Abstract
The current study tested the hypothesis that modification in central hemodynamics during short-term continuous positive airway pressure (CPAP) application was accompanied by altered firing patterns of sympathetic nerve activity in CHF patients and healthy subjects.
Muscle sympathetic nerve activity (MSNA), hemodynamic and ventilatory parameters were obtained from 8 healthy middle aged subjects and 7 CHF patients. Action potentials (APs) were extracted from MSNA neurograms, quantified as AP frequency and classified into different sized clusters. While on CPAP at 10 cm H2O, multi-unit MSNA, AP frequency and mean burst area/min increased in healthy middle aged subjects (p < 0.05) whereas CPAP had no effect on these variables in CHF patients. In conclusion, the impact of CPAP on central hemodynamics in healthy individuals elicited a moderate activation of sympathetic neurons through increased AP firing frequency, whereas in CHF patients both hemodynamics and MSNA remained unaltered.
Keywords: Action potential detection, Microneurography, End-expiratory positive pressure, Human
1. Introduction
The long-term use of continuous positive airway pressure (CPAP) has been associated with clinical benefit in patients with chronic heart failure (CHF) and is recognized as a novel non-pharmacological therapeutic option in this condition, which is characterized by a high mortality rate and limited treatment options (Usui et al., 2005; Naughton et al., 1995a). The improvement in left ventricular function with CPAP in CHF patients has been ascribed to increased intrathoracic pressure and associated reduction in left ventricular transmural pressure (Naughton et al., 1995b; Baratz et al., 1992; Bradley et al., 1992). However, data on short-term hemodynamic effects of CPAP in CHF patients as well as in healthy subjects are still controversial and inconsistent. Improvement of cardiac index (CI) and stroke volume index (SVI) was found in CHF patients with increased pulmonary capillary wedge pressure (PCWP) after 10 min of CPAP; however, CHF patients with normal PCWP exhibited an opposite effect (De et al., 1995). Naughton et al. (1995b) reported that acute CPAP produced a reduction in CI and SVI in healthy subjects but no change in CHF patients. Several other studies found no beneficial effects of short-term use of CPAP in CHF (Buckle et al., 1992; Davies et al., 1993).
The responses of the autonomic nervous system after short-term application of the CPAP appear to be different between CHF patients and healthy individuals. In young subjects with normal cardiac filling pressures, a positive end-expiratory pressure induced by CPAP causes reduced venous return, followed by a decrease in cardiac output (CO) in accordance with Frank-Starling mechanism. Diminished CO leads to unloading of aortic baroreceptors and a reflexive increase in the muscle sympathetic nerve activity (MSNA) (Naughton et al., 1998; Tanaka et al., 1994). Contrary to healthy subjects, the short-term effects of CPAP in CHF are less consistent. Positive end-expiratory pressure in CHF patients with increased cardiac filling pressures may cause a reduction in afterload (due to lowering of the left ventricular transmural pressure) and result in increased CO without the need for sympathetic outflow augmentation (Naughton et al., 1995b).
However, sympathetic neural firing patterns and activation strategies of the sympathetic nervous system during CPAP are not completely understood. In contrast to the idea that sympathoinhibition should occur if CPAP enhances cardiac index (as above), Heindl et al. (2001) found a modest increase in multi-unit MSNA burst frequency in both CHF patients and healthy control subjects. Recently, our studies have explored the potential importance of quantifying actual action potential (AP) patterns in the postganglionic sympathetic nerve activity as an indicator of sympathetic patterns, rather than just the frequency of integrated bursts (Steinback et al., 2010; Salmanpour et al., 2010, 2011; Breskovic et al., 2011; Maslov et al., 2012).Specifically, multi-unit MSNA bursts are composed of several sympathetic neurons firing at the same time with burst-by-burst variations in the numbers of APs recruited (Salmanpour et al., 2010). Traditional quantification of MSNA as bursts frequency or burst incidence provides only general impression about sympathetic nerve activity without insight into neural firing patterns of sympathetic neurons contributing to sympathetic burst.
The purpose of the present study was to investigate short-term effects of CPAP on the firing strategies of sympathetic neurons measured by microneurography in CHF patients and in healthy, gender and age matched controls. We hypothesized that modification in the sympathetic nerve firing during short-term CPAP would reflect a response to altered central hemodynamics.
2. Methods
2.1. Subjects
Seven patients with moderate CHF and eight healthy age and gender-matched control subjects were included in the study. Patients were recruited from the Department of Cardiology, University Hospital of Split and from the Croatian Register of Heart Failure Patients (Croatian Cardiology Society). Eligibility was determined using the following criteria: age 20–75 years, left ventricular ejection fraction (LVEF) < 40%, New York Heart Association classes I–III, stable condition (no rales on auscultation or tibial edema) with no recent (1 month) history of decompensation or hospitalization, and stable pharmacotherapy (3 months). Exclusion criteria were: atrial fibrillation, pace maker dependence, history of smoking or alcoholism and history of comorbidities (kidney disease, obstructive lung disease, cerebrovascular insult, severe anemia Hgb < 90 g/l). Health volunteers free of cardiovascular disease were matched for age and gender and recruited as control subjects. All subjects gave written informed consent to participate in the study that was conducted in accordance with the Declaration of Helsinki and was approved by the research ethics board at The University of Split, School of Medicine.
2.2. Measurements
All blood tests were done in Biochemical laboratory at University Hospital Split. ECLIA (Electrochemiluminescence immunoassay; analyzer Cobas E601; Roche Diagnostics GMBH, Mannheim; Germany) was used to obtain pro-BNP.
