Abstract
Translation of the isoleucine codon AUA in most prokaryotes requires a modified C (lysidine or agmatidine) at the wobble position of tRNA2Ile to base pair specifically with the A of the AUA codon but not with the G of AUG. Recently, a Bacillus subtilis strain was isolated in which the essential gene encoding tRNAIle-lysidine synthetase was deleted for the first time. In such a strain, C34 at the wobble position of tRNA2Ile is expected to remain unmodified and cells depend on a mutant suppressor tRNA derived from tRNA1Ile, in which G34 has been changed to U34. An important question, therefore, is how U34 base pairs with A without also base pairing with G. Here, we show (i) that unlike U34 at the wobble position of all B. subtilis tRNAs of known sequence, U34 in the mutant tRNA is not modified, and (ii) that the mutant tRNA binds strongly to the AUA codon on B. subtilis ribosomes but only weakly to AUG. These in vitro data explain why the suppressor strain displays only a low level of misreading AUG codons in vivo and, as shown here, grows at a rate comparable to that of the wild-type strain.
INTRODUCTION
The genetic code consists of 16 four-codon boxes in which the four codons in a box differ from one another in the 3′ terminal nucleotide. In 14 of the 16 boxes, all four codons either specify the same amino acid or are split into two sets of two codons; those ending in pyrimidines specifying one amino acid and those ending in purines specifying a different amino acid (1,2). The Wobble hypothesis of Crick proposes how a single tRNA with G in the first position of the anticodon (also called the wobble base) can read codons ending in U or C and how a tRNA with U (or a modified U) can read codons ending in A or G (3–5). The AUN codon box specifying isoleucine and methionine is unique in that three of the four codons, AUU, AUC and AUA, specify isoleucine, whereas the fourth codon, AUG, specifies methionine. This organization raises the question of how the AUA codon is read by an isoleucine tRNA without also reading the AUG codon for methionine.
The strategy used by various organisms to read isoleucine codons is kingdom-specific. Most eukaryotic cells contain two isoleucine tRNAs, the one with the anticodon IAU (tRNA ; I = inosine) reads all three isoleucine codons following the Wobble hypothesis (3), whereas the other with the anticodon ψAψ (tRNA
; I = inosine) reads all three isoleucine codons following the Wobble hypothesis (3), whereas the other with the anticodon ψAψ (tRNA ; ψ = pseudouridine) is thought to read only AUA (6). A possible explanation for the presence of two tRNAs which can read AUA in eukaryotes is inefficient decoding of AUA by tRNA
; ψ = pseudouridine) is thought to read only AUA (6). A possible explanation for the presence of two tRNAs which can read AUA in eukaryotes is inefficient decoding of AUA by tRNA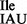 (7,8). Prokaryotes, which also contain two isoleucine tRNAs, have, however, evolved a different strategy for reading the three isoleucine codons. In most bacteria and archaea, a tRNA with the anticodon GAU (tRNA1
(7,8). Prokaryotes, which also contain two isoleucine tRNAs, have, however, evolved a different strategy for reading the three isoleucine codons. In most bacteria and archaea, a tRNA with the anticodon GAU (tRNA1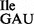 ), reads two of the isoleucine codons (AUU and AUC) following the Wobble hypothesis, whereas another tRNA with the anticodon C*AU reads the third isoleucine codon AUA. C* is derived from C and has been identified as lysidine in bacterial isoleucine tRNA (tRNA2
), reads two of the isoleucine codons (AUU and AUC) following the Wobble hypothesis, whereas another tRNA with the anticodon C*AU reads the third isoleucine codon AUA. C* is derived from C and has been identified as lysidine in bacterial isoleucine tRNA (tRNA2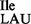 ; L = lysidine) (9,10) and agmatidine in archaeal isoleucine tRNA (tRNA2
; L = lysidine) (9,10) and agmatidine in archaeal isoleucine tRNA (tRNA2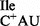 ; C+ = agmatidine) (11–13). In both cases, an amino acid, lysine (in bacteria) and a decarboxylated arginine (in archaea), replaces the C2-oxo group of C34, the wobble base. The modification of C34 to lysidine or agmatidine in tRNA2Ile results in a dual specificity switch of the tRNA in aminoacylation and in codon binding: while the unmodified tRNA with C34 is aminoacylated in vitro with methionine by methionyl-tRNA synthetase (MetRS) and reads the AUG codon, the modified tRNA is aminoacylated with isoleucine by isoleucyl-tRNA synthetase (IleRS) and reads the AUA codon (14–16).
; C+ = agmatidine) (11–13). In both cases, an amino acid, lysine (in bacteria) and a decarboxylated arginine (in archaea), replaces the C2-oxo group of C34, the wobble base. The modification of C34 to lysidine or agmatidine in tRNA2Ile results in a dual specificity switch of the tRNA in aminoacylation and in codon binding: while the unmodified tRNA with C34 is aminoacylated in vitro with methionine by methionyl-tRNA synthetase (MetRS) and reads the AUG codon, the modified tRNA is aminoacylated with isoleucine by isoleucyl-tRNA synthetase (IleRS) and reads the AUA codon (14–16).
Why have bacteria and archaea evolved a mechanism to use an isoleucine tRNA with a modified C34 in the anticodon to exclusively base pair with A instead of using an isoleucine tRNA with U34? Is it because a tRNA which contains U or a modified U in the wobble position cannot read the AUA codon without also misreading the AUG codon (4)? A possible answer to these questions could come from the analysis of codon recognition properties of isoleucine tRNAs from the very few bacterial and archaeal species, whose genomes encode an isoleucine tRNA with the anticodon UAU (tRNA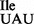 ) but not C*AU, such as Nanoarchaeum equitans, Korarchaeum sp., Mycoplasma mobile, Bifidobacterium adolescentis, Neorickettsia sennetsu and others [summarized in (17)]. These selected organisms are also distinguished by the absence of genes encoding tRNAIle-lysidine synthetase (TilS) in bacteria or tRNAIle-agmatidine synthetase (TiaS) in archaea, responsible for the biosynthesis of lysidine or agmatidine, respectively.
) but not C*AU, such as Nanoarchaeum equitans, Korarchaeum sp., Mycoplasma mobile, Bifidobacterium adolescentis, Neorickettsia sennetsu and others [summarized in (17)]. These selected organisms are also distinguished by the absence of genes encoding tRNAIle-lysidine synthetase (TilS) in bacteria or tRNAIle-agmatidine synthetase (TiaS) in archaea, responsible for the biosynthesis of lysidine or agmatidine, respectively.
The recent isolation of a mutant tRNA1Ile gene in Bacillus subtilis in which the anticodon sequence GAT has been mutated to TAT (17) has provided us with the opportunity to study the properties, including the codon binding properties, of an isoleucine tRNA carrying U in the wobble position. This mutant tRNA, henceforth called mutant tRNA1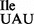 , was isolated as a suppressor in a B. subtilis strain in which the gene encoding TilS had been deleted. In the absence of TilS, the wobble base of the isoleucine tRNA containing the CAU anticodon is expected to remain unmodified and cells depend on the mutant tRNA1
, was isolated as a suppressor in a B. subtilis strain in which the gene encoding TilS had been deleted. In the absence of TilS, the wobble base of the isoleucine tRNA containing the CAU anticodon is expected to remain unmodified and cells depend on the mutant tRNA1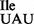 for translation of the AUA codon. The availability of B. subtilis strains carrying the suppressor mutation in the isoleucine tRNA gene has allowed us to investigate (i) whether U34 in the mutant tRNA is modified, and (ii) whether the mutant tRNA is specific for AUA or also misreads AUG.
for translation of the AUA codon. The availability of B. subtilis strains carrying the suppressor mutation in the isoleucine tRNA gene has allowed us to investigate (i) whether U34 in the mutant tRNA is modified, and (ii) whether the mutant tRNA is specific for AUA or also misreads AUG.
