PAT, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinol. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- 39.Sewter CP, Blows F, Vidal-Puig A, O’Rahilly S. Regional differences in the response of human pre-adipocytes to PPARgamma and RXRalpha agonists. Diabetes. 2002;51:718–728. doi: 10.2337/diabetes.51.3.718. [DOI] [PubMed] [Google Scholar]
- 40.Mathews SG, Phillips DIW. Minireview: transgenerational inheritance of the stress response; a new frontier in stress research. Endocrinol. 2010;151:7–13. doi: 10.1210/en.2009-0916. [DOI] [PubMed] [Google Scholar]
- 41.Jammes H, Junien C, Chavette-Palmer P. Epigenetic control of development and expression of quantitative traits. Reprod Fert Dev. 2011;23:64–74. doi: 10.1071/RD10259. [DOI] [PubMed] [Google Scholar]
- 42.Loehle M, Schwab M, Kadner S, et al. Dose-response effects of betamethasone on maturation of the fetal sheep lung. Am J Obstet Gynecol. 2010;202:e1–e7. doi: 10.1016/j.ajog.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]