Blood pressure was measured with the use of photo-plethysmography (Finometer, Finapress Medical Systems, Arnhem, Netherlands). From the continuous blood pressure measurement, the arterial pulse wave was analyzed by an improved method of Wesseling (Modelflow program) which computes changes in left ventricular stroke volume (SV) from the pulsatile systolic area (Jellema et al., 1999). The values of systolic (SBP) and diastolic (DBP) blood pressures obtained by the photoplethysmographic method were gauged using the mercury sphygmomanometer. The heart rate (HR) was obtained by electrocardiogram (ECG, Bioamp, ADInstruments, Castle Hill, Australia) and CO was computed as SV times HR. Arterial oxygen saturation (SaO2) was monitored continuously by pulse oximetry (Poet II, Criticare Systems,Waukesha, WI, USA), with the probe placed on the middle finger. A pneumatic respiratory belt, located around the chest at the level of the xiphoid process, was coupled to a differential pressure transducer (Prignitz Mikrosystemtechnik, Wittenberge, Germany) and was used to monitor thoracic movements. Respiratory parameters including tidal volume (VT) and breathing frequency (Bf) were measured continuously with a breath-by-breath analyzer (AMIS2000, Innovision A/S, Odense, Denmark). Ventilatory volume (VE) was computed as VT times Bf.
Multi-unit MSNA of postganglionic sympathetic neurons was recorded using microneurography. A tungsten microelectrode was inserted percutaneously into the peroneal nerve while the reference electrode was inserted 1–3 cm from the recording site. Small adjustments of the active electrode were made until multi-unit bursts occurred. Confirmation that the recorded signal represented MSNA was determined by the absence of skin paresthesia and presence of a signal that increased in response to voluntary apnea but not during arousal to a loud noise (Vallbo et al., 2004). The nerve signal was amplified (100 000 times), bandpass filtered (band pass 700–2000 Hz), rectified, integrated using 0.1 s time constant and sampled at 10 000 Hz (Powerlab/16SP; ADInstruments) and stored for subsequent analysis using Chart software (version 5.5.6.7).
2.3. Protocol
The study was conducted in two consecutive days. On the 1st day of the study, upon arrival to the laboratory, subjects were informed about procedures and potential risks. A brief history, physical examination and ECG were obtained from each subject. All participants underwent anthropometric measurements and performed a dynamic spirometry test (Quark PFT; Cosmed, Rome, Italy). LVEF was determined using two-dimensional echocardiography (Vivid Q, GE, Milwaukee, WI USA). Blood samples were withdrawn from the antecubital vein to measure levels of probrain natriuretic peptide (pro-BNP). Additionally, all subjects were instructed in the use of CPAP (BiPAP Vision, Respironics, Pittsburg, PA, USA) to familiarize them with the device and minimize potential hemodynamic effects of anxiety. To maximize generalizability of data, the CHF patients continued on standard pharmacotherapy during the protocol. On the 2nd day of the study, subjects were placed in supine position and after instrumentation they were given 10 min of quite rest. After stabilization of hemodynamic parameters, subjects began breathing through a mouthpiece with a pneumatic one-way valve connected to a breath-by-breath respiratory gas analyzer with nose clip in place. All subjects breathed room air for 10 min to obtain baseline values prior to administering CPAP for 5 min at each of two levels of positive pressure (5 and 10 cm H2O), followed by 10 min of recovery on room air.
2.4. Data acquisition and analysis
Hemodynamic, ventilatory and MSNA parameters were analyzed and averaged during the last minute of baseline, during the first minute (start) and last minute (end) of each CPAP level (5 and 10 cm H2O) and during the last 1 min of recovery. Integrated bursts of MSNA were identified as exhibiting pulse-synchrony, having a signal-to-noise ratio (SNR) of at least 2:1 with respect to a previous period of neural silence and having characteristic rising and falling slopes. Multiunit-MSNA was quantified as burst frequency by counting the number of bursts during a specified period of time, as burst incidence by expressing the number of burst that occurred per 100 heart beats, and as mean burst area/min (Sundlof and Wallin, 1977). Action potentials (APs) were detected and extracted from the filtered raw MSNA signal using the continuous wavelet transform (CWT) technique for AP detection as reported previously (Steinback et al., 2010; Salmanpour et al., 2010, 2011; Breskovic et al., 2011; Maslov et al., 2012). Briefly, the method uses an original mother wavelet, derived from an average human AP. The program specifies the location of the AP whereby it can be extracted in a 3.2 ms window and then measured for its peak-to-peak amplitude. Probability of spike summation with concurrent APs has been established (<2.3%) and suspected spikes are eliminated from the analysis. Only raw neurograms with signal-to-noise ratio (SNR) ≥3 were studied for AP detection (Salmanpour et al., 2010). Extracted APs were quantified as AP frequency (number of APs per minute), as AP incidence (number of APs per 100 heart beats), as AP/burst (number of AP in each burst), and AP size. Action potentials were also ordered in families (clusters) based on their peak-to-peak amplitude.