Here, we describe the characterization of two B. subtilis mutant strains lacking tilS and carrying the suppressor tRNA genes. We show that one of the mutant strains grows at a rate comparable to that of the wild-type strain. We also describe the purification and analysis of tRNA1Ile from this B. subtilis mutant strain. We have used biochemical and mass spectroscopic analyses to show that U34 in the wobble position of the mutant tRNA1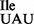 is not modified. We also show that this mutant tRNA binds well to the AUA codon on B. subtilis ribosomes but binds only weakly to AUG, suggesting that it has an inherently low potential of misreading the AUG codon and of incorporating isoleucine in place of methionine. The B. subtilis strains deleted of tilS (17) used in this work represent the only examples of deletion of this essential gene. Therefore, in parallel, we have also purified and analyzed tRNA2Ile from one of the B. subtilis strains lacking tilS to confirm that it lacks the C34 to L34 base modification in the anticodon and has the expected aminoacylation and codon binding properties.
is not modified. We also show that this mutant tRNA binds well to the AUA codon on B. subtilis ribosomes but binds only weakly to AUG, suggesting that it has an inherently low potential of misreading the AUG codon and of incorporating isoleucine in place of methionine. The B. subtilis strains deleted of tilS (17) used in this work represent the only examples of deletion of this essential gene. Therefore, in parallel, we have also purified and analyzed tRNA2Ile from one of the B. subtilis strains lacking tilS to confirm that it lacks the C34 to L34 base modification in the anticodon and has the expected aminoacylation and codon binding properties.
MATERIALS AND METHODS
Isolation of total tRNA from B. subtilis
Bacillus subtilis strains were grown in LB medium at 37°C with aeration; cells were harvested by centrifugation at an OD600 of 1.5–1.8 and immediately used for RNA isolation. All steps were carried out at 4°C unless otherwise noted. Bacillus subtilis cells from a 3 L-culture were pelleted and resuspended in 90 ml of extraction buffer [1 mM Tris–HCl or 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) pH 7.5, 10 mM Mg(OAc)2]. A total of 100 ml of acid phenol (Ambion) was added and the suspension was placed on a nutator for 30 min. The mixture was centrifuged for 30 min at 10 000g, the aqueous layer was adjusted to 1 M lithium chloride and left on ice for 2 h. After centrifugation (30 min, 10 000g), tRNAs were recovered from the supernatant by precipitation with ethanol. The tRNA was washed extensively with 70% ethanol and resuspended in 10 mM HEPES, pH 7.5. Typically 250 to 350 A260 units of total tRNA, mostly free of rRNA, were obtained from a 3 L culture. The quality of the total tRNA preparation was confirmed by agarose gel electrophoresis and in vitro aminoacylation with isoleucine or methionine. For isolation of total tRNA under acidic conditions, cells were resuspended in ice-cold 0.1 M sodium acetate, pH 5.0. After extraction of total RNA using Trizol (Invitrogen) following the manufacturer’s instructions, large rRNAs were removed by precipitation with lithium chloride as described above. After several washes with ethanol, the tRNA was resuspended in 10 mM sodium acetate, pH 5.0, and stored at −80°C. A portion of the material was subjected to deacylation by the addition of Tris–HCl, pH 9.5, to a final concentration of 0.1 M. The deacylation reaction was performed at 37°C for 90–120 min; deacylated tRNAs were re-precipitated with ethanol and stored in 10 mM HEPES, pH 7.5.
Purification of isoleucine tRNAs from B. subtilis
Purification of individual isoleucine tRNAs was carried out essentially as described (18). Briefly, the tRNA of interest is purified in a two step-procedure involving (i) affinity chromatography using a 5′-biotinylated DNA oligonucleotide immobilized to streptavidin sepharose resin followed by (ii) purification of the highly enriched tRNA by electrophoresis on a native polyacrylamide gel. For ∼400 A260 units of total tRNA, ∼0.8 ml of streptavidin sepharose resin (Pharmacia) and 20 A260 units of the DNA oligonucleotide (IDT) complementary to nucleotides 54–76 of the tRNA of interest were used. tRNA and sepharose resin were mixed in a buffer containing 1.2 M NaCl, 30 mM HEPES, pH 7.5, and 15 mM ethylenediaminetetraacetic acid (EDTA) (6 × NHE). Following a denaturation step at 70°C for 30 min, the tRNA was allowed to bind to the oligonucleotide on the resin by lowering the temperature from 70°C to 30°C (∼3°C/min). After 30 min at 30°C, the resin was washed several times with 3 × NHE buffer at 37°C. For elution of the bound tRNA, the resin was suspended in 0.5–1 ml of 0.1 × NHE buffer and incubated at 65°C for 5 min. The resin was centrifuged and the supernatant was collected as eluted tRNA. The elution step was repeated 10–15 times. Supernatant fractions containing tRNA were pooled, concentrated and used for electrophoresis on a native 10% polyacrylamide gel. The tRNA was detected by UV shadowing, eluted from the gel and dialyzed extensively against 5 mM ammonium acetate, pH 5.5. The dialyzed tRNA was concentrated by evaporation, precipitated with ethanol and the precipitate was washed several times with ethanol.
Analysis of B. subtilis tRNA by polyacrylamide gel electrophoresis and northern blotting
RNAs were analyzed by acid–urea polyacrylamide gel electrophoresis (PAGE) (19) and native PAGE [10–15% polyacrylamide in Tris/Borate/EDTA (TBE) buffer] followed by staining with ethidium bromide or northern blotting. The transfer of tRNA onto Hybond-XL (GE Healthcare) or Nytran SPC (Whatman) has been described (19). Northern blots were analyzed by autoradiography and phosphorimaging using Imagequant software.
Cloning and expression of B. subtilis IleRS and MetRS in Escherichia coli
The genes for B. subtilis MetRS and IleRS were amplified by polymerase chain reaction using Pfu Turbo DNA polymerase (Stratagene) from B. subtilis 168 genomic DNA and inserted into pET15b (Novagen) under control of the T7 promoter. B. subtilis MetRS and IleRS containing NH2-terminal His6-tags were expressed in E. coli BL21(DE3) and purified by affinity chromatography using Talon resin (Clontech) following the manufacturer’s protocol for batch–gravity flow purification of proteins.
In vitro aminoacylation and biotinylation of tRNA
0.002–0.5 A260 of tRNA were aminoacylated in vitro with L-isoleucine or L-methionine as described below using purified B. subtilis IleRS or MetRS at a final concentration of 0.05 μM. Reaction mixtures contained: (i) for IleRS, 50 mM HEPES, pH 7.5, 10 mM MgCl2, 5 mM ATP, 0.1 μg/μl bovine serum albumin (BSA) and 100 μM L-isoleucine; and (ii) for MetRS, 50 mM imidazole, pH 7.6, 150 mM NH4Cl, 15 mM MgCl2, 10 mM ATP, 0.1 μg/μl BSA and 100 μM L-methionine. Incubation was at 37°C for 30–60 min. Reaction products were examined by acid–urea PAGE/northern blot analysis as described. Alternatively, in vitro aminoacylations were carried out in the presence of radiolabeled amino acids using 25 μM L-14C-isoleucine (ARC), 5 μM L-3H-isoleucine (ARC) or 20 μM L-methionine/L-35S-methionine (Perkin Elmer). At various time points, aliquots were removed and analyzed by precipitation with trichloroacetic acid (TCA) followed by liquid scintillation counting of TCA-precipitable counts. In vitro biotinylation of tRNAs was as described before (12).
