Abstract
UDP-glucuronosytransferase-2B10 (UGT2B10) is the primary catalyst of nicotine glucuronidation. To develop a predictive genetic model of nicotine metabolism, the conversion of deuterated (D2)-nicotine to D2-nicotine-glucuronide, D2-cotinine, D2-cotinine-glucuronide, and D2-trans-3'-hydroxycotinine were quantified in 188 European Americans, and the contribution of UGT2B10 genotype to variability in first-pass nicotine glucuronidation assessed, following a procedure previously applied to nicotine C-oxidation. The proportion of total nicotine converted to nicotine-glucuronide (D2-nicotine-glucuronide/ (D2-nicotine +D2-nicotine-glucuronide +D2-cotinine +D2-cotinine-glucuronide +D2-trans-3'-hydroxycotinine)) was the primary phenotype. The variant, rs61750900T (D67Y) (minor allele frequency (MAF) = 10%), is confirmed to abolish nicotine glucuronidation activity. Another variant, rs112561475G (N397D) (MAF = 2%), is significantly associated with enhanced glucuronidation. rs112561475G is the ancestral allele of a well-conserved amino acid, indicating that the majority of human UGT2B10 alleles are derived hypomorphic alleles. CYP2A6 and UGT2B10 genotype explain 53% of the variance in oral nicotine glucuronidation in this sample. CYP2A6 and UGT2B10 genetic variants are also significantly associated with un-deuterated (D0) nicotine glucuronidation in subjects smoking ad libitum. We find no evidence for further common variation markedly influencing hepatic UGT2B10 expression in European Americans.
Keywords: UGT2B10, nicotine, cotinine, metabolism, glucuronidation, CYP2A6
Introduction
Despite reduced prevalence, tobacco use remains the largest cause of preventable mortality in the US [1]. Smoking phenotypes are highly complex, but they are also strongly influenced by heritable factors [2-4]; therefore genetic studies provide a powerful tool to reveal the underlying biology of nicotine dependence and smoking cessation, which may lead to improved treatments. Genetic associations with DNA variants in the chromosomal region 19q13, surrounding the nicotine metabolism gene Cytochrome P450 2A6 (CYP2A6), exemplify both the difficulties and opportunities in identifying further genes and pathways related to nicotine dependence and cessation. 19q13 is among the loci most consistently associated with smoking phenotypes, identified in multiple large genome-wide association studies (GWAS) of cigarette consumption, and related pulmonary disease [5-7]. The region also demonstrated the strongest association in a recent candidate gene study of smoking cessation[8].
In most smokers, the primary pathway of nicotine metabolism is CYP2A6-catalyzed 5'-oxidation [9] (Fig. 1). The iminium ion product of this reaction is further oxidized by either CYP2A6 or aldehyde oxidase to cotinine [10], and cotinine is then further metabolized by CYP2A6 to trans-3′-hydroxycotinine [11]. There are two additional pathways of nicotine metabolism; flavin monooxygenase (FMO)- catalyzed N-oxidation and the UDP-glucuronsyltransferase (UGT)-catalyzed N-glucuronidation (Fig. 1). UGT2B10 is an efficient catalyst of the N-glucuronidation of both nicotine and cotinine [12]. Nicotine glucuronide typically accounts for <10% of a total nicotine dose excreted, but in some smokers the nicotine-glucuronide may account for up to 40% of the excreted dose [13]. Glucuronide metabolites of nicotine and cotinine together account for on average 25% of the nicotine metabolites excreted in urine, ranging between 0.8 and 84%[13, 14]. An O-glucuronide conjugate of trans 3'-hydroxycotinine is excreted, but on average only 20% of the total trans 3'-hydroxycotinine is a glucuronide conjugate [9]. The primary metabolite of nicotine in plasma is cotinine, which is typically present at ten times the concentration of nicotine and 3-4x the concentration of 3'-hydroxycotinine [9]. Plasma levels of nicotine N-glucuronide have not previously been quantified. We have previously quantified cotinine-N-glucuronide indirectly and reported the mean level to be about 10% of the total cotinine [15].
Figure 1.
Nicotine metabolism pathways with primary associated metabolic enzymes.
Because the psychoactive properties of nicotine closely correlate with its plasma concentration, dependent smokers titrate nicotine intake to maintain target nicotine blood levels [16-18]. Therefore, heritable differences in nicotine half-life are likely to explain some of the observed differences in cigarettes per day (CPD) and related behaviors between fast and slow nicotine metabolizers and individuals who differ in CYP2A6 genotype [14, 19-26]. The other pathways of nicotine metabolism may also contribute to smoking behaviors and we have recently found evidence that genetic variation in FMO3, the gene encoding the primary catalyst of nicotine-N-oxidation, is associated with differences in cigarette consumption[27]. Genetic variation in glucuronidation has been suggested to influence individual variability in total nicotine metabolism [28] and ethnic differences in glucuronidation and in UGT2B10 genotype [29] are reported to be associated with differences in cigarette consumption[15]; but with the exception of a non-synonymous variant, rs61750900 (D67Y) [12, 15], common variants in UGT2B10 have not been comprehensively tested. Here we report the results of a controlled nicotine metabolism study performed in 188 European Americans. Nicotine and its metabolites were analyzed in the plasma of smokers and non-smokers orally administered deuterated nicotine to evaluate the effects of UGT2B10 genotype upon nicotine and cotinine glucuronidation. Our prior investigation that explained ~70% of the variation in first-pass nicotine C-oxidation with CYP2A6 haplotype[30] demonstrated this as a robust method to determine the relative activities of different alleles of hepatic enzymes regarding metabolism of specific substrates. The primary aim of this study is to detail the association between UGT2B10 genotype and nicotine glucuronidation by controlling for the indirect effects of CYP2A6 genotype.
Materials and methods
Study Subjects
Participants were recruited from the Collaborative Genetic Study of Nicotine Dependence (COGEND)[31], for an oral nicotine metabolism experiment as previously described[30]. All were self-identified as being of European American ancestry, and race was verified using EIGENSTRAT[32]. Sample demographics were previously described[31, 33]. Subjects were given 2 mg of [3',3'-D2] nicotine (synthesized and purified to >99.4% as described previously[30]) in 4 oz of orange juice. Plasma used in these experiments was collected 240 minutes later and frozen at −20°C until analysis. Subjects who were current smokers were allowed to smoke 2h following the administration of [3',3'-D2] nicotine. The study complies with the Code of Ethics of the World Medical Association and obtained informed consent from participants and approval from the appropriate institutional review boards.
Nicotine Metabolite Measurements
D2-nicotine, D2 -cotinine, D2-trans-3'-hydroxycotinine, non-deuterated (D0)-nicotine, D0-cotinine, and D0-trans-3'-hydroxycotinine were analyzed by liquid chromatography tandem mass spectrometry (LC/MS/MS), as previously described [30, 34]. Plasma concentrations of all but D0-nicotine have been reported previously [30].