2.5. Statistical analysis
Results are expressed as means ± SD. The main effects of group (CHF group vs. control group) and time (baseline vs. start and end of each CPAP level) on MSNA parameters were assessed with a Mixed analysis of variance (ANOVA). Friedman ANOVA was used to evaluate the main effect of time (baseline vs. start and end of CPAP level 10 cm H2O) on MSNA, hemodynamic and ventilatory variables in each group. In case of significance, pair-wise comparisons were assessed using the Wilcoxon test. Baseline anthropometric, hemodynamic, ventilatory and MSNA data between the groups were compared using Mann–Whitney U test. Relationships between CPAP responses in MSNA parameters (burst frequency, burst incidence, AP frequency, mean burst area/min and AP/burst; dependent variables) and hemodynamic variables (DBP, SV and CO; independent variables) were analyzed by linear regression. The inter-subject variation was accounted for by multiple regression, with subjects as dummy variables. The remaining, intra-subject relationships were evaluated as the partial correlation coefficients (univariate analyses). To further account for mutual associations of independent variables, the associations of SV and CO were adjusted for DBP and the associations of DBP were adjusted for SV and CO (multivariate analyses). p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
The anthropometric and clinical characteristics of the 7 CHF patients and 8 healthy controls are listed in Table 1. By design, the two groups were comparable for gender and age. As expected, CHF group had significantly higher integrated MSNA burst frequency and higher burst incidence at baseline (Table 2). Furthermore AP detection analysis exposed a three-fold higher AP frequency and AP incidence in the CHF patients when compared to controls (p < 0.05). Compared with Control, total MSNA activity, expressed as mean burst area/min, was greater by 150% in CHF patients (p < 0.05). Except for mean arterial pressure (MAP) and SBP which were by ~14 mmHg and by ~27 mmHg higher in controls than in CHF patients (p < 0.05), the groups did not differ in DBP, HR, SV, CO, VT, Bf and VE at baseline (Table 3).
Table 1.
Anthropometric and clinical characteristics of the subjects
| CHF patients (N=7) | Controls (N = 8) | |
|---|---|---|
| Anthropometrics | ||
| Age (years) | 61 ± 9 | 53 ±7 |
| Gender (male/female) | 5/2 | 6/2 |
| Height (cm) | 173 ±0.1 | 180 ±0.1 |
| Weight (kg) | 88 ±12 | 78 ±13 |
| BMI | 29 ±3* | 24 ± 3 |
| pro-BNP (pmol/l) | 84.7 ± 39 | |
| Spirometry | ||
| VC (% predicted) | 89 ±7* | 111 ±7 |
| FEV1 (predicted) | 87 ±12* | 108 ±7 |
| Echocardiography | ||
| LVEF (%) | 35 ± 4 | - |
| LVDd (mm) | 75 ±9 | - |
| NYHA class I | 1 | |
| I | 1 | - |
| II | 5 | - |
| III | 1 | - |
| Etiology | ||
| Dilated cardiomyopathy | 3 | - |
| Coronary artery disease | 3 | - |
| Idiopathic | 1 | |
| Pharmacotherapy | ||
| ACEi | 5 | - |
| AT1 receptor blockers | 1 | - |
| Diuretics | 7 | - |
| Digitalis | 1 | - |
| β- blockers | 4 | - |
| Nitrates | 2 |
Values are mean ± SD. BMI indicates body mass index; pro-BNP, pro brain natriuretic peptide; VC, vital capacity; FEV1, forced expiratory volume in one second; LVEF, left ventricular ejection fraction; LVDd, left ventricular diastolic diameter; NYHA, New York Heart Association; ACEi, angiotensin converting enzyme inhibitor; AT1 receptor blockers, blockers of type 1 receptors of angiotensin II.
p <0.05 different from controls.
Table 2.
Difference in baseline sympathetic parameters between groups
| CHF patients (N=7) |
Controls (N=8) |
|
|---|---|---|
| Burst frequency (burst/min) | 55 ± 9* | 33 ± 8 |
| Burst incidence (burst/100 heart beats) | 85 ± 11* | 48 ± 11 |
| Mean burst area/min | 5± 1* | 2± 1 |
| AP frequency (APs/min) | 746 ± 366* | 263 ± 116 |
| AP incidence (APs/100 heart beats) | 1138 ± 559* | 364 ± 164 |
| AP/burst | 13 ±5 | 8± 2 |
| Distinct clusters/burst | 5± 1* | 4 ± 0.7 |
Values are mean ± SD.
p <0.05 different from controls
Table 3.
Hemodynamic and ventilatory responses to CPAP 5 and 10cm H2O
| Hemodynamic and ventilatory parameters |
CHF patients (N=7) |
Controls (N=8) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | CPAP 5 cm H2O start |
CPAP 5 cm H2O end |
CPAP 10 cm H2O start |
CPAP 10cm H2O end |
Recovery | Baseline | CPAP 5 cm H2O start |
CPAP 5 cm H2O end |
CPAP 10 cm H2O start |
CPAP 10cm H2O end |
Recovery | ||
| MAP (mmHg) | Mean SD |
84.1† 12 |
82.8 11 |
86.4 15 |
84.0 12 |
83.3 13 |
84.4 14 |
97.8 8 |
100.2 9 |
101.8 9 |
102.3 7 |
102.9 8 |
99.7 9 |
| SBP(mmHg) | Mean SD |
122.5† 21 | 135.5* 26 |
135.0* 23 |
131.6* 16 |
130.1* 15 |
133.5 26 |
148.0 19 |
153.5 23 |
156.2 23 |
158.7 21 |
157.5 20 |
150.1 23 |
| DBP (mmHg) | Mean SD |
63.4 8 |
66.2 12 |
65.4 13 |
64.8 13 |
63.8 13 |
64.8 11 |
71.1 7 |
74.7* 6 |
75.2 5 |
77.1* 4 |
77.4* 6 |
73.2 6 |
| SV (ml) | Mean SD |
94.5 19 |
91.1 22 |
90.1 23 |
92.1 23 |
93.6 25 |
95.8 22 |
111.0 17 |
101.7* 14 |
102.1* 10 |
96.5* 12 |
95* 12 |
105.5 14 |
| HR (bpm) | Mean SD |
64 7 |
65 8 |
63 7 |
68 5 |
68 6 |
64 7 |
67 8 |
66 7 |
66 7 |
65 7 |
66 12 |
65 7 |
| CO (l/min) | Mean SD |
6.1 2 |
5.9 1 |
5.7 2 |
6.1 1 |
6.3 2 |
6.1 1 |
7.5 2 |
6.7 1 |
6.7* 1 |
6.2* 1 |
6.2* 1 |
6.8 1 |
| VT (ml) | Mean SD |
640 382 |
700 220 |
800 550 |
951 556 |
980 560 |
520 392 |
825 106 |
1274* 385 |
1177 407 |
1358* 277 |
1155* 322 |
769 116 |
| Bf(bpm) | Mean SD |
16 6 |
16 7 |
16 7 |
19 9 |
16 10 |
19 8 |
12 3 |
13 3 |
15* 3 |
16* 4 |
16* 4 |
12 3 |
| VE (l/min) | Mean SD |
8.8 3 |
10.1 3 |
10.4 5 |
14.8 5 |
12.7 5 |
8.1 4 |
10.0 2 |
16.7* 4 |
16.8* 5 |
20.7* 6 |
7.8* 4 |
9.9 3 |
MAP indicates mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; SV, stroke volume; HR, heart rate; CO, cardiac output; VT, tidal volume; Bf, breathing frequency; VE, ventilatory volume.