Template-dependent binding of aminoacylated tRNAs to ribosomes
The preparation of ribosomes from wild-type B. subtilis 168 and the binding of aminoacylated tRNAs to ribosomes were carried out essentially as described (20,21) with modifications as follows. For the preparation of ribosomes, B. subtilis cells were harvested in mid-log phase (OD600 0.5–0.6), resuspended in buffer A (20 mM HEPES, pH 7.4, 10 mM MgCl2, 100 mM NH4Cl and 6 mM 2-mercaptoethanol) and lysed by two passes through a French Press at 12 000psi. The cell lysate was cleared by two consecutive centrifugations for 15 min at 10 000g. The resulting supernatant was centrifuged for 30 min at 30 000g followed by ultracentrifugation at 100 000g for 3 h. The ribosome pellet was washed with buffer B (20 mM HEPES, pH 7.4, 10 mM MgCl2, 0.5 M NH4Cl and 6 mM 2-mercaptoethanol) by gentle shaking on ice. Ribosomes were re-pelleted and the final ribosome pellet was resuspended in buffer C (50 mM HEPES, pH 7.4, 10 mM MgCl2, 70 mM NH4Cl, 30 mM KCl and 1 mM dithiothreitol), dialyzed extensively against the same buffer, divided into aliquots, flash frozen and stored at −80°C. All steps above were carried out at 4°C unless otherwise mentioned.
Prior to use, aliquots of frozen ribosome stock were thawed, ‘activated’ by incubation at 42°C for 10 min and then cooled to room temperature (25°C). 2.5 μM ribosomes were pre-incubated with 0–300 μM mRNA (IDT) in buffer C for 5 min at room temperature in a 10 or 20 μl reaction. Radiolabeled aminoacylated tRNAs were added (∼1500 cpm of 3H-Ile-tRNAs; 4000 cpm 35S-Met-tRNAs), and incubation was continued for 30 min at room temperature. Reactions were terminated with 0.5 ml of ice-cold buffer C and filtered through nitrocellulose membranes (Millipore HA 0.45 μm). Preparation of 3H-Ile-tRNAs and 35S-Met-tRNAs was as described above. The averages from three to five independent experiments are shown.
Analysis of 5’-32P-labeled tRNA by partial RNase T1 and A digestion and alkali hydrolysis
Purified tRNAs were dephosphorylated with calf intestinal alkaline phosphatase (NEB) and subsequently labeled at the 5′ terminus with 32P using T4-PNK (NEB) following standard procedures (22). After denaturing gel purification, 5’-32P-labeled tRNA was mixed with non-radiolabeled tRNA and subjected to heat-denaturation at 65°C for 5 min in the respective reaction buffer suitable for partial RNase T1 or A digestion. Partial digestion of tRNA with RNase T1: 1 μg of tRNA in 50 mM Tris–HCl, pH 7.5, and 7 M urea was pre-incubated at 50°C for 5 min before RNase T1 (10–15 U; Ambion) was added and samples were incubated at 50°C for 10 min. Partial digestion of tRNA with RNase A: 1 μg of tRNA in 10 mM Tris–HCl, pH 7.5, and 1 mM EDTA was pre-incubated at 37°C for 5 min before RNase A (0.004 U; Ambion) was added and samples were incubated at 37°C for 5 min. Similarly, 1 μg tRNA was subjected to partial alkaline hydrolysis at 95°C for 2–4 min in a buffer containing 33 mM sodium carbonate/bicarbonate buffer, pH 9.2. Reactions were stopped by quick-freezing on dry ice, and reaction products were analyzed by denaturing 8–10% PAGE followed by autoradiography.
Analysis of 5′-32P-nucleotides by thin layer chromatography
For the analysis of 5′-32P-nucleotides isolated from 5′-32P-labeled fragments derived from nucleotides 34 to 37 of the anticodon loop, purified tRNAs were first subjected to partial alkali hydrolysis as described above, fragments produced were 5′-end labeled with 32P using T4-PNK (NEB) and run on a denaturing 8% polyacrylamide gel (23–26). Fragments corresponding to nucleotides 34–37 of the anticodon loop were eluted from the gel, digested with nuclease P1 and the 5′-terminal 32P-labeled nucleotide of each fragment was determined by thin layer chromatography (TLC) (22,27). The nuclease P1 (Sigma) digestion was carried out in 50 mM ammonium acetate, pH 5.0, for 6–8 h at 37°C. Samples were quick frozen on dry ice and lyophilized under vacuum. Cellulose F plates (Merck) were used for TLC analysis. Two different solvent systems were used for separation: (A) isobutyric acid:concentrated ammonia:water (66:1:33) (v:v:v) and (B) isopropanol:concentrated HCl:water (70:15:15) (v:v:v). For two dimensional analysis, samples were first run in solvent A for 16 h, plates were dried overnight and then run in solvent B for 28 h. TLC plates were analyzed by autoradiography. Non-radiolabeled nucleotide standards were added to samples and visualized by UV shadowing.
Mass spectral analysis of purified isoleucine tRNAs
For oligonucleotide sequence analysis, 1 μg of tRNA was digested with 50 U of RNase T1 (Worthington Biochemical Corp.) in 20 mM ammonium acetate, pH 5.3, for 2 h at 37°C. The digestion products were separated using a Thermo Surveyor HPLC system with a Waters XBridge C18 1.0 × 150 mm column at 40 μl/min with a gradient of 400 mM 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), 8.15 mM triethylamine (TEA), pH 7.0, and 400 mM HFIP, 8.15 mM TEA:methanol (50:50) (v:v), pH 7.0. The eluent was directed into a Thermo LTQ-XL for collection of mass spectra using a capillary temperature of 275°C, spray voltage of 4.5 kV, sheath gas, auxiliary gas and sweep gas at 25, 14 and 10 arbitrary units, respectively. Collision-induced dissociation (CID) tandem mass spectrometry with a normalized collision energy of 35% was used in data-dependent mode to obtain sequence information from the RNase T1 digestion products as previously described (28). The data-dependent scan was performed on the most abundant ions and each ion was selected for CID for up to 15 scans for 30 s before it was put on a dynamic exclusion list for 30 s.
