Abstract
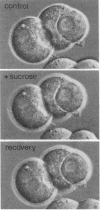
Hepatocyte couplets were isolated by collagenase perfusion from rat liver. Between adjacent cells, the bile canaliculus forms a closed space into which secretion occurs. As in intact liver, Mg2+-ATPase is localized at the canalicular lumen, the organic anion fluorescein is excreted, and secretion is modified by osmotic gradients. By passing a microelectrode through one cell into the canalicular vacuole, a transepithelial potential profile was obtained. In 27 cell couplets the steady-state intracellular (-26.3 +/- 5.3 mV) and intracanalicular (-5.9 +/- 3.3 mV) potentials were recorded at 37 degrees C with reference to the external medium. Input resistances were determined within the cell (86 +/- 23 M omega) and in the bile canalicular lumen (32 +/- 17 M omega) by passing current pulses through the microelectrode. These data define electrical driving forces for ion transport across the sinusoidal, canalicular, and paracellular barriers and indicate ion permeation across a leaky paracellular junctional pathway. These findings indicate that the isolated hepatocyte couplet is an effective model for electrophysiologic studies of bile secretory function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth C. A., Schwarz L. R. Transcellular transport of fluorescein in hepatocyte monolayers: evidence for functional polarity of cells in culture. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4985–4987. doi: 10.1073/pnas.79.16.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H. J., Boyer J. L. Bile salts: a determinant of the bile-peritoneal electrical potential difference in the rat. Gastroenterology. 1973 Dec;65(6):943–948. [PubMed] [Google Scholar]
- Blatt M. R., Slayman C. L. KCl leakage from microelectrodes and its impact on the membrane parameters of a nonexcitable cell. J Membr Biol. 1983;72(3):223–234. doi: 10.1007/BF01870589. [DOI] [PubMed] [Google Scholar]
- Blitzer B. L., Ratoosh S. L., Donovan C. B., Boyer J. L. Effects of inhibitors of Na+-coupled ion transport on bile acid uptake by isolated rat hepatocytes. Am J Physiol. 1982 Jul;243(1):G48–G53. doi: 10.1152/ajpgi.1982.243.1.G48. [DOI] [PubMed] [Google Scholar]
- Boyer J. L. New concepts of mechanisms of hepatocyte bile formation. Physiol Rev. 1980 Apr;60(2):303–326. doi: 10.1152/physrev.1980.60.2.303. [DOI] [PubMed] [Google Scholar]
- Duffy M. C., Blitzer B. L., Boyer J. L. Direct determination of the driving forces for taurocholate uptake into rat liver plasma membrane vesicles. J Clin Invest. 1983 Oct;72(4):1470–1481. doi: 10.1172/JCI111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSNER E., NOVIKOFF A. B., MASEK B. Adenosinetriphosphatase and 5-nucleotidase activities in the plasma membrane of liver cells as revealed by electron microscopy. J Biophys Biochem Cytol. 1958 Nov 25;4(6):711–716. doi: 10.1083/jcb.4.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J. Canalicular bile salt-independent bile formation: concepts and clues from electrolyte transport in rat liver. Am J Physiol. 1983 Mar;244(3):G233–G246. doi: 10.1152/ajpgi.1983.244.3.G233. [DOI] [PubMed] [Google Scholar]
- Graf J., Giebisch G. Intracellular sodium activity and sodium transport in necturus gallbladder epithelium. J Membr Biol. 1979 Jun 7;47(4):327–355. doi: 10.1007/BF01869743. [DOI] [PubMed] [Google Scholar]
- Graf J., Petersen O. H. Cell membrane potential and resistance in liver. J Physiol. 1978 Nov;284:105–126. doi: 10.1113/jphysiol.1978.sp012530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Arias I. M. Taurocholate transport by rat liver sinusoidal membrane vesicles: evidence of sodium cotransport. Hepatology. 1982 Sep-Oct;2(5):572–579. doi: 10.1002/hep.1840020510. [DOI] [PubMed] [Google Scholar]
- LEIBOVITZ A. THE GROWTH AND MAINTENANCE OF TISSUE-CELL CULTURES IN FREE GAS EXCHANGE WITH THE ATMOSPHERE. Am J Hyg. 1963 Sep;78:173–180. doi: 10.1093/oxfordjournals.aje.a120336. [DOI] [PubMed] [Google Scholar]
- Layden T. J., Elias E., Boyer J. L. Bile formation in the rat: the role of the paracellular shunt pathway. J Clin Invest. 1978 Dec;62(6):1375–1385. doi: 10.1172/JCI109258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London C. D., Diamond J. M., Brooks F. P. Electrical potential differences in the biliary tree. Biochim Biophys Acta. 1968 Apr 29;150(3):509–517. doi: 10.1016/0005-2736(68)90151-x. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., HAUSMAN D. H., PODBER E. The localization of adenosine triphosphatase in liver: in situ staining and cell fractionation studies. J Histochem Cytochem. 1958 Jan;6(1):61–71. doi: 10.1177/6.1.61. [DOI] [PubMed] [Google Scholar]
- Oshio C., Phillips M. J. Contractility of bile canaliculi: implications for liver function. Science. 1981 May 29;212(4498):1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. The effect of glucagon on the liver cell membrane potential. J Physiol. 1974 Jun;239(3):647–656. doi: 10.1113/jphysiol.1974.sp010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Oshio C., Miyairi M., Katz H., Smith C. R. A study of bile canalicular contractions in isolated hepatocytes. Hepatology. 1982 Nov-Dec;2(6):763–768. doi: 10.1002/hep.1840020603. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Van Amelsvoort J. M., Van Dam K. Amino acid transport in plasma-membrane vesicles from rat liver. Characterization of L-alanine transport. Eur J Biochem. 1980 Apr;105(2):217–224. doi: 10.1111/j.1432-1033.1980.tb04492.x. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Putney J. W., Jr Does calcium mediate the increase in potassium permeability due to phenylephrine or angiotensin II in the liver? J Pharmacol Exp Ther. 1978 Dec;207(3):669–676. [PubMed] [Google Scholar]
- Wondergem R. Transmembrane potential of rat hepatocytes in primary monolayer culture. Am J Physiol. 1981 Nov;241(5):C209–C214. doi: 10.1152/ajpcell.1981.241.5.C209. [DOI] [PubMed] [Google Scholar]