p <0.05 different from baseline.
p <0.05 different from controls.
3.2. Hemodynamic response to CPAP
CPAP at 5 and 10 cm H2O levels did not influence DBP, HR, SV or CO in the CHF patients, but caused an immediate increase in SBP at 5 cm H2O that was sustained during the start and end of the 10 cm H2O level (Table 3). Control group demonstrated an abrupt decrease in SV and CO with 5 cm H2O of CPAP which was sustained during both measurement points of CPAP 10 cm H2O (p < 0.05). In contrast, DBP increased at the beginning of CPAP 5 cm H2O and then rose again during the 10 cm H2O of CPAP (p < 0.05). SaO2 did not change with CPAP in either group. In both groups, all hemodynamic parameters returned to baseline values during recovery after cessation of CPAP.
3.3. Ventilatory response to CPAP
5 cm H2O of CPAP caused a 449 ml augmentation of VT in control subjects (Table 3) that was maintained through the 10 cm H2O of CPAP (p < 0.05). Changes in VE in healthy subjects followed a similar trend during CPAP. Bf was increased at the end of level 5 cm H2O of CPAP and continued to be increased during level 10 cm H2O (p < 0.05). All ventilatory parameters returned to baseline levels during recovery. In contrast to the control group, ventilatory parameters were not modified by CPAP in the CHF patients, although VT tended to be increased during 10 cm H2O of CPAP compared to baseline (p = 0.07). SaO2 remained unchanged in both groups during CPAP breathing.
3.4. Sympathetic nervous response to CPAP
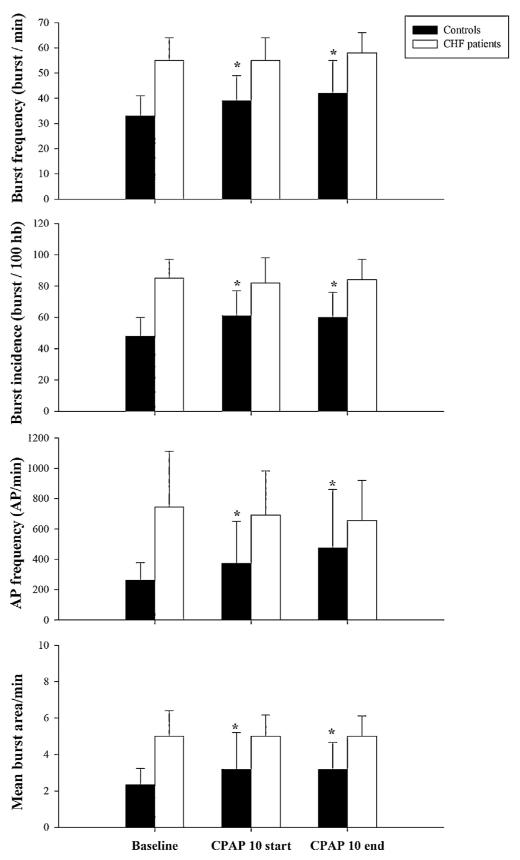
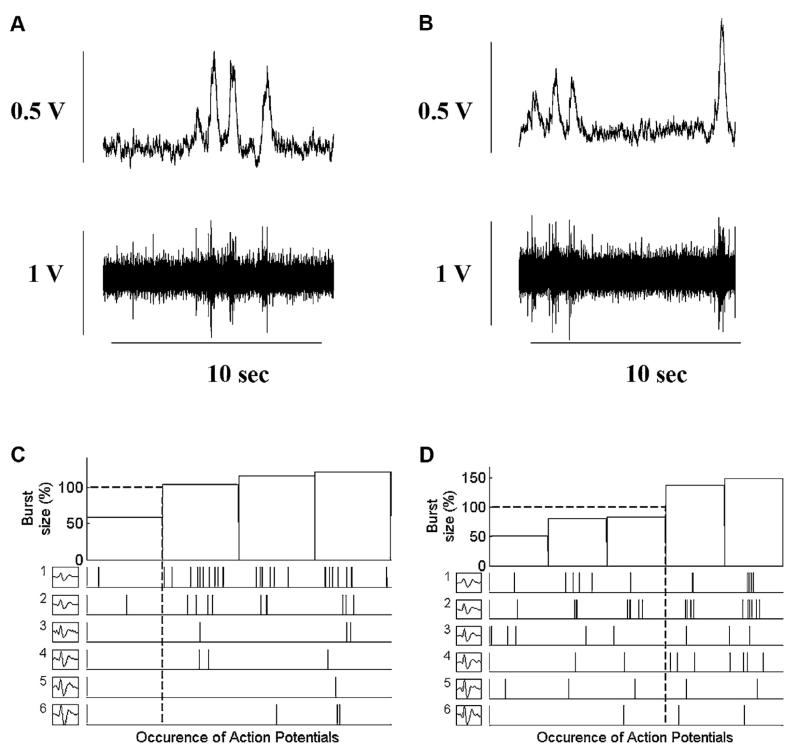
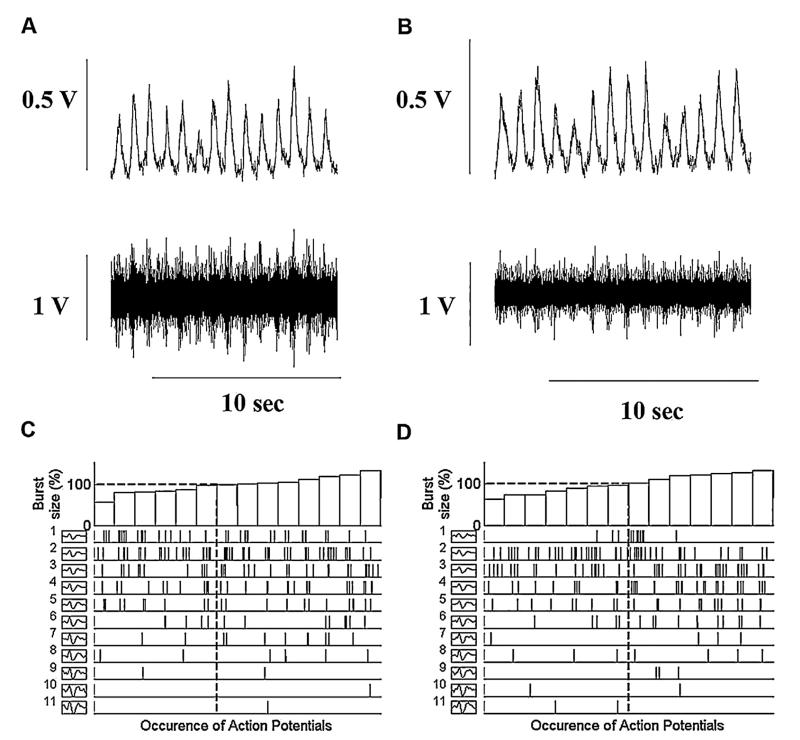
A main effect of group (CHF vs. controls) was observed at baseline for burst frequency, burst incidence, mean burst area/min, AP firing frequency, and AP incidence (p < 0.05). In the control group, compared to baseline, integrated MSNA burst frequency was increased by ~18% and burst incidence by ~27% at the beginning of 10 cm H2O of CPAP and remained elevated until the end of CPAP (p < 0.05) (Fig. 1). Simultaneously, with multi-unit MSNA changes, AP firing frequency was increased by ~41% at the beginning of CPAP 10 cm H2O and by ~80% at the end of CPAP when compared to baseline levels (p < 0.05). There was a trend toward increase in the number of AP/burst during CPAP in the control group (8 ± 2, 9 ± 4 