RESULTS
Characterization and growth phenotypes of B. subtilis strains lacking tilS
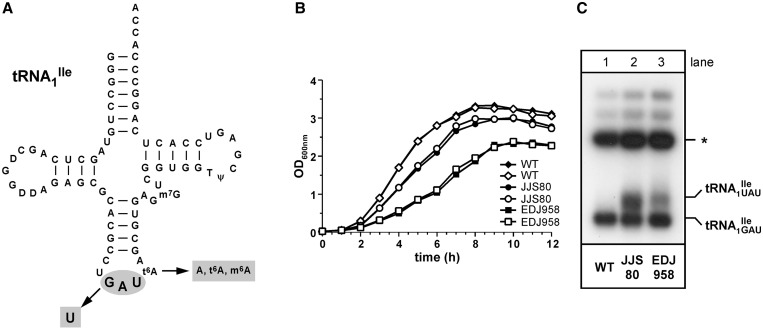
Wild-type B. subtilis 168 contains a total of four genes for two isoleucine tRNAs (29), three of which encode tRNA1Ile (anticodon: GAU; tRNA1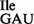 ; Figure 1A) for translation of AUC and AUU codons, and one of which encodes tRNA2Ile (anticodon: CAU, which is post-translationally modified to LAU, L = lysidine; tRNA2
; Figure 1A) for translation of AUC and AUU codons, and one of which encodes tRNA2Ile (anticodon: CAU, which is post-translationally modified to LAU, L = lysidine; tRNA2 ; Supplementary Figure S1A) for translation of the AUA codon. Recently, two mutant strains of B. subtilis, JJS80 and EDJ958, were isolated in which the gene encoding TilS had been deleted (17). The resulting absence of TilS activity leads to a lack of modification of C34 to lysidine in tRNA2
; Supplementary Figure S1A) for translation of the AUA codon. Recently, two mutant strains of B. subtilis, JJS80 and EDJ958, were isolated in which the gene encoding TilS had been deleted (17). The resulting absence of TilS activity leads to a lack of modification of C34 to lysidine in tRNA2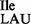 , (i.e. synthesis of tRNA2
, (i.e. synthesis of tRNA2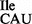 ), thereby rendering it inactive for translation of AUA codons. The survival of these B. subtilis strains was ensured through the presence of a mutant tRNA derived from one of the three tRNA1
), thereby rendering it inactive for translation of AUA codons. The survival of these B. subtilis strains was ensured through the presence of a mutant tRNA derived from one of the three tRNA1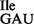 genes with a change in the anticodon sequence from GAT to TAT (Supplementary Table S1), allowing for the synthesis of a new cellular tRNA1Ile species with a UAU anticodon (tRNA1
genes with a change in the anticodon sequence from GAT to TAT (Supplementary Table S1), allowing for the synthesis of a new cellular tRNA1Ile species with a UAU anticodon (tRNA1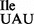 ), most likely responsible for translation of AUA codons.
), most likely responsible for translation of AUA codons.
Figure 1.
tRNA1Ile in Bacillus subtilis wild-type strain 168 and mutant strains JJS80 and EDJ958 lacking tilS. (A) Cloverleaf structure of wild-type tRNA1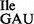 . The changes in mutant tRNA1
. The changes in mutant tRNA1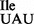 are indicated. Location of modified nucleosides is based on TLC and LC-MS analysis presented in this study. D (dihydrouridine); t6A (N6-threonylcarbamoyladenosine); m6A (N6-methyladenosine); m7G (7-methylguanosine); T (5-methyluridine); ψ (pseudouridine). (B) Growth analysis of B. subtilis wild-type and mutant strains. Cells were grown in LB medium at 37°C with aeration and cell growth was monitored over the course of 12 h. (C) Total tRNA was isolated from B. subtilis wild-type and mutant strains and analyzed by native PAGE (15%). 0.5 A260 of total tRNA were applied per lane. tRNAs were detected by northern hybridization using a 5′-32P-labeled DNA oligonucleotide directed against nucleotides 1–20 of tRNA1Ile which is identical in all tRNA1Ile species. A tRNA1Ile precursor species is indicated by asterisk.
are indicated. Location of modified nucleosides is based on TLC and LC-MS analysis presented in this study. D (dihydrouridine); t6A (N6-threonylcarbamoyladenosine); m6A (N6-methyladenosine); m7G (7-methylguanosine); T (5-methyluridine); ψ (pseudouridine). (B) Growth analysis of B. subtilis wild-type and mutant strains. Cells were grown in LB medium at 37°C with aeration and cell growth was monitored over the course of 12 h. (C) Total tRNA was isolated from B. subtilis wild-type and mutant strains and analyzed by native PAGE (15%). 0.5 A260 of total tRNA were applied per lane. tRNAs were detected by northern hybridization using a 5′-32P-labeled DNA oligonucleotide directed against nucleotides 1–20 of tRNA1Ile which is identical in all tRNA1Ile species. A tRNA1Ile precursor species is indicated by asterisk.
Bacillus subtilis mutant strains JJS80 and EDJ958 were grown in LB medium at 37°C and their growth was monitored over a 12 h period. The mutant strain JJS80, in which the trnO gene carries the GAT to TAT mutation (Supplementary Table S1), grows almost wild-type like, while the mutant strain EDJ958, in which the trnB gene carries the same GAT to TAT mutation (Supplementary Table S1) grows slower and saturates at a lower cell density (Figure 1B). Total tRNA was isolated from both mutant strains and analyzed by PAGE followed by northern blot hybridization using a probe directed against the 5′ terminal portion of tRNA1Ile, which detects both wild-type and mutant tRNA1Ile species. Separation on a 15% native polyacrylamide gel allowed the visualization of a slower migrating tRNA species consistent with the presence of the tRNA1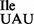 (Figure 1C). The abundance of tRNA1
(Figure 1C). The abundance of tRNA1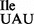 varied substantially between JJS80 and EDJ958 and reflects most likely different transcription efficiencies for the different chromosomal loci from which the mutant tRNA1
varied substantially between JJS80 and EDJ958 and reflects most likely different transcription efficiencies for the different chromosomal loci from which the mutant tRNA1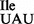 is derived from in the respective strain. Since the mutant strain JJS80 consistently showed better growth than EDJ958 and produced more of tRNA1
is derived from in the respective strain. Since the mutant strain JJS80 consistently showed better growth than EDJ958 and produced more of tRNA1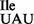 , all experiments described below were carried out with JJS80.
, all experiments described below were carried out with JJS80.
Aminoacylation properties of tRNA1Ile and tRNA2Ile from wild-type B. subtilis 168 and mutant strain JJS80 lacking tilS
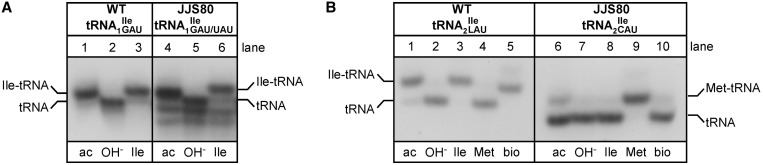
Total tRNA was isolated from B. subtilis JJS80, under acidic conditions, deacylated and re-aminoacylated using purified recombinant aminoacyl-tRNA synthetases (Figure 2). tRNAs were analyzed by acid–urea PAGE/northern blotting. tRNA1Ile from both the wild-type and mutant strain are aminoacylated with isoleucine by IleRS (Figure 2A). Although tRNA1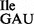 and tRNA1
and tRNA1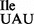 could not be clearly separated under acid–urea PAGE conditions, it is noteworthy that total tRNA isolated from JJS80 contains two additional faster migrating species, which most likely correlate with different modifications at position 37 (see below) and/or conformational variants of the mutant tRNA1
could not be clearly separated under acid–urea PAGE conditions, it is noteworthy that total tRNA isolated from JJS80 contains two additional faster migrating species, which most likely correlate with different modifications at position 37 (see below) and/or conformational variants of the mutant tRNA1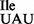 .
.
Figure 2.