For the quantitation of the nicotine and cotinine N-glucuronides, 50 μl plasma was mixed with 450 μl of 1.5 % formic acid in water. Internal standards, nicotine-D3 N-β-D-glucuronide, 2.85 pmol (Toronto Research Chemicals) and cotinine- D3 N-β-D-glucuronide, 1.34 pmol, (Toronto Research Chemicals) were added to each sample. The samples were vortexed and loaded on an Oasis MCX column (10 mg Waters Corporation, Milford, MA) that had been activated by washing with 1 ml methanol then 1ml of 1.5 % formic acid. The columns were washed with 0.5 ml 1.5 % formic acid in water then the glucuronides were eluted with 0.5 ml of 5% ammonium hydroxide in methanol. The eluant was immediately acidified with 5 μl formic acid. Samples were evaporated to dryness under a gentle stream of nitrogen, resuspended in 40 μl of 0.1% TFA in methanol and stored at −20°C until analysis. The LC/MS/MS analysis was similar to that used previously for the quantitation of cotinine [30] except the mobile phase was 80.7% acetonitrile, 18% water, 1.25% formic acid and 0.01% TFA. The mass transitions monitored were as follows: D0-Nicotine glucuronide (m/z 339→163), D2-nicotine glucuronide (m/z 341→165), D3-nicotine glucuronide (m/z 342→166), D0-cotinine glucuronide (m/z 353→177), D2-cotinine glucuronide (m/z 355→179), D3-cotinine glucuronide (m/z 356→180).
The concentrations of the D0 and D2 metabolites were calculated from the ratio of the peak area for each metabolite mass-transition to the peak area of its respective D3-internal standard. The presence of isotopomers of non-deuterated glucuronides with the same nominal mass as the D2-glucuronides result in a contribution to the D2-glucuronide SRM signal from non-deuterated glucuronides present in the sample. Using standards, the percent contribution was determined empirically to be 0.54% for nicotine glucuronide and 0.9% for cotinine-glucuronide. These percentages were used to correct for the contribution of D0-nicotine glucuronide and D0-cotinine glucuronide to the mass transitions monitored for the analysis of D2 nicotine glucuronide and D2-cotinine glucuronide.
The relative recovery for nicotine glucuronide, based on recovery of the internal standard and a standard curve, was 60% to 90%. However, the recovery of cotinine glucuronide was highly variably, and ranged from 0-90%. The mean relative recovery compared to nicotine glucuronide was 42% ± 31% (n=430). For 61 samples the recovery was too low to obtain an accurate value for the cotinine glucuronides. The limit of quantitation was 0.5 pmol/ml for nicotine glucuronide and 1 pmol/ml for cotinine glucuronide (when recovery was >20%). All samples were analyzed in duplicate in sets of 20 with a positive control. Samples were excluded if the recovery of the internal standard was < 20% or if duplicates did not agree within 10%. The positive control was a smoker's plasma from a previous study in which nicotine and cotinine glucuronide concentrations were determined indirectly as the difference in concentration before and after treatment of the sample with base, which hydrolyzes the glucuronide conjugates releasing the aglycon [15]. The concentrations of nicotine glucuronide and of cotinine glucuronide in the positive control samples were 23.9 ± 2.2 and 91.5 ± 17.1 pmol/ml respectively (n=12).
Genotyping
CYP2A6 genotyping was previously described [30]. All variants with minor allele frequency >0.5% in the exons of UGT2B10 were genotyped. rs61750900 was previously determined using a custom designed array as part of a larger study[31]. rs61749966, rs112561475, and rs4694358 were genotyped using the KBioscience Competitive Allele Specific PCR genotyping system (KASPar, KBioscience, Hoddesdon, Herts, UK) following standard procedures with custom designed primers (Supplemental Table 1). KASPar assays were set up as 8μl reactions and measured with the 7900HT Fast Real Time PCR System (Applied Biosytems, Foster City, CA, USA). rs17146905 was genotyped and rs4694358 genotype was confirmed by Sanger Sequencing using PCR primers that flank the UGT2B10 3’ UTR, ( GATGTGATTGGGTTCCTGCT and TTGACAAGGTAGACTTCGAAAGG ). All SNP genotypes conformed to Hardy-Weinberg equilibrium. UGT2B10 copy number was not determined, as copy variation in this gene is reported to be very rare in Europeans (undetected in 180 subjects, i.e. <0.3% frequency[35]). UGT2B10 haplotypes were determined using PHASE version 2.1.1[19, 20].
Statistical Analysis
All statistical analyses (t-tests, linear regression) were performed using the software package ‘R’ (R Foundation for Statistical Computing, Vienna, Austria). Metabolite phenotypes are calculated using measured metabolites in terms of picomoles/ml plasma. For analysis, valid measurements below the limits of detection were assumed to be zero; three of eight subjects with nicotine-glucuronide measurements below the limits of detection were UGT2B10*2 homozygotes. For regression analyses, genetic variables, such as CYP2A6*1A or UGT2B10*2, are treated as number of alleles per subject (0,1 or 2). All CYP2A6 haplotypes are placed into four categories based on statistically significant differences between them regarding metabolism of nicotine to cotinine [30] i.e. 1) the null alleles CYP2A6*2,*4,*12 and *38, 2) CYP2A6*1A, 3) CYP2A6*9 and 4) all other haplotypes, termed normal alleles. All regression analyses are performed on genetic variables relative to the null CYP2A6 category. To display and compare the relative nicotine-glucuronidation among groups of subjects after accounting for CYP2A6 genotype, the residual variance for each subject was determined from the linear model: phenotype ~ (CYP2A6 Normal alleles) + (CYP2A6*1A alleles) + (CYP2A6*9 alleles). The residuals are then displayed for each UGT2B10 diplotype, such as UGT2B10*1/*1 or UGT2B10*1/*2. Direct comparisons between UGT2B10 diplotype groups were also performed on the residuals for each subject. All t-tests performed were two-sided. Measures of statistical significance are not altered to correct for multiple testing.
Allelic expression study
DNA and RNA extracted from de-identified non-cancerous liver biopsy samples were previously described[36]. cDNAs and genomic DNAs for heterozygotes for the assayed SNPs were arrayed together in the same 384-well plate in triplicate, and were run on an ABI-7900 real-time PCR system under standard conditions with custom designed assays for rs4694358 (forward primer: GAAGCTGGAAAACCTGATAGATAGGAA; reverse primer: TGTCACAGGAAGAAAGAAATCTTGCA; reporter 1: TTGCTGGAATCAGCTGAAGT; reporter 2: TGCTGGAATCAACTGAAGT), and rs61750900 (forward primer: TGGCATCTTCAGCTTCCATTCTTTT; reverse primer: TCAGTTTTAGTTAAAGATGTAGGATAAACTTCAAGTT; reporter 1: ATCCCAACGACTCATC; reporter 2: TGATCCCAACTACTCATC). The relative expression of both alleles for each expression marker was determined by subtracting the smaller cycle threshold (Ct, the number of cycles at which PCR products generated exceed a defined threshold) value of one allele PCR reaction from the larger Ct value of the other allele PCR reaction (dCt). dCt values were obtained as an average of two or three reactions for each cDNA sample and were normalized against the overall average ratio obtained for heterozygous gDNAs for each assay.