and 10 ± 5; Baseline, CPAP 10start and CPAP 10end respectively) that did not reach statistical significance. However number of AP/burst remain the same in the CHF patients during CPAP (13 ± 6, 12 ± 4 and 12 ± 4; Baseline, CPAP 10start and CPAP 10end respectively). In both groups the overall number of distinct clusters of APs was similar during CPAP as during baseline breathing. Original recordings of the integrated and filtered MSNA data at baseline (panels A) and during CPAP 10 cm H2O (panels B) are shown for one healthy subject in Fig. 2 and for one CHF patient in Fig. 3. The occurrence of individual AP clusters as a function of burst amplitude at baseline (panel C) and during CPAP (panel D) in one healthy middle aged subject is shown in Fig. 2 and in one CHF patient is shown in Fig. 3. In the healthy subject, AP clusters of sympathetic neurons present at baseline increased firing frequency during CPAP, while the number of different clusters of sympathetic neurons was unaltered with CPAP (6 AP clusters at baseline and 6 during CPAP breathing). As shown in Fig. 3, sympathetic pattern remained unaltered in CHF patients while on CPAP.
Fig. 1.
Multi-unit MSNA and action potential (AP) firing frequency during baseline, beginning (start) of the CPAP 10 cm H2O and at the end of CPAP 10 cm H2O in healthy subjects (dark bars) and in chronic heart failure (CHF) patients (light bars). Values are mean±SD. * p < 0.05 compared to controls.
Fig. 2.
Occurrence of postganglionic sympathetic APs for each AP cluster as a function of burst size between baseline (plots A and C) and during the CPAP (plots B and D) in one healthy control subject. Plots A and B provide the integrated and filtered raw muscle sympathetic nerve activity (MSNA) tracings during baseline and CPAP breathing used to display the range of integrated sympathetic bursts ordered by burst size as a percentage of baseline (C and D, top panels) together with the occurrence of postganglionic sympathetic action potentials as a function of integrated burst size for each action potential cluster (C and D, lower panels). Dashed lines represent the mean of integrated burst sizes (horizontal) and corresponding action potential cluster range (drop lines). Note the same number of different clusters present at baseline and during the CPAP breathing (6).
Fig. 3.