Acid–urea PAGE/northern blot analysis of isoleucine tRNAs from Bacillus subtilis wild-type and mutant strain JJS80 lacking tilS. (A) Analysis of tRNA1Ile from B. subtilis wild-type (WT; lanes 1–3) and mutant strain JJS80 (lanes 4–6). tRNA1Ile was visualized by northern hybridization using a 32P-labeled DNA oligonucleotide complementary to the 5′ portion of tRNA1Ile. (B) Analysis of tRNA2Ile from B. subtilis wild-type (WT; lanes 1–5) and mutant strain JJS80 (lanes 6–10). tRNA2Ile was visualized by northern hybridization using a 32P-labeled DNA oligonucleotide complementary to the 3′ portion of tRNA2Ile. 0.1 A260 of total tRNA were applied per lane. ac, tRNA isolated under acidic condition; OH-, deacylation by base treatment; Ile, in vitro aminoacylation using IleRS; Met, in vitro aminoacylation using MetRS; bio, in vitro biotinylation of lysidine.
tRNA2Ile from the mutant strain showed a clear shift in mobility by acid–urea PAGE compared to wild-type tRNA2Ile, consistent with the lack of lysidine at position 34 (Figure 2B, compare lanes 1 and 6). Also, tRNA2Ile from the mutant strain showed a significant amount of deacylation during isolation and work-up compared to wild-type tRNA2Ile, possibly indicative of a change in amino acid specificity from isoleucine to methionine since the ester link between methionine and tRNA is much weaker than the ester link between isoleucine and tRNA (30). However, it is also possible that tRNA2Ile from the mutant strain is not as good a substrate for aminoacylation in vivo and, therefore, appears only partially aminoacylated. As expected, tRNA2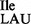 from the wild-type B. subtilis strain could be aminoacylated in vitro with isoleucine but not with methionine (Figure 2B, lanes 3 and 4), whereas tRNA2
from the wild-type B. subtilis strain could be aminoacylated in vitro with isoleucine but not with methionine (Figure 2B, lanes 3 and 4), whereas tRNA2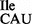 from JJS80 could not be aminoacylated with isoleucine but could be aminoacylated instead with methionine (Figure 2B, lanes 8 and 9). Furthermore, the presence of lysidine in wild-type tRNA2
from JJS80 could not be aminoacylated with isoleucine but could be aminoacylated instead with methionine (Figure 2B, lanes 8 and 9). Furthermore, the presence of lysidine in wild-type tRNA2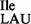 could be confirmed by reaction of the free NH2 group in lysidine with an N-hydroxysuccinimide ester derivative of biotin giving rise to a clear mobility shift (12); in contrast, tRNA2
could be confirmed by reaction of the free NH2 group in lysidine with an N-hydroxysuccinimide ester derivative of biotin giving rise to a clear mobility shift (12); in contrast, tRNA2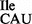 from the mutant strain could not be biotinylated due to the lack of lysidine (Figure 2B, compare lanes 5 and 10).
from the mutant strain could not be biotinylated due to the lack of lysidine (Figure 2B, compare lanes 5 and 10).
Purification of tRNA1Ile and tRNA2Ile from wild-type B. subtilis 168 and mutant strain JJS80 lacking tilS
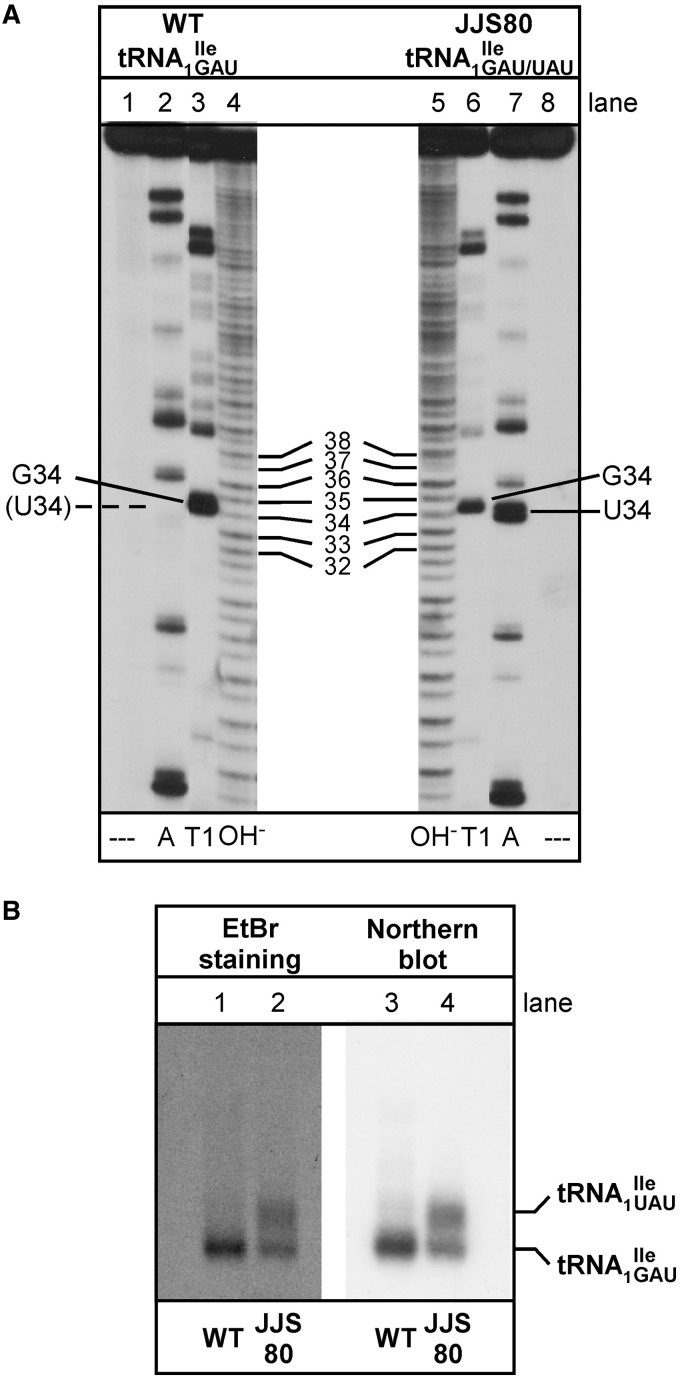
Wild-type and mutant isoleucine tRNA1Ile and tRNA2Ile were purified from the B. subtilis wild-type strain 168 and mutant strain JJS80, respectively. The extent of purification of various tRNAs was assessed by in vitro aminoacylation using B. subtilis IleRS and MetRS, with 14C-isoleucine or 35S-methionine acceptance higher than 1200 pmole/A260 unit for all tRNAs (Supplementary Table S2). The homogeneity of purified tRNAs was further verified by partial RNase T1 and RNase A digestion of 5′-32P-labeled tRNAs (31–33) (Figure 3A and Supplementary Figure S1B), confirming that none of the samples contained detectable levels of contaminating tRNAs except for the desired mutant tRNA1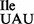 (see below).
(see below).
Figure 3.
Analysis of tRNA1Ile purified from Bacillus subtilis wild-type and mutant strain JJS80. (A) Characterization of purified tRNA1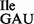 from B. subtilis wild-type (WT; lanes 1–4) and tRNA1
from B. subtilis wild-type (WT; lanes 1–4) and tRNA1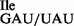 from JJS80 (lanes 5–8) by RNase A and T1 analysis. The 5′-32P-labeled tRNA was partially digested with 0.004 U of RNase A (lanes 2 and 7) and 10 U of RNase T1 (lanes 3 and 6). Lanes 4 and 5 show a partial alkali digest; lanes 1 and 8 show undigested control reactions. 32P-labeled fragments were separated by denaturing PAGE and visualized by autoradiography. (B) Analysis of purified tRNA1
from JJS80 (lanes 5–8) by RNase A and T1 analysis. The 5′-32P-labeled tRNA was partially digested with 0.004 U of RNase A (lanes 2 and 7) and 10 U of RNase T1 (lanes 3 and 6). Lanes 4 and 5 show a partial alkali digest; lanes 1 and 8 show undigested control reactions. 32P-labeled fragments were separated by denaturing PAGE and visualized by autoradiography. (B) Analysis of purified tRNA1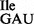 and tRNA1
and tRNA1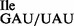 by 15% native PAGE. tRNAs were visualized by staining with ethidium bromide (EtBr) and northern hybridization using a universal tRNA1Ile probe. 0.01 A260 of total tRNA were applied per lane.
by 15% native PAGE. tRNAs were visualized by staining with ethidium bromide (EtBr) and northern hybridization using a universal tRNA1Ile probe. 0.01 A260 of total tRNA were applied per lane.