Results
UGT2B10 polymorphisms and haplotypes
Subjects (n=188) were analyzed for the five exonic polymorphisms with MAF >0.05% in Europeans [37]. A summary of haplotypes defined by these polymorphisms is presented in Table 1.
Table 1.
UGT2B10 Haplotypes
| Haplotype name | rs61749966 (N37N) | rs61750900 (D67Y) | rs112561475 (N481D) | rs17146905 (3’-UTR) | rs4694358 (3’-UTR) | alleles | frequency |
|---|---|---|---|---|---|---|---|
| *1A | T | G | A | A | T | 288 | 76.6% |
| *1B | T | G | A | A | C | 42 | 11.2% |
| *2A | C | T | A | G | T | 36 | 9.6% |
| *3 | T | G | G | A | T | 8 | 2.1% |
| *1C | T | G | A | G | T | 1 | 0.3% |
| *2B | C | T | A | A | T | 1 | 0.3% |
Polymorphic sites analyzed are given at the top of each column by rs number, and further relevant description. Haplotypes are ordered by frequency. For convenient reference in the text, haplotype names are designated by the authors based on previously published nomenclature.
Nicotine metabolite measurements
A method was developed to directly quantify nicotine-N-glucuronide and cotinine-N-glucuronide in plasma. Due to relatively poor recovery of cotinine-N-glucuronide, accurate quantitation of this metabolite was possible in only 126 of the genotyped samples. Plasma concentrations of nicotine, cotinine and their glucuronide conjugates are summarized in Table 2. D2-Nicotine glucuronide was present in plasma at a concentration similar to that of D2-nicotine, whereas D2-cotinine glucuronide on average was present at <4% of the D2-cotinine concentration. In smokers, the mean plasma concentration of D0-nicotine glucuronide was <20% (Table 1) of the D0-nicotine concentration. The mean plasma D0-cotinine glucuronide concentration in smokers was <5% of the cotinine concentration. The difference in D0-cotinine between males and females was not significant after controlling for weight; but the higher levels of cotinine-glucuronide among smokers was significant (p=0.004). Smokers and current non-smokers in this study do not significantly differ in weight (p=0.5)[30].
Table 2.
Plasma concentrations of D2-nicotine, D2-nicotine-glucuronide, D2-cotinine, D2-cotinine-glucuronide, D2-3OH-cotinine, D0-nicotine D0-nicotine-glucuronide, D0-cotinine, D0-cotinine-glucuronide and D0-3OH-cotininea
| D2-nicotine | D2-nicotine-glucuronide | D2-cotinine | D2-cotinine-glucuronide | D2-3OH-cotinine | D0-nicotine | D0-nicotine-glucuronide | D0-cotinine | D0-cotinine-glucuronide | D0-3OH-cotinine | |
|---|---|---|---|---|---|---|---|---|---|---|
| All Subjects (192b/131c) | 5.1 ±3.5 | 3.9 ±2.3 | 136 ±37.4 | 4.6 ±2.6 | 13.7 ±8.9 | |||||
| Males (92/61) | 5.1 ±2.8 | 3.7 ±2.0 | 117±28.5* | 3.9 ±2.4 | 10.0 ±5.4 | |||||
| Females (100/70) | 5.2 ±4.1 | 4.2 ±2.6 | 152 ±36.5* | 5.1 ±2.6 | 17.0 ±10.0 | |||||
| Current smokersd | ||||||||||
| All (103/72) | 5.0 ±3.6 | 4.1 ±2.4** | 133 ±39.2 | 5.1 ±2.9*** | 13.9 ±9.4 | 186 ±86.5 | 30.9 ±31.2 | 1890 ±1080 | 87.9 ±68.6 | 558.3 ±349.8 |
| Male (53/32) | 4.6 ±2.8 | 4.0 ±2.1 | 115 ±29.0 | 4.8 ±2.8 | 10.8 ±6.2 | 196 ±93.6 | 36.1 ±39.5 | 2020 ±1180 | 95.8 ±80.8 | 579.3 ±388.8 |
| Female (50/40) | 5.4 ±4.3 | 4.4 ±2.7 | 150 ±40.4 | 5.4 ±3.0 | 17.1 ±11.0 | 175 ±77.7 | 25.3 ±17.5 | 1750 ±951 | 81.5 ±57.2 | 536.9 ±308.0 |
| Non-current smokers | ||||||||||
| All (88/58) | 5.3 ±3.4 | 3.6 ±2.2** | 139 ±35.2 | 3.9 ±1.9*** | 13.4 ±8.4 | |||||
| Male (38/28) | 5.7 ±2.8 | 3.2 ±1.8 | 118±28.3 | 2.9 ±1.4 | 8.7 ±3.8 | |||||
| Female (50/30) | 4.9 ±3.8 | 4.0 ±2.4 | 154±32.3 | 4.8 ±1.8 | 17.0 ±9.1 | |||||
Measures taken from plasma collected 240 min following the oral administration of deuterated nicotine (D2-nicotine) and analyzed by LC/MS/MS. Values are expressed as means ± standard deviation in pico-moles/ml. trans 3′-hydroxycotinine (3-OHcotinine).
Number of subjects with measures of D2-nicotine, D2-nicotine-glucuronide, D2-cotinine, D2-trans 3′-hydroxycotinine, D0-nicotine-glucuronide, D0-nicotine, D0-cotinine, and D0-trans 3′-hydroxycotinine, includes subjects without genotypes who were not further analyzed.
Number of subjects with measures of D2-cotinine-glucuronide, and D0-cotinine-glucuronide, includes subjects without genotypes who were not further analyzed.
Current smokers includes all subjects with mean undeuterated cotinine > 2 ng/ml, excluding one subject using the nicotine patch.
males vs. females, p = 0.45 corrected for weight
current smokers vs. non-current smokers, p = 0.09
current smokers vs. non-current smokers, p = 0.004
The indirect effect of CYP2A6 genotype upon nicotine glucuronidation
The administration of deuterated nicotine allows for direct measurement of the conversion of nicotine to its metabolites. Although CYP2A6 is not directly involved in glucuronidation, the large majority of nicotine is metabolized to cotinine by CYP2A6 in most smokers, and the activity of parallel metabolism pathways can have indirect effects on substrate: metabolite ratios. Therefore we began by testing the relationship between CYP2A6 genotype and in vivo nicotine glucuronidation.