Occurrence of postganglionic sympathetic APs for each AP cluster as a function of burst size between baseline (plots A and C) and during the CPAP (plots B and D) in one CHF patient. Plots A and B provide the integrated and filtered raw muscle sympathetic nerve activity (MSNA) tracings during baseline and CPAP breathing used to display the range of integrated sympathetic bursts ordered by burst size as a percentage of baseline (C and D, top panels) together with the occurrence of postganglionic sympathetic action potentials as a function of integrated burst size for each action potential cluster (C and D, lower panels). Dashed lines represent the mean of integrated burst sizes (horizontal) and corresponding action potential cluster range (drop lines). Note the same number of different clusters present at baseline and during the CPAP breathing (11).
3.5. Association of sympathetic and hemodynamic responses to CPAP
The medium sized linear correlations between sympathetic and hemodynamic responses to CPAP were observed in controls, but not in patients (Table 4); the greatest were between CO and burst incidence (r = −0.68; p = 0.01), CO and mean burst area (r = −0.58; p = 0.01) and DBP and burst incidence (r = −0.6; p = 0.01). Adjustment for mutual association of hemodynamic variables diminished theses associations. Notably, since the heart rate was practically unaffected, to avoid over adjustment, the stroke volume and cardiac output were not mutually controlled for.
Table 4.
Linear correlation coefficients (p-values) between muscle sympathetic nerve activity (MSNA) and hemodynamic variables during CPAP performed in healthy individuals
| MSNA parameters | DBP |
SV |
CO |
|||
|---|---|---|---|---|---|---|
| Raw | Adjusteda | Raw | Adjustedb | Raw | Adjustedb | |
| Burst incidence | −0.6 (0.01) | −0.05 (0.8) | −0.69(0.02) | −0.43 (0.09) | −0.68(0.01) | −0.45 (0.07) |
| Burst frequency | −0.41 (0.09) | −0.16(0.5) | −0.4(0.1) | −0.09 (0.7) | −0.38(0.12) | −0.13 (0.6) |
| Burst frequency Mean burst area/min | −0.43 (0.79) | −0.11 (0.68) | −0.52(0.032) | −0.32 (0.22) | −0.58(0.01) | −0.42 (0.9) |
| AP frequency AP/burst | −0.26 (0.3) | −0.17 (0.53) | −0.29 (0.25) | −0.12 (0.64) | −0.36 (0.15) | −0.36 (0.15) |
| AP/burst | −0.09 (0.71) | −0.05 (0.84) | −0.17 (0.49) | −0.19 (0.46) | −0.29 (0.24) | −0.33 (0.2) |
Data are from 7 healthy subjects (dummy variables) during baseline, CPAP 10 H2O start and CPAP 10 H2O end. DBP, diastolic blood pressure; SV, stroke volume; CO, cardiac output.
Adjusted for stroke volume and cardiac output.
Adjusted for diastolic blood pressure.
4. Discussion
The main finding of the present study was that in healthy middle-aged subjects, short-term application of 5 and 10 cm H2O level of CPAP caused a reduction of SV and CO followed by an increase in MSNA burst incidence as well as firing frequency of sympathetic APs. However, CPAP did not affect the number of different sized clusters of APs within a sympathetic burst. In contrast, short-term application of CPAP did not alter MSNA or hemodynamics in CHF patients.
Despite small number of measurements (3 repeated measurements-during baseline, start and end of CPAP 10 H2O in 7 subjects), linear correlations were observed between sympathetic and hemodynamic responses in the control group, especially in the case of CO and DBP. This substantiates the hypothesis that CPAP-induced decreases in cardiac output reflexively triggered the observed sympathetic responses in healthy subjects.
To date, information regarding firing strategies of sympathetic neurons during different cardiovascular and respiratory stimuli in CHF patients and in healthy middle-aged individuals is very limited. This is the first study to report the activation pattern of post-anglionic sympathetic neurons during CPAP in CHF patients and in healthy middle aged individuals. An important contribution to our understanding of sympathetic firing strategies in healthy individuals and in CHF patients was made by Macefield et al. (1994, 2002) who used a single-unit method and showed that when active, sympathetic neurons fire once within a burst of activity. While this single unit approach suggests whether or not a neuron becomes more or less active, it cannot address the question about firing strategies of all sympathetic neurons contributing to a sympathetic burst or about latent populations of sympathetic neurons that may become active in response to physiological stress such as hypotension, CPAP or low body negative pressure (LBNP)application. The AP detection technique used in the present study demonstrates the pattern of activity of all AP within each sympathetic burst and also provides information regarding size of each AP and the appearance of new, larger APs within a given neurogram. A diminished venous return and consequent decrease in CO are associated with CPAP, as well with LBNP (Braunwald et al., 1957; Peters et al., 1997). When applied in young healthy subjects, both conditions cause hemodynamic stress and baroreceptor unloading with increase in multi-unit MSNA (Tanaka et al., 1994; Tripathi et al., 1989). The current results support these earlier observations and extend the issue to an examination of AP patterns. One of the questions addressed in the current study was whether or not such hemodynamic stress would be sufficient to recruit additional “subpopulations” of larger and faster-conducting APs. Previously, conditions of large hemodynamic stress such as −80 mmHg lower body suction or prolonged breath-hold (Steinback et al., 2010; Salmanpour et al., 2011) demonstrated such a reserve of efferent sympathetic axons. However, smaller stressors such as LBNP of −60 mmHg, in young healthy subjects elicited an increase in the multi-unit MSNA as well as AP firing frequency of the sympatheticneurons without recruiting new, additional clusters of sympathetic neurons (Salmanpour et al., 2011). In the current study, breathing at 10 cm H2O CPAP caused a decrease in CO by ~18% and a corresponding rise in MSNA burst frequency which would be considered a neural response to a modest cardiovascular stress. Increased AP firing frequency in healthy subjects during CPAP was due to increased activity of already active clusters of sympathetic neurons without recruitment of new, larger sympathetic neurons. The same number of clusters of sympathetic neurons was active before and during CPAP with higher firing rate during CPAP as shown in Fig. 2C and D. Observations from the above-mentioned studies and from the current study suggest that recruitment of latent subpopulation of larger sympathetic neurons is probably reserved for conditions of higher sympathetic needs like severe orthostatic stress or chemoreflex challenge (Steinback et al., 2010; Salmanpour et al., 2011; Breskovic et al., 2011; Maslov et al., 2012)
As expected, CHF patients exhibited higher baseline levels of sympathetic activity compared to the control participants. We have shown recently that high baseline sympathetic outflow in CHF patients is achieved by both a high number of APs per burst and a high number of different clusters of sympathetic neurons per burst (Maslov et al., 2012). In the current study the AP detection technique demonstrated ~3-fold higher AP frequency and AP incidence in CHF patients’ baseline neurograms if compared with controls.