In general, 2.5–3 A260 units of purified tRNA1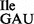 and 0.5 A260 of purified tRNA2
and 0.5 A260 of purified tRNA2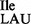 or tRNA2
or tRNA2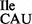 were obtained from ∼400 A260 of total RNA. Although, wild-type tRNA1
were obtained from ∼400 A260 of total RNA. Although, wild-type tRNA1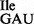 and mutant tRNA1
and mutant tRNA1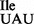 could be separated by native PAGE (Figures 1C and 3B) under analytical conditions, we were unable to do so under preparative conditions. Therefore, most of the in vitro experiments described in this study, except in the last section, were carried out with a mixture of both wild-type and mutant tRNA, which herein is referred to as tRNA1
could be separated by native PAGE (Figures 1C and 3B) under analytical conditions, we were unable to do so under preparative conditions. Therefore, most of the in vitro experiments described in this study, except in the last section, were carried out with a mixture of both wild-type and mutant tRNA, which herein is referred to as tRNA1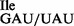 . Typically, 2.5 A260 of purified tRNA1
. Typically, 2.5 A260 of purified tRNA1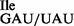 were obtained from ∼400 A260 of total RNA; based on the intensity of ethidium bromide-stained bands or northern blot (Figure 3B, lanes 2 and 4), this sample contained ∼50% of the mutant tRNA1
were obtained from ∼400 A260 of total RNA; based on the intensity of ethidium bromide-stained bands or northern blot (Figure 3B, lanes 2 and 4), this sample contained ∼50% of the mutant tRNA1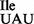 species as estimated by the analytical PAGE analysis.
species as estimated by the analytical PAGE analysis.
Characterization of tRNA2Ile from B. subtilis lacking tilS
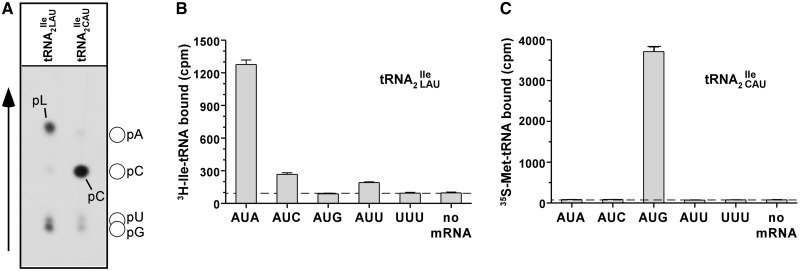
The absence of lysidine at position 34 of the mutant tRNA2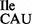 was further established by TLC analysis of the nucleotide at the wobble position as described in ‘Materials and Methods’ section. Figure 4A shows a clear difference in the mobility of lysidine originating from the wild-type tRNA2
was further established by TLC analysis of the nucleotide at the wobble position as described in ‘Materials and Methods’ section. Figure 4A shows a clear difference in the mobility of lysidine originating from the wild-type tRNA2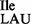 and the mobility of the unmodified C from the mutant tRNA2
and the mobility of the unmodified C from the mutant tRNA2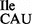 . The mobility of the unmodified C was confirmed by non-radiolabeled standards that were run in parallel and visualized by UV shadowing.
. The mobility of the unmodified C was confirmed by non-radiolabeled standards that were run in parallel and visualized by UV shadowing.
Figure 4.
Lysidine is absent in tRNA2Ile from Bacillus subtilis JJS80 lacking tilS. (A) 1D TLC analysis of the wobble position 34 in tRNA2Ile purified from B. subtilis wild-type and JJS80. Purified wild-type tRNA2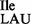 and mutant tRNA2
and mutant tRNA2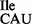 were partially hydrolyzed by alkali, the 5′ termini of the fragments were 32P-labeled using T4-PNK. 32P-labeled fragments were subsequently digested with nuclease P1 and the nature of the 5′ terminal nucleotide was determined by TLC. The solvent used was isobutyric acid:concentrated ammonia:water (66:1:33) (v:v:v). The mobility of each nucleotide (pA, pC, pG, pU) was confirmed with non-radiolabeled standards used as internal markers and visualized by UV shadowing. (B and C) Template-dependent binding of purified wild-type 3H-Ile-tRNA2
were partially hydrolyzed by alkali, the 5′ termini of the fragments were 32P-labeled using T4-PNK. 32P-labeled fragments were subsequently digested with nuclease P1 and the nature of the 5′ terminal nucleotide was determined by TLC. The solvent used was isobutyric acid:concentrated ammonia:water (66:1:33) (v:v:v). The mobility of each nucleotide (pA, pC, pG, pU) was confirmed with non-radiolabeled standards used as internal markers and visualized by UV shadowing. (B and C) Template-dependent binding of purified wild-type 3H-Ile-tRNA2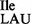 (B) and mutant 35S-Met-tRNA2
(B) and mutant 35S-Met-tRNA2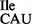 (C) to ribosomes isolated from B. subtilis. Oligonucleotides used were AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU; the oligonucleotide concentration was 200 μM.
(C) to ribosomes isolated from B. subtilis. Oligonucleotides used were AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU; the oligonucleotide concentration was 200 μM.
Template-dependent binding of purified and aminoacylated 3H-Ile-tRNA2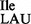 and 35S-Met-tRNA2
and 35S-Met-tRNA2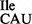 to B. subtilis ribosomes was performed with five different oligonucleotides; AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU. Wild-type tRNA2
to B. subtilis ribosomes was performed with five different oligonucleotides; AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU. Wild-type tRNA2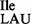 showed binding to AUA, and weaker binding to AUC and AUU; in contrast, tRNA2
showed binding to AUA, and weaker binding to AUC and AUU; in contrast, tRNA2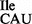 isolated from the tilS mutant strain showed binding only to AUG (Figure 4B and C).
isolated from the tilS mutant strain showed binding only to AUG (Figure 4B and C).
Characterization of U34 in the mutant tRNA1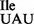
As described above, mutant tRNA1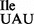 was purified from JJS80 as a mixture of wild-type tRNA1
was purified from JJS80 as a mixture of wild-type tRNA1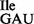 and mutant tRNA1
and mutant tRNA1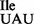 (tRNA1
(tRNA1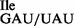 ; Figure 3B) and most in vitro experiments were carried out by direct side-by-side comparison of the tRNA1
; Figure 3B) and most in vitro experiments were carried out by direct side-by-side comparison of the tRNA1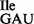 and tRNA1
and tRNA1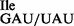 . To verify the presence of tRNA1
. To verify the presence of tRNA1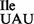 in the purified tRNA1
in the purified tRNA1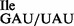 sample, purified 5′-32P-labeled tRNAs were subjected to partial digestion with RNases T1 and A (31–33) (Figure 3A). While RNase T1 digests confirmed the presence of G at position 34 of both the tRNA1
sample, purified 5′-32P-labeled tRNAs were subjected to partial digestion with RNases T1 and A (31–33) (Figure 3A). While RNase T1 digests confirmed the presence of G at position 34 of both the tRNA1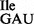 and the tRNA1
and the tRNA1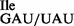 preparations (Figure 3A, lanes 3 and 6), digests with RNase A confirmed the presence of U34 in the tRNA1
preparations (Figure 3A, lanes 3 and 6), digests with RNase A confirmed the presence of U34 in the tRNA1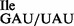 but not in the tRNA1
but not in the tRNA1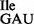 preparation (Figure 3A, compare lanes 2 and 7).
preparation (Figure 3A, compare lanes 2 and 7).
The nature of U34 in the mutant tRNA1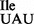 was examined by mass spectral (MS) and TLC studies. First, purified tRNA1
was examined by mass spectral (MS) and TLC studies. First, purified tRNA1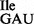 and tRNA1
and tRNA1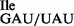 were digested completely with RNase T1 and the fragments produced were subjected to LC-MS/MS analysis. Due to the mutation in the anticodon from G34 to U34, digestion with RNase T1 yields a different cleavage pattern for the wild-type and the mutant tRNA preparations (Table 1 and Supplementary Figure S2). In case of the wild-type tRNA, the anticodon loop is cleaved into two fragments, CCUGp and AUAAGp, while the mutant tRNA produces a mixture of three related 9-mers containing CCUUAUAAGp (Table 1). LC-MS/MS analysis showed clearly, that U34 in the mutant tRNA is not modified (Table 1 and Supplementary Figure S3), unless it is modified to ψ, a mass-silent modification. Also, the modification at position 37 showed some interesting heterogeneity; in addition to t6A, both m6A and unmodified A are present in RNase T1 digests of the mutant but not of the wild-type tRNA (Table 1 and Supplementary Figure S3). Based on the results obtained from the RNase T1/MS analysis for the entire tRNA molecule (Supplementary Table S3), cloverleaf structures for wild-type and mutant tRNA1Ile were assembled (Figure 1A).
were digested completely with RNase T1 and the fragments produced were subjected to LC-MS/MS analysis. Due to the mutation in the anticodon from G34 to U34, digestion with RNase T1 yields a different cleavage pattern for the wild-type and the mutant tRNA preparations (Table 1 and Supplementary Figure S2). In case of the wild-type tRNA, the anticodon loop is cleaved into two fragments, CCUGp and AUAAGp, while the mutant tRNA produces a mixture of three related 9-mers containing CCUUAUAAGp (Table 1). LC-MS/MS analysis showed clearly, that U34 in the mutant tRNA is not modified (Table 1 and Supplementary Figure S3), unless it is modified to ψ, a mass-silent modification. Also, the modification at position 37 showed some interesting heterogeneity; in addition to t6A, both m6A and unmodified A are present in RNase T1 digests of the mutant but not of the wild-type tRNA (Table 1 and Supplementary Figure S3). Based on the results obtained from the RNase T1/MS analysis for the entire tRNA molecule (Supplementary Table S3), cloverleaf structures for wild-type and mutant tRNA1Ile were assembled (Figure 1A).
Table 1.
RNA sequencing of the anticodon loop of wild-type tRNA1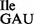 and mutant tRNA1
and mutant tRNA1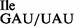 by MS analysis of RNase T1 fragments
by MS analysis of RNase T1 fragments
 |
Modified nucleosides: t6A (N6-threonylcarbamoyladenosine); m6A (N6-methyladenosine).
Note that the mutant tRNA1Ile sample is a mixture of wild-type tRNA1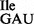 and mutant tRNA1
and mutant tRNA1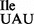 . Fragments that are unique within the mutant sample are highlighted in gray. The analysis of RNase T1-fragments derived from the entire tRNA molecule is shown in Supplementary Table S3.
. Fragments that are unique within the mutant sample are highlighted in gray. The analysis of RNase T1-fragments derived from the entire tRNA molecule is shown in Supplementary Table S3.
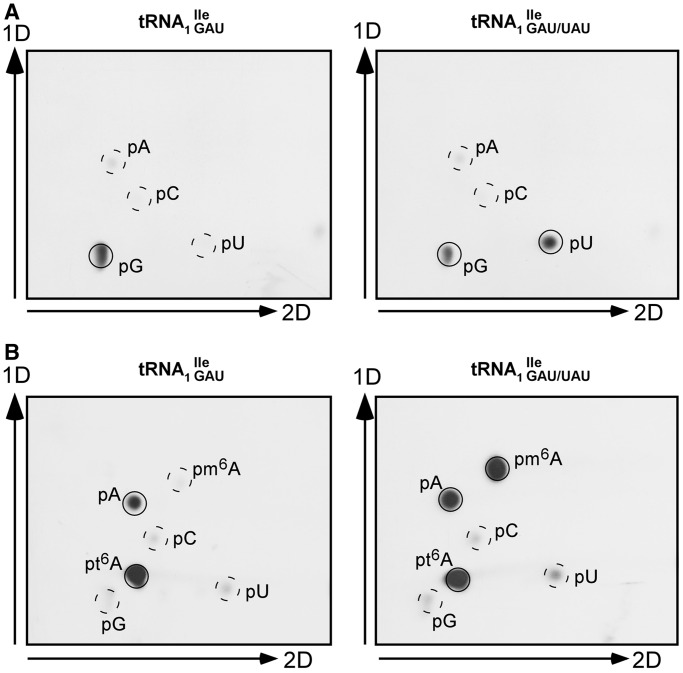
These results were verified independently by TLC analyses of 5′-32P-nucleotides isolated from 5′-32P-labeled fragments derived from nucleotides 34 to 37 of the anticodon loop of both wild-type and mutant tRNA1Ile (Figure 5 and Supplementary Figure S4). Both, 1D and 2D-TLC corroborated and extended the results obtained from the RNase T1/MS analysis: (i) the mutant tRNA1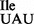 contains an unmodified U34 and not ψ34, which separates clearly from U (34) (Figure 5A and Supplementary Figure S4); and (ii) the presence of unmodified A and two different modifications, m6A and t6A, at position 37 were confirmed in the mutant tRNA1
contains an unmodified U34 and not ψ34, which separates clearly from U (34) (Figure 5A and Supplementary Figure S4); and (ii) the presence of unmodified A and two different modifications, m6A and t6A, at position 37 were confirmed in the mutant tRNA1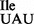 , whereas wild-type tRNA1
, whereas wild-type tRNA1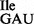 contained mostly t6A and some A (Figure 5B and Supplementary Figure S4). Since MS analysis of RNase T1 digests of wild-type tRNA1
contained mostly t6A and some A (Figure 5B and Supplementary Figure S4). Since MS analysis of RNase T1 digests of wild-type tRNA1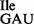 indicated no unmodified A at position 37, the presence of a small amount of A at this position is most likely due to some contamination by the neighboring fragment on the polyacrylamide gel, carrying an A at position 38.
indicated no unmodified A at position 37, the presence of a small amount of A at this position is most likely due to some contamination by the neighboring fragment on the polyacrylamide gel, carrying an A at position 38.
Figure 5.
Characterization of nucleotides present at position 34 (A) and 37 (B) of purified wild-type and mutant Bacillus subtilis tRNA1Ile by 2D TLC. Purified tRNAs were hydrolyzed and labeled with 32P as described for Figure 4. The 5’-32P-labeled nucleotides obtained after nuclease P1 digest were separated by 2D TLC, using an isobutyric acid:concentrated ammonia:water (66:1:33) (v:v:v) solvent for the first dimension and an isopropanol:concentrated HCl:water (70:15:15) (v:v:v) solvent for the second dimension. The mobility of each nucleotide (pA, pC, pG, pU, pm6A, pt6A) was confirmed with non-radiolabeled standards used as internal markers and visualized by UV shadowing. Note that the mutant tRNA1Ile sample is a mixture of wild-type tRNA1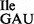 and mutant tRNA1
and mutant tRNA1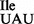 .
.
Codon binding properties of the mutant tRNA1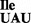 in the presence of B. subtilis ribosomes
in the presence of B. subtilis ribosomes
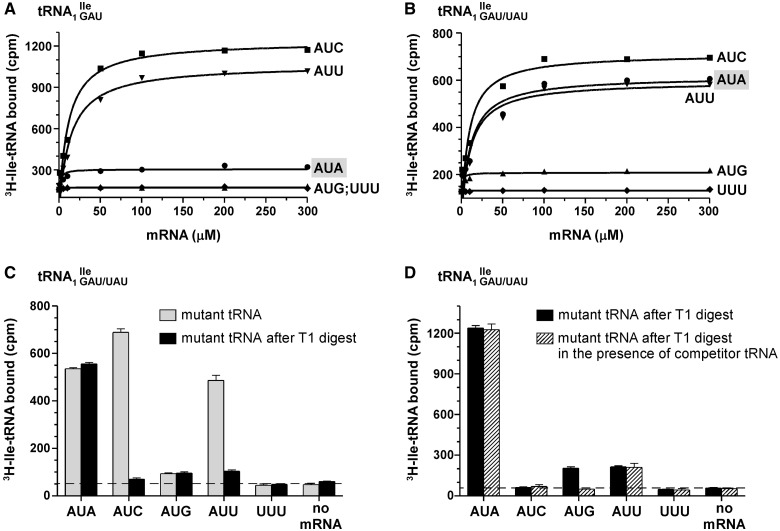
Template-dependent binding of purified and aminoacylated wild-type and mutant 3H-Ile-tRNA1Ile to B. subtilis ribosomes was performed. As expected, wild-type tRNA1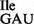 showed binding to AUC and AUU codons and to a very minor extent to AUA (Figure 6A). In contrast, the mutant tRNA1Ile, which is a mixture of tRNA1
showed binding to AUC and AUU codons and to a very minor extent to AUA (Figure 6A). In contrast, the mutant tRNA1Ile, which is a mixture of tRNA1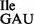 and tRNA1
and tRNA1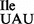 , bound strongly to AUA, AUC and AUU (Figure 6B). Compared to the negative control (UUU-containing mRNA), the mutant tRNA also showed slightly elevated but weak binding to the methionine codon AUG (Figure 6B).
, bound strongly to AUA, AUC and AUU (Figure 6B). Compared to the negative control (UUU-containing mRNA), the mutant tRNA also showed slightly elevated but weak binding to the methionine codon AUG (Figure 6B).
Figure 6.
Template-dependent binding of purified wild-type 3H-Ile-tRNA1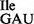 and mutant 3H-Ile-tRNA1
and mutant 3H-Ile-tRNA1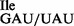 to ribosomes isolated from Bacillus subtilis. Oligonucleotides used were AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU. (A) Wild-type tRNA1
to ribosomes isolated from Bacillus subtilis. Oligonucleotides used were AUG AUA, AUG AUC, AUG AUG, AUG AUU and AUG UUU. (A) Wild-type tRNA1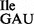 and (B) mutant tRNA1
and (B) mutant tRNA1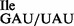 sample; note that the mutant sample is a mixture of wild-type tRNA1
sample; note that the mutant sample is a mixture of wild-type tRNA1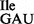 and mutant tRNA1
and mutant tRNA1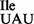 . (C) The mutant 3H-Ile-tRNA1
. (C) The mutant 3H-Ile-tRNA1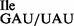 sample was treated with RNase T1 under native conditions to specifically inactivate the wild-type 3H-Ile-tRNA1Ile by cleavage at G34; the mutant tRNA1Ile containing U34 is resistant to this treatment; (D) an equimolar amount of non-radioactive competitor Met-tRNA2
sample was treated with RNase T1 under native conditions to specifically inactivate the wild-type 3H-Ile-tRNA1Ile by cleavage at G34; the mutant tRNA1Ile containing U34 is resistant to this treatment; (D) an equimolar amount of non-radioactive competitor Met-tRNA2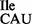 was added to RNase T1-treated mutant tRNA. The oligonucleotide concentration in (C) and (D) was 200 μM. Note that in (D) the mutant tRNA1
was added to RNase T1-treated mutant tRNA. The oligonucleotide concentration in (C) and (D) was 200 μM. Note that in (D) the mutant tRNA1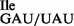 sample was treated with RNase T1 first, followed by aminoacylation with 3H-Ile, resulting in a doubling of 3H-Ile-tRNA1
sample was treated with RNase T1 first, followed by aminoacylation with 3H-Ile, resulting in a doubling of 3H-Ile-tRNA1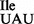 -specific counts present in the ribosome binding experiments.
-specific counts present in the ribosome binding experiments.
To remove the wild-type tRNA1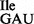 from the mixture of wild-type and mutant tRNA1Ile, tRNA1
from the mixture of wild-type and mutant tRNA1Ile, tRNA1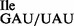 was treated with RNase T1 in the presence of Mg++ under mild conditions to inactivate specifically the wild-type tRNA1
was treated with RNase T1 in the presence of Mg++ under mild conditions to inactivate specifically the wild-type tRNA1 by cleavage at position 34 (35). RNase T1 treatment of tRNA1
by cleavage at position 34 (35). RNase T1 treatment of tRNA1 produced a ‘nicked’ tRNA, which was inactive in both in vitro aminoacylation (Supplementary Figure S5A) and binding to ribosomes (Supplementary Figure S5C). The efficiency of RNase T1 cleavage at G34 is clearly demonstrated by the fact that aminoacylated wild-type Ile-tRNA1
produced a ‘nicked’ tRNA, which was inactive in both in vitro aminoacylation (Supplementary Figure S5A) and binding to ribosomes (Supplementary Figure S5C). The efficiency of RNase T1 cleavage at G34 is clearly demonstrated by the fact that aminoacylated wild-type Ile-tRNA1 lost essentially all ribosome binding activity subsequent to RNase T1-treatment (Supplementary Figure S5C; black bars). In contrast, binding of the mutant tRNA1
lost essentially all ribosome binding activity subsequent to RNase T1-treatment (Supplementary Figure S5C; black bars). In contrast, binding of the mutant tRNA1 containing U34 to AUA is resistant to this treatment (Figure 6C). RNase T1 treatment of tRNA1
containing U34 to AUA is resistant to this treatment (Figure 6C). RNase T1 treatment of tRNA1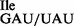 led to a loss of isoleucine acceptance of ∼40% (Supplementary Figure S5B), consistent with the level of contaminating wild-type tRNA1
led to a loss of isoleucine acceptance of ∼40% (Supplementary Figure S5B), consistent with the level of contaminating wild-type tRNA1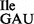 species (Figure 3B). The resulting highly enriched mutant tRNA1
species (Figure 3B). The resulting highly enriched mutant tRNA1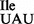 showed strong binding to AUA and weak binding to AUG and AUU, while binding to AUC was completely eliminated (Figure 6C; black bars).
showed strong binding to AUA and weak binding to AUG and AUU, while binding to AUC was completely eliminated (Figure 6C; black bars).
Ribosome binding of the highly enriched tRNA1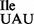 sample was also carried out in the presence of an equimolar amount of competitor tRNA, in this case Met-tRNA2
sample was also carried out in the presence of an equimolar amount of competitor tRNA, in this case Met-tRNA2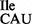 , which reduced binding of Ile-tRNA1
, which reduced binding of Ile-tRNA1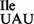 to AUG, but not AUA or AUU, to background levels (Figure 6D; hatched bars). Weak binding to the AUU-containing mRNA leaves open the possibility that the mutant tRNA1
to AUG, but not AUA or AUU, to background levels (Figure 6D; hatched bars). Weak binding to the AUU-containing mRNA leaves open the possibility that the mutant tRNA1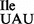 can base-pair, albeit weakly, with an AUU codon.
can base-pair, albeit weakly, with an AUU codon.
We also performed ribosome binding assays using the mutant tRNA1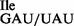 and ribosomes isolated from E. coli. The mutant Ile-tRNA1
and ribosomes isolated from E. coli. The mutant