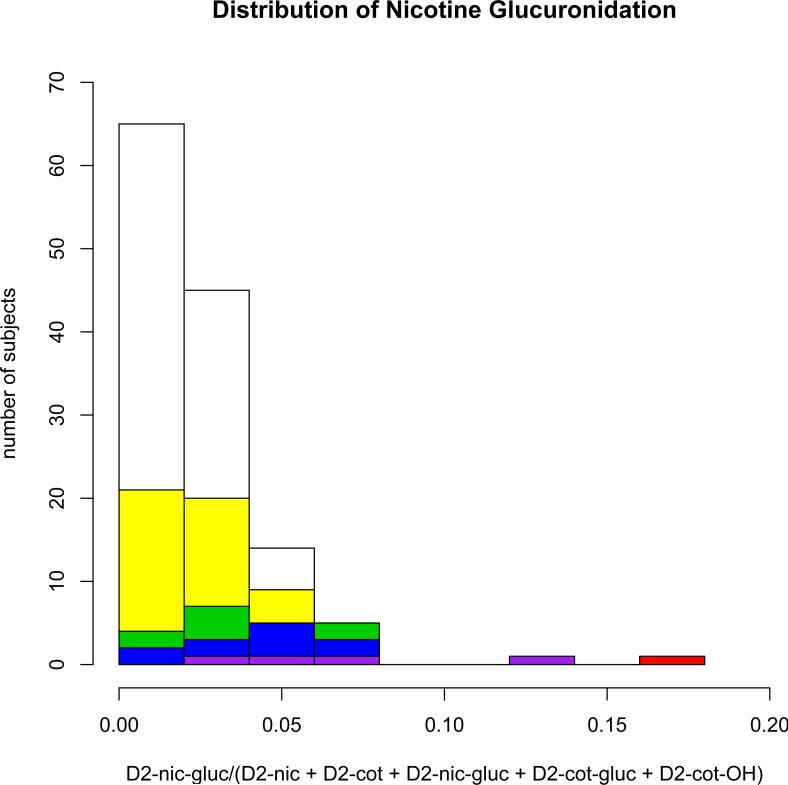
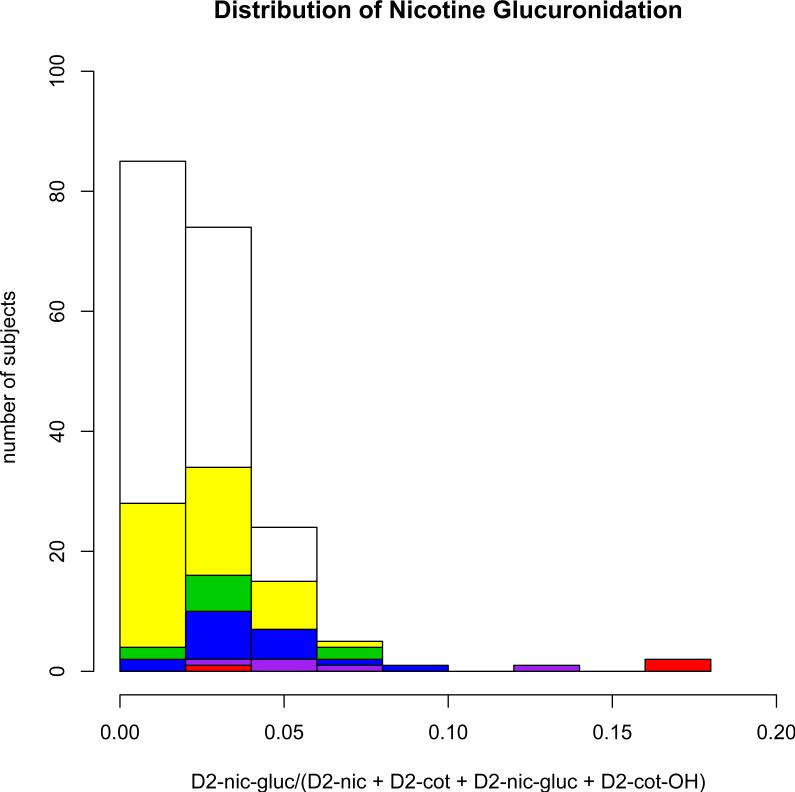
The primary phenotype analyzed here is the fraction of D2-nicotine equivalents converted to D2 nicotine-glucuronide, i.e. D2-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-cotinine-glucuronide + D2-trans-3'-hydroxycotinine) (Figure 2). All results refer to this ratio unless otherwise specified. Additionally, because D2-cotinine-glucuronide measurements were only available for 126 genotyped subjects, to increase power, D2-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-trans-3'-hydroxycotinine) is also analyzed (Figure 3).
Figure 2.
The Distribution of deuterated (D2)-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-cotinine-glucuronide + D2-trans-3'-hydroxycotinine) measured 4 hours after oral D2-nicotine administration among European American subjects displayed as a cumulative histogram color-coded by CYP2A6 diplotype class. White = normal/normal (n=69), Yellow = normal/intermediate (n=34), Green = intermediate/intermediate (n=8), Blue = normal/null (n=10), Purple = intermediate/null (n=4), Red = null/null (n=1). Normal haplotypes are *1B, *1D,*1H and *14. Intermediate haplotypes are *1A and *9. Null haplotypes are *2, *4, *12 and *38. Genotype is not further differentiated.
Figure 3.
The Distribution of deuterated (D2)-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-trans-3'-hydroxycotinine) measured 4 hours after oral D2-nicotine administration among European American subjects displayed as a cumulative histogram color-coded by CYP2A6 diplotype class. White = normal/normal (n=102), Yellow = normal/intermediate (n=51), Green = intermediate/intermediate (n=10), Blue = normal/null (n=17), Purple = intermediate/null (n=5), Red = null/null (n=3). Normal haplotypes are *1B, *1D,*1H and *14. Intermediate haplotypes are *1A and *9. Null haplotypes are *2, *4, *12 and *38. Genotype is not further differentiated.
Our previous analysis of CYP2A6 haplotype and nicotine C-oxidation justified dividing all CYP2A6 alleles in European Americans into four categories with statistically significantly different activities: 1) null alleles CYP2A6*2, *4, *12, and *38, 2) CYP2A6*1A associated with reduced mRNA splicing efficiency [36], 3) CYP2A6*9 associated with lower expression due to variation in the 5’ TATA box [38], and 4) all other fully-active alleles (*1B, *1D, *1H, *14, *1X2). The CYP2A6 null and fully-active allele categories are both highly significantly associated with the nicotine-glucuronide ratio in separate single variable analyses (Table 3). In a combined multivariate model, relative to null CYP2A6 alleles, the other three allele categories are all highly-significantly associated with reduced glucuronidation (Table 3), and combined CYP2A6 genotype alone explains 43% (R2=0.43) of the variance in the nicotine-glucuronide : nicotine equivalents ratio (p=2.4×10−15, n=126, Figure 2). CYP2A6 genotype also explains 37% of the ratio excluding cotinine-glucuronide from the denominator (p<2×10−16, n=183, Figure 3) demonstrating the superior sensitivity of the phenotype that includes the more complete measure of nicotine equivalents.