Higher AP firing rates were associated with higher multi-unit MSNA as well as higher number of distinct active clusters of sympathetic neurons. CPAP did not alter sympathetic firing activity in CHF group in a manner seen in healthy subjects, as central hemodynamics were not changed during CPAP in CHF group and there was no need to defend systemic blood pressure. When administrated nocturnally over a period of one month in CHF patients with obstructive sleep apnea, CPAP lowers daytime MSNA (Usui et al., 2005) and plasma norepinephrine spillover (Naughton et al., 1995a). These sympathoinhibitory responses are most likely attributed to attenuation of apnea related hypoxia and arousal from sleep. In contrast to these effects of long-term CPAP use, an opposite (Heindl et al., 2001) or unchanged (Naughton et al., 1998) effect on sympathetic outflow was reported during short-term CPAP use. The current results also indicate that acute CPAP has no sympathoexcitatory or inhibitory effect in CHF patients. The likely explanation for unchanged MSNA in CHF patients relates to the unaltered cardiac output in this group. An increased cardiac filling pressures characterizes CHF; thus, short positive pressure breathing with CPAP decreases left ventricular transmural pressure, unloads the inspiratory muscles and reduces myocardial oxygen demand without affecting CO (Naughton et al., 1998, 1995b). In these conditions there is no need for further augmentation of MSNA. Another potential explanation for unaltered MSNA in CHF patients is that sympathoinhibitory effect of CPAP on pulmonary-stretch receptors (Harada et al., 2011) and stimulation of pulmonary vagal afferents (Seals et al., 1990) was counterbalanced with cardiopulmonary baroreceptor unloading and reflex sympathoexcitation (Heindl et al., 2001). While VT was increased in healthy subjects with CPAP, it remained unaltered in CHF patients. These findings are in line with previous studies using short-term CPAP in CHF patients (Naughton et al., 1998; Heindl et al., 2001; Lalande et al., 2012), showing that brief use of CPAP affects cardiac filling and transmural pressures more so than ventilatory parameters. In healthy subjects increased firing of sympathetic neurons during CPAP was likely associated with diminished CO that was not seen in our CHF patients. Therefore hemodynamic effect of short-term CPAP on sympathetic nerve activity seems to override the possible ventilatory sympathoinhibitory effect of increased VT in healthy individuals.
This study emphasized the acute effects of CPAP on MSNA discharge, using the expected variations in cardiovascular stress between healthy control and CHF patients. Examination of the neurophysiologic basis of sympathoinhibition in CHF patients with prolonged CPAP treatment would require additional research. In this study, the pharmacological therapy of the CHF patients was preserved to avoid rebound cardiovascular responses and associated baroreceptor-mediated effects on sympathetic nerve traffic and also to maintain comparative consistency with previous work in this population (Lalande et al., 2012; Macefield et al., 1999; Seals et al., 1993).
Our findings lead us to two conclusions. First, brief application of CPAP in healthy middle age individuals increases firing of sympathetic neurons that were already present in the neurograms prior to CPAP application, without recruiting an additional subpopulation of sympathetic neurons, known to be reserved for high physiological stress circumstances (Steinback et al., 2010; Breskovic et al., 2011). These findings indicate a previously unrecognized effect of CPAP on firing strategies of sympathetic nervous system in healthy middle aged individuals. Second, short-term application of CPAP in CHF patients had no effect on central hemodynamics that would require modifications in sympathetic outflow, as observed in healthy subjects.
Acknowledgments
The authors thank Ante Obad, MD, PhD; Nediljko Pivac, MD, PhD and Darija Bakovic, MD, PhD for their help in recruitment of CHF patients. We also thank Vladimir Ivancev, MD, PhD; Ivana Banic, and Branka Runtic MD for their assistance in data collection.
This study was supported by the Croatian Ministry of Science, Education and Sports (Grant #216-2160133-0130) to Z.D. J.K.S. was supported by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research Team Grant in Physical Activity, Mobility and Neural Health (Grant #217532). JKS is a Canada Research Chair in Integrative Physiology of Exercise and Health. B.D.J. and T.P.O. are supported by the National Institute of Health Grant #HL71478 and American Heart Association Grant #12GNT11630027.
References
- Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102:1397–1401. doi: 10.1378/chest.102.5.1397. [DOI] [PubMed] [Google Scholar]
- Bradley TD, Holloway RM, McLaughlin PR, Ross BL, Walters J, Liu PP. Cardiac output response to continuous positive airway pressure in congestive heart failure. American Review of Respiratory Disease. 1992;145:377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Binion JT, Morgan WL, Jr., Sarnoff SJ. Alterations in central blood volume and cardiac output induced by positive pressure breathing and counteracted by metaraminol (aramine) Circulation Research. 1957;5:670–675. doi: 10.1161/01.res.5.6.670. [DOI] [PubMed] [Google Scholar]
- Breskovic T, Steinback CD, Salmanpour A, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic neurons during breath-holding at different lung volumes in apnea divers and controls. Autonomic Neuroscience. 2011;164:74–81. doi: 10.1016/j.autneu.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Buckle P, Millar T, Kryger M. The effect of short-term nasal CPAP on Cheyne-Stokes respiration in congestive heart failure. Chest. 1992;102:31–35. doi: 10.1378/chest.102.1.31. [DOI] [PubMed] [Google Scholar]
- Davies RJ, Harrington KJ, Ormerod OJ, Stradling JR. Nasal continuous positive airway pressure in chronic heart failure with sleep-disordered breathing. American Review of Respiratory Disease. 1993;147:630–634. doi: 10.1164/ajrccm/147.3.630. [DOI] [PubMed] [Google Scholar]
- De HA, Liu PP, Benard DC, Bradley TD. Haemodynamic effects of continuous positive airway pressure in humans with normal and impaired left ventricular function. Clinical Science (London) 1995;88:173–178. doi: 10.1042/cs0880173. [DOI] [PubMed] [Google Scholar]
- Harada D, Joho S, Oda Y, Hirai T, Asanoi H, Inoue H. Short term effect of adaptive servo-ventilation on muscle sympathetic nerve activity in patients with heart failure. Autonomic Neuroscience. 2011;161:95–102. doi: 10.1016/j.autneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Heindl S, Dodt C, Krahwinkel M, Hasenfuss G, Andreas S. Short term effect of continuous positive airway pressure on muscle sympathetic nerve activity in patients with chronic heart failure. Heart. 2001;85:185–190. doi: 10.1136/heart.85.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellema WT, Imholz BP, Oosting H, Wesseling KH, van Lieshout JJ. Estimation of beat-to-beat changes in stroke volume from arterial pressure: a comparison of two pressure wave analysis techniques during head-up tilt testing in young healthy men. Clinical Autonomic Research. 1999;9:185–192. doi: 10.1007/BF02330482. [DOI] [PubMed] [Google Scholar]
- Lalande S, Luoma CE, Miller AD, Johnson BD. Expiratory loading improves cardiac output during exercise in heart failure. Medicine and Science in Sports and Exercise. 2012;44:2309–2314. doi: 10.1249/MSS.0b013e318267bb5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG, Vallbo AB. The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. Journal of Physiology. 1994;481(Pt 3):799–809. doi: 10.1113/jphysiol.1994.sp020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Rundqvist B, Sverrisdottir YB, Wallin BG, Elam M. Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation. 1999;100:1708–1713. doi: 10.1161/01.cir.100.16.1708. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Elam M, Wallin BG. Firing properties of single postganglionic sympathetic neurones recorded in awake human subjects. Autonomic Neuroscience. 2002;95:146–159. doi: 10.1016/s1566-0702(01)00389-7. [DOI] [PubMed] [Google Scholar]
- Maslov PZ, Breskovic T, Brewer DN, Shoemaker JK, Dujic Z. Recruitment pattern of sympathetic muscle neurons during premature ventricular contractions in heart failure patients and controls. American Journal of Physiology –Regulatory, Integrative and Comparative Physiology. 2012;303:R1157–R1164. doi: 10.1152/ajpregu.00323.2012. [DOI] [PubMed] [Google Scholar]
- Naughton MT, Liu PP, Bernard DC, Goldstein RS, Bradley TD. Treatment of congestive heart failure and Cheyne-Stokes respiration during sleep by continuous positive airway pressure. American Journal of Respiratory and Critical Care Medicine. 1995a;151:92–97. doi: 10.1164/ajrccm.151.1.7812579. [DOI] [PubMed] [Google Scholar]
- Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995b;91:1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- Naughton MT, Floras JS, Rahman MA, Jamal M, Bradley TD. Respiratory correlates of muscle sympathetic nerve activity in heart failure. Clinical Science (London) 1998;95:277–285. [PubMed] [Google Scholar]
- Peters JK, Lister G, Nadel ER, Mack GW. Venous and arterial reflex responses to positive-pressure breathing and lower body negative pressure. Journal of Applied Physiology. 1997;82:1889–1896. doi: 10.1152/jappl.1997.82.6.1889. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Shoemaker JK. Spike detection in human muscle sympathetic nerve activity using a matched wavelet approach. Journal of Neuroscience Methods. 2010;193:343–355. doi: 10.1016/j.jneumeth.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R, Shoemaker JK. Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. Journal of Neurophysiology. 2011;105:2830–2842. doi: 10.1152/jn.00814.2010. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circulation Research. 1990;67:130–141. doi: 10.1161/01.res.67.1.130. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circulation Research. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. Journal of Physiology. 2010;588:4825–4836. doi: 10.1113/jphysiol.2010.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. Journal of Physiology. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Sagawa S, Miki K, Claybaugh JR, Shiraki K. Changes in muscle sympathetic nerve activity and renal function during positive-pressure breathing in humans. American Journal of Physiology. 1994;266:R1220–R1228. doi: 10.1152/ajpregu.1994.266.4.R1220. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Mack G, Nadel ER. Peripheral vascular reflexes elicited during lower body negative pressure. Aviation Space and Environmental Medicine. 1989;60:1187–1193. [PubMed] [Google Scholar]
- Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, Floras JS. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. Journal of the American College of Cardiology. 2005;45:2008–2011. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. Journal of Applied Physiology. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]