Table 3.
Variables predicting D2-nicotine glucuronidation individually, and when included in a multivariate regression model
| Phenoytpe | Percent of total D2-nicotine converted to nicotine-glucuronidea (n= 126 subjects/ 252 chromosomes) | Percent of D2-nicotine (excluding cotinine-glucuronide) converted to nicotine-glucuronideb (n= 188 subjects/ 376 chromosomes) | |||||
|---|---|---|---|---|---|---|---|
| nc | Modeled individually | multivariate model | nc | multivariate model | |||
| Variable | R2 | p | Parameter estimate | p | p | ||
| CYP2A6 normal allelesd | 182 | 0.20 | 7.5×10−8 | −0.040 ± 0.004 | <2×10−16 | 272 | <2×10−16 |
| CYP2A6*1A | 37 | 0 | 0.3 | −0.033 ± 0.004 | 7.1×10−12 | 53 | 3.1×10−13 |
| CYP2A6*9 | 17 | 0 | 0.3 | −0.033 ± 0.005 | 5.7×10−9 | 23 | 4.8×10−8 |
| UGT2B10*2 (67Y) | 26 | 0.08 | 4.6×10−4 | −0.014 ± 0.003 | 1.0×10−5 | 39 | 1.1×10−5 |
| UGT2B10*3 (481D) | 6 | 0.02 | 0.07 | 0.014 ± 0.006 | 0.02 | 8 | 0.047 |
| Current smokinge | 72 | 0 | 0.5 | 0.004 ± 0.003 | 0.1 | 103 | 0.045 |
| Total adjusted R2 optimum model | 0.53 | 0.46 | |||||
Results of linear regression analyses treating CYP2A6 null alleles (CYP2A6*2, *4, *12 and *38) and UGT2B10*1 as the reference. Genetic variables are coded as number of haplotypes per subject (0,1,2). Negative parameter estimates indicate reduced nicotine glucuronidation relative to the reference genotype.
Deuterated (D2)-(nicotine-glucuronide / (nicotine + nicotine-glucuronide + cotinine + cotinine-glucuronide + trans-3′-hydroxycotinine))
Deuterated (D2)-(nicotine-glucuronide / (nicotine + nicotine-glucuronide + cotinine + trans-3′-hydroxycotinine))
number of alleles
all CYP2A6 alleles excluding CYP2A6*1A, *9 and assumed null alleles CYP2A6*2, *4, *12 and *38.
Current smoking status as stratified by a mean D0-cot >2ng/ml.
The relationship between nicotine glucuronidation and UGT2B10 haplotype indicates that the large majority of UGT2B10 alleles have reduced function
Four common UGT2B10 haplotypes are defined by the five exonic variants with MAF ≥0.5% in Europeans. In a multivariate analysis with CYP2A6 genotype variables, both UGT2B10 haplotypes defined by the non-synonymous variants, UGT2B10*2 and *3 (Table 1), are significantly associated with altered nicotine glucuronidation (p = 1.0×10−5 and 0.02 respectively, Table 3). The minor allele of rs61750900 (D67Y) is associated with reduced nicotine-glucuronide confirming its previous identification as a UGT2B10 null allele [12]. Indeed, D2-nicotine-glucuronide was below the limits of detection in all measured rs61750900 homozygotes (n=3). By contrast, the minor allele of rs112561475 (N481D) is associated with significantly increased nicotine-glucuronide. Interestingly, the relatively infrequent minor allele (2%) is the ancestral allele [39], and the more common derived allele is predicted to be ‘damaging’ by protein structure conservation predictive tools[40, 41]. Direct comparison also shows that diplotypes including UGT2B10*2 differ significantly from other UGT2B10 genotypes after controlling for CYP2A6 genotype (*2/*2 vs. *1/*1 p<10−16 ; *3/*1 vs. *2/*1 p=0.03; *3/*1 vs. *2/*2 p=0.02; *2/*1 vs. *1/*1 p=1.0×10−4, Figure 4). Together in a multivariate model, the two non-synonymous variants account for 10% (R2=0.10) of the total variance in the nicotine-glucuronide: nicotine equivalents ratio ( D2-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-cotinine-glucuronide + D2-trans-3'-hydroxycotinine) p=3.9x10−4, n=126).
Figure 4.
UGT2B10 diplotypes associated with nicotine glucuronidation (deuterated (D2)-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-cotinine-glucuronide + D2-trans-3'-hydroxycotinine) after accounting for CYP2A6 genotype. Data points are the residual values for each subject after applying the model including three CYP2A6 variables (CYP2A6 normal alleles, CYP2A6*1A, and CYP2A6*9). The boxplots provide summaries of the data distributions for each group of (n) subjects defined by UGT2B10 diplotype. A box represents the interquartile range, which includes 50% of values. The line across the box indicates the median. The whisker lines extend to the highest and lowest values that are within 1.5x the interquartile range. Further outliers are marked with circles.
The second-most common haplotype (UGT2B10*1B), is defined by a variant in the 3’-UTR, rs4694358-C (Table 1). With either nicotine glucuronidation phenotype (Table 3) UGT2B10*1B does not significantly differ from the other reference haplotype (UGT2B10*1A). In multivariate analyses including the CYP2A6 variables, UGT2B10*2 and UGT2B10*3, the parameter estimate for UGT2B10*1B is −0.001 ± 0.003, p=0.7 for the primary phenotype.
We also repeated the multivariate analysis of UGT2B10 haplotypes with the primary phenotype in CYP2A6 normal homozygotes only. In this subset of subjects which are assumed to have near uniform CYP2A6 activity, the UGT2B10*2 allele is also significantly associated with reduced nicotine glucuronidation (estimate= −0.011 ± 0.003, p=7.9×10−5); the association between the UGT2B10*3 allele and glucuronidation is not statistically significant in this group (estimate= 0.006 ± 0.006, p=0.25). Together, the two non-synonymous UGT2B10 variants account for 20% (R2=0.20) of the total variance in the primary phenotype in CYP2A6 normal homozygotes.
CYP2A6 and UGT2B10 genotype together account for the majority of variance in oral nicotine glucuronidation
In a previous investigation of CYP2A6 we identified a weak association between the metabolism of nicotine to cotinine and smoking status, along with a more robust association between faster metabolism and female sex, consistent with prior reports [42-44]. Here we do not find sex significantly associated with nicotine glucuronidation, either treated individually (p=0.64), or in a multivariate model with UGT2B10 and CYP2A6 variables (p=0.45). Current smoking status is not significantly associated with the primary phenotype (p=0.1), although there is a suggestion of an association with greater nicotine glucuronidation using the phenotype D2-nicotine-glucuronide/ (D2-nicotine + D2-nicotine-glucuronide + D2-cotinine + D2-trans-3'-hydroxycotinine) (p=0.045, Table 3). Altogether an optimum multivariate model including genetic variants in UGT2B10 and CYP2A6 with smoking status, explains 53% of the variance in the primary phenotype (R2=0.53 Table 3).
As in our previous investigation of nicotine-C-oxidation [30], to demonstrate the robustness of the parameter estimates in the resultant predictive model of nicotine glucuronidation (Table 3), subjects were stratified by smoking status and the parameter estimates generated from current smokers only (n=72) were used to predict the phenotype in current non-smokers (n=58), and vice-versa, and similarly in males (n=61) and females(n=70). Parameter estimates for CYP2A6 and UGT2B10 (Table 3) variables based on measurements made only in current smokers predicted phenotype in non-current (former) smokers with an adjusted R2 of 0.48, and equivalent estimates in non-current smokers predicted phenotype in current smokers with an adjusted R2 of 0.53. The power of estimates based on each group to predict phenotype in the other indicates that the relative influence of genotypes does not differ between smokers and non-current smokers. Similarly, parameters estimated in males predicted phenotype in females with an adjusted R2 of 0.47, and vice-versa with an adjusted R2 of 0.46.
Variables associated with cotinine glucuronidation and ad libitum nicotine glucuronidation
Cotinine, the primary initial metabolite of nicotine, is also glucuronidated to cotinine-glucuronide, as well as oxidized to trans-3'-hydroxycotinine; prior evidence indicates that the primary catalysts of these conversions are also UGT2B10 and CYP2A6 respectively. We therefore tested those variables significantly associated with deuterated nicotine glucuronidation with regard to a metric of cotinine glucuronidation: D2-cotinine-glucuronide / (D2-cotinine + D2-cotinine-glucuronide + D2-trans-3'-hydroxycotinine). The relative size and direction of the effects of these variables upon cotinine glucuronidation paralleled their effects upon nicotine glucuronidation (Tables 3 & 4). Together, genetic variants in UGT2B10 and CYP2A6, and smoking status explain 15% (R2=0.15 Table 4) of the variance in this cotinine-glucuronidation metric.
Table 4.
Variables predicting D2-cotinine glucuronidationa following oral D2-nicotine administration in a multivariate regression model (n=126 subjects)
| Variable | nb | Parameter estimate | p |
|---|---|---|---|
| CYP2A6 normal allelesc | 182 | −0.012 ± 0.005 | 0.02 |
| CYP2A6*1A | 37 | −0.012 ± 0.006 | 0.04 |
| CYP2A6*9 | 17 | −0.015 ± 0.007 | 0.03 |
| UGT2B10*2 (67Y) | 26 | −0.012 ± 0.004 | 0.003 |
| UGT2B10*3 (481D) | 6 | 0.016 ± 0.008 | 0.06 |
| Current smokingd | 72 | 0.010 ± 0.004 | 0.006 |
| Total adjusted R2 | 0.15 | ||
Results of linear regression analyses treating CYP2A6 null alleles (CYP2A6*2, *4, *12 and *38) and UGT2B10*1 as the reference. Genetic variables are coded as number of haplotypes per subject (0,1,2). Negative parameter estimates indicate reduced nicotine glucuronidation relative to the reference genotype.
Deuterated (D2)-(cotinine-glucuronide / (cotinine + cotinine-glucuronide + trans-3′-hydroxycotinine))
number of alleles
all CYP2A6 alleles excluding CYP2A6*1A, *9 and assumed null alleles CYP2A6*2, *4, *12 and *38.
Current smoking status as stratified by a mean D0-cot >2ng/ml.
To determine the contribution of these variables to nicotine glucuronidation derived from ad libitum smoking we also pursued a metric of nicotine glucuronidation based on the non-deuterated metabolites of nicotine we had measured, i.e. D0-nicotine-glucuronide / (D0-nicotine + D0-nicotine-glucuronide + D0-cotinine + D0-cotinine-glucuronide + D0-trans-3'-hydroxycotinine)). Again, the relative size and direction of the effects of these variables upon ad libitum nicotine glucuronidation paralleled their effects upon oral nicotine glucuronidation (Tables 3 & 5). Altogether, genetic variants explain 37% of the variance (R2=0.37 Table 5).
Table 5.
Variables predicting ad libitum D0-nicotine glucuronidationa in a multivariate regression model in current smokers
| Variable | nb | Parameter estimate | p |
|---|---|---|---|
| CYP2A6 normal allelesc | 88 | −0.009 ± 0.003 | 0.003 |
| CYP2A6*1A | 16 | −0.007 ± 0.003 | 0.02 |
| CYP2A6*9 | 6 | −0.007 ± 0.004 | 0.09 |
| UGT2B10*2 (67Y) | 11 | −0.007 ± 0.002 | 0.0007 |
| UGT2B10*3 (481D) | 2 | 0.012 ± 0.005 | 0.02 |
| Total adjusted R2 | 0.37 | ||
Results of linear regression analyses treating CYP2A6 null alleles (CYP2A6*2, *4, *12 and *38) and UGT2B10*1 as the reference. Genetic variables are coded as number of haplotypes per subject (0,1,2). Negative parameter estimates indicate reduced nicotine glucuronidation relative to the reference genotype.
Non-deuterated (D0)-(nicotine-glucuronide / (nicotine-glucuronide + nicotine + cotinine + cotinine-glucuronide + trans-3′-hydroxycotinine))
number of alleles
all CYP2A6 alleles excluding CYP2A6*1A, *9 and assumed null alleles CYP2A6*2, *4, *12 and *38.
Differences in allelic mRNA expression among UGT2B10 haplotypes
Allele-specific gene expression assays performed in heterozygous tissues can robustly determine the relative mRNA expression levels of two alleles by avoiding confounding factors that affect total gene expression such as sample quality, diet or disease. Thus the relative expression of different alleles may be demonstrated in fairly small numbers of subjects heterozygous for assayable exonic SNPs. We were interested to find evidence of further common polymorphism in the UGT2B10 locus that might influence gene function by mechanisms unrelated to the amino acid sequence, i.e. differences in mRNA transcription, splicing efficiency, or stability. Therefore ninety-nine European American liver samples were genotyped for the SNPs rs4694358 and rs61750900 (Table 1). Among these individuals, we identified nineteen rs4694358 heterozygotes and fifteen rs61750900 heterozygotes. None were heterozygous for both SNPs. In both groups, TaqMan allelic expression assays revealed relatively little heterogeneity among differences in relative allelic expression; the total range of dCt (the difference in the number of cycles at which PCR products generated from each allele exceed a defined threshold) was 0.49 for rs4694358 and 0.72 for rs61750900, corresponding to 1.4 and 1.6-fold differences in mRNA levels respectively (Figure 5). For comparison, in a recent study in which we confirmed differences in allelic expression among CYP2A6 haplotypes associated with a modest difference in nicotine metabolism, the range of dCt equaled 2.1, or a 4.3-fold difference in mRNA levels[36]. Therefore, we conclude that there is no evidence for further common variation in the UGT2B10 locus in European Americans associated with functionally-relevant differences in UGT2B10 allelic mRNA expression.
Figure 5.
UGT2B10 relative allelic expression in rs4694358 (n=19) and rs61750900 (n=15) heterozygous liver cDNAs. Data points are the difference in Ct (the number of cycles at which products generated exceed a defined threshold) between PCR reactions for one allele minus the other normalized against the average ratio obtained for all heterozygous gDNAs for each assay. 0.0 equals no difference.
Discussion
Variation in nicotine metabolism and nicotine metabolism genes has been associated with differences in cigarette consumption [15, 19, 22, 27, 45], smoking cessation [26] and liability for smoking-related disease [7, 46]. Although the majority of nicotine metabolism in most smokers is catalyzed by one enzyme, CYP2A6, there is evidence that other nicotine metabolism pathways may significantly impact smoking behavior[15, 27]. Nicotine glucuronidation has been shown to vary widely among subjects as well as among ethnic groups [13, 29, 47]. Therefore, to assess the contribution of nicotine glucuronidation to total nicotine metabolism, we quantified the plasma concentration of the glucuronide conjugates of both nicotine and cotinine in subjects who had been administered D2-nicotine. These subjects have been previously analyzed for CYP2A6 genotype and CYP2A6-generated nicotine metabolites [30].
The glucuronide conjugates of cotinine and nicotine have been quantified in the urine of smokers in many studies [13, 15, 28, 30]. However, in all cases quantitation was by an indirect method, which determines nicotine and cotinine concentrations before and after treatment with β-glucuronidase. The same method has been used to measure plasma cotinine glucuronide concentrations in two studies [15, 48]. In those studies about 10% of plasma cotinine was conjugated, compared to the 4-5% reported here for D2 and D0 cotinine. In contrast, the glucuronide metabolite accounted for more than 40% of the total D2-nicotine and 15% of the D0-nicotine. Although, the plasma concentration of nicotine glucuronide is significantly lower than the concentration of the products of CYP2A6-catalyzed metabolism, cotinine and trans 3'-hydroxycotinine, it accounts for a significant proportion of the total circulating nicotine and might serve as a reservoir for free nicotine.
Our analyses demonstrate that the majority of variation in nicotine glucuronidation in European Americans following oral nicotine administration can be predicted by a few non-synonymous polymorphisms in the UGT2B10 gene, in addition to the large indirect influence of variation in the CYP2A6 gene. This study focused on variants in the UGT2B10 exons. Non-exonic variants that might alter UGT2B10 gene function were not investigated, but the small differences in allelic expression among common haplotypes demonstrated from human liver derived cDNAs indicate that it is unlikely that further common variants occur among Europeans with a marked impact upon UGT2B10 function. Undetectable levels of glucuronidated nicotine among the UGT2B10*2 (D67Y) homozygotes in this study also strongly indicates that other UGT enzymes do not significantly contribute to hepatic nicotine glucuronidation, despite the modest reported in vitro nicotine metabolism activities of other UGTs [49, 50].
Our results confirm prior evidence that UGT2B10*2 (D67Y) is a null allele lacking all nicotine glucuronidation activity. A surprising result of this study however is the apparently greater activity of the infrequent (MAF = 2%) UGT2B10*3 (N481D) allele when compared to the UGT2B10 reference allele previously assumed to possess full activity. Sequence similarity in primates and the high conservation of amino acid 481 across other species indicates that the less-frequent (MAF = 2%) *3 allele is in fact the ancestral allele despite the fact that the hypomorphic reference allele comprises the vast majority of UGT2B10 alleles in both European and African Americans [37]. Examples exist of metabolism enzyme gene variants detrimental to enzymatic function that have become ubiquitous in some but not all ethnic groups [51, 52]; this allele may serve as an example of a ‘damaging’ mutation that has come to predominate in most ethnic groups without reaching fixation.
This is the first study to investigate nicotine glucuronidation using orally-administered deuterated nicotine in both smoking and smoking-abstinent subjects. The effect of current smoking upon the parallel nicotine metabolism pathway, C-oxidation, has been controversial, with published evidence supporting both inductive [53, 54] and inhibitory effects [55]. Our prior investigation of nicotine C-oxidation in this sample indicated a clinically insignificant induction of nicotine C-oxidation associated with current smoking [30]. Here we detect a potential weak induction of nicotine glucuronidation and a relatively robust induction of cotinine glucuronidation associated with current smoking. CYP2A6 activity is also reportedly induced by estrogen[56], and this is believed to explain the consistent difference in nicotine C-oxidation between men and women[42-44]. We find no evidence for a similar sex difference in nicotine glucuronidation efficiency.
To our knowledge, this is the first study to examine the influence of genetic variation upon metabolism that includes polymorphisms in genes contributing to two metabolic pathways competing for the same substrate and utilizing direct measurement of both relevant metabolites. The comprehensiveness of this analysis is key because, although the effects of UGT2B10 haplotypes upon nicotine metabolism appear to be robust, the majority of variation in nicotine glucuronidation is still explained by polymorphism in a gene, CYP2A6, that does not encode a glucuronosytransferase enzyme. In light of this large indirect influence upon levels of measurable glucuronidated nicotine metabolites, it would be difficult to properly assess the association between nicotine-glucuronidation and variation in UGT2B10 without accounting for CYP2A6 genotype, and statistical power to detect the effects of infrequent alleles like UGT2B10*3 would be greatly reduced. Overall our results demonstrate the value of thoroughly determining the contribution of genotype to functional variation in one gene or pathway to act as a covariate to detect the effects of further genetic variation upon another related pathway.
Supplementary Material
Acknowledgments
The authors wish to thank and mention the following: Investigators directing data collection for COGEND are Laura Bierut, Naomi Breslau, Dorothy Hatsukami, and Eric Johnson; data management is organized by Nancy Saccone and John Rice; laboratory analyses are led by Alison Goate; data collection was supervised by Tracey Richmond. Katherine Wickham and Nicole Thomas (University of Minnesota) assisted in the analysis of nicotine metabolites. LC/MS/MS analysis was carried out in the Analytical Biochemistry Core of the University of Minnesota Cancer Center, supported in part by CA-77598.
Sources of support: the National Institute of Mental Health (5T32MH014677-33 to AJB); and the National Cancer Institute (P01 CA-089392 to LJB); and the National Institute on Drug Abuse (K02 DA-021237 to LJB) and the NIH grant from the National Cancer Institute (P01 CA-89392 to SEM).
Footnotes
Disclosures: Drs. Goate and Bierut are listed as inventors on a patent (US 20070258898) covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–7. doi: 10.1080/14622299050011811. discussion S69-70. [DOI] [PubMed] [Google Scholar]
- 3.Li MD. The genetics of smoking related behavior: a brief review. Am J Med Sci. 2003;326:168–73. doi: 10.1097/00000441-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:383–93. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 5.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, et al. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet. 2012;21:947–57. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37:641–50. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 10.von Weymarn LB, Brown KM, Murphy SE. Inactivation of CYP2A6 and CYP2A13 during nicotine metabolism. J Pharmacol Exp Ther. 2006;316:295–303. doi: 10.1124/jpet.105.091306. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3'-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277:1010–5. [PubMed] [Google Scholar]
- 12.Chen G, Blevins-Primeau AS, Dellinger RW, Muscat JE, Lazarus P. Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 Codon 67 (Asp>Tyr) polymorphism. Cancer Res. 2007;67:9024–9. doi: 10.1158/0008-5472.CAN-07-2245. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SE, Link CA, Jensen J, Le C, Puumala SS, Hecht SS, et al. A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:1617–23. [PubMed] [Google Scholar]
- 14.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicoticomparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 15.Berg JZ, von Weymarn LB, Thompson EA, Wickham KM, Weisensel NA, Hatsukami DK, et al. UGT2B10 genotype influences nicotine glucuronidation, oxidation, and consumption. Cancer Epidemiol Biomarkers Prev. 2010;19:1423–31. doi: 10.1158/1055-9965.EPI-09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. 2000;66:553–8. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 17.Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86:294–300. doi: 10.1016/j.drugalcdep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. J Natl Cancer Inst. 2004;96:844–52. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 19.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Ho MK, Tyndale RF. Overview of the pharmacogenomics of cigarette smoking. Pharmacogenomics J. 2007;7:81–98. doi: 10.1038/sj.tpj.6500436. [DOI] [PubMed] [Google Scholar]
- 21.Malaiyandi V, Goodz SD, Sellers EM, Tyndale RF. CYP2A6 genotype, phenotype, and the use of nicotine metabolites as biomarkers during ad libitum smoking. Cancer Epidemiol Biomarkers Prev. 2006;15:1812–9. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 22.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol Psychiatry. 2006;11:400–9. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–4. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 24.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–7. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 26.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloom AJ, Murphy SE, Martinez M, von Weymarn LB, Bierut LJ, Goate A. Effects upon in-vivo nicotine metabolism reveal functional variation in FMO3 associated with cigarette consumption. Pharmacogenet Genomics. 2013;23:62–8. doi: 10.1097/FPC.0b013e32835c3b48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lessov-Schlaggar CN, Benowitz NL, Jacob P, Swan GE. Genetic influences on individual differences in nicotine glucuronidation. Twin Res Hum Genet. 2009;12:507–13. doi: 10.1375/twin.12.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg JZ, Mason J, Boettcher AJ, Hatsukami DK, Murphy SE. Nicotine metabolism in African Americans and European Americans: variation in glucuronidation by ethnicity and UGT2B10 haplotype. J Pharmacol Exp Ther. 2010;332:202–9. doi: 10.1124/jpet.109.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom J, Hinrichs AL, Wang JC, von Weymarn LB, Kharasch ED, Bierut LJ, et al. The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics. 2011;21:403–16. doi: 10.1097/FPC.0b013e328346e8c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–56. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3',3'-d(2)]-nicotine and [3',3'-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:1–8. doi: 10.1016/j.jchromb.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, et al. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–12. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloom AJ, Harari O, Martinez M, Zhang X, McDonald SA, Murphy SE, et al. A compensatory effect upon splicing results in normal function of the CYP2A6*14 allele. Pharmacogenet Genomics. 2013 doi: 10.1097/FPC.0b013e32835caf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.(ESP) NGESP Exome Variant Server.
- 38.Yoshida R, Nakajima M, Nishimura K, Tokudome S, Kwon JT, Yokoi T. Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6*9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin Pharmacol Ther. 2003;74:69–76. doi: 10.1016/S0009-9236(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 39.Meyer LR, Zweig AS, Hinrichs AS, Karolchik D, Kuhn RM, Wong M, et al. The UCSC Genome Browser database: extensions and updates 2013. Nucleic Acids Res. 2013;41:D64–9. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 41.Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–7. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]
- 42.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–8. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–30. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Kandel DB, Hu MC, Schaffran C, Udry JR, Benowitz NL. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol. 2007;165:901–10. doi: 10.1093/aje/kwm010. [DOI] [PubMed] [Google Scholar]
- 45.Minematsu N, Nakamura H, Furuuchi M, Nakajima T, Takahashi S, Tateno H, et al. Limitation of cigarette consumption by CYP2A6*4, *7 and *9 polymorphisms. Eur Respir J. 2006;27:289–92. doi: 10.1183/09031936.06.00056305. [DOI] [PubMed] [Google Scholar]
- 46.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291:1196–203. [PubMed] [Google Scholar]
- 48.de Leon J, Diaz FJ, Rogers T, Browne D, Dinsmore L, Ghosheh OH, et al. Total cotinine in plasma: a stable biomarker for exposure to tobacco smoke. J Clin Psychopharmacol. 2002;22:496–501. doi: 10.1097/00004714-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 49.Kuehl GE, Murphy SE. N-glucuronidation of nicotine and cotinine by human liver microsomes and heterologously expressed UDP-glucuronosyltransferases. Drug Metab Dispos. 2003;31:1361–8. doi: 10.1124/dmd.31.11.1361. [DOI] [PubMed] [Google Scholar]
- 50.Kaivosaari S, Toivonen P, Hesse LM, Koskinen M, Court MH, Finel M. Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol Pharmacol. 2007;72:761–8. doi: 10.1124/mol.107.037093. [DOI] [PubMed] [Google Scholar]
- 51.Veeramah KR, Thomas MG, Weale ME, Zeitlyn D, Tarekegn A, Bekele E, et al. The potentially deleterious functional variant flavin-containing monooxygenase 2*1 is at high frequency throughout sub-Saharan Africa. Pharmacogenet Genomics. 2008;18:877–86. doi: 10.1097/FPC.0b013e3283097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whetstine JR, Yueh MF, McCarver DG, Williams DE, Park CS, Kang JH, et al. Ethnic differences in human flavin-containing monooxygenase 2 (FMO2) polymorphisms: detection of expressed protein in African-Americans. Toxicol Appl Pharmacol. 2000;168:216–24. doi: 10.1006/taap.2000.9050. [DOI] [PubMed] [Google Scholar]
- 53.Haley NJ, Sepkovic DW, Hoffmann D. Elimination of cotinine from body fluids: disposition in smokers and nonsmokers. Am J Public Health. 1989;79:1046–8. doi: 10.2105/ajph.79.8.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kyerematen GA, Morgan ML, Chattopadhyay B, deBethizy JD, Vesell ES. Disposition of nicotine and eight metabolites in smokers and nonsmokers: identification in smokers of two metabolites that are longer lived than cotinine. Clin Pharmacol Ther. 1990;48:641–51. doi: 10.1038/clpt.1990.208. [DOI] [PubMed] [Google Scholar]
- 55.Benowitz NL, Jacob P., 3rd Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin Pharmacol Ther. 1993;53:316–23. doi: 10.1038/clpt.1993.27. [DOI] [PubMed] [Google Scholar]
- 56.Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935–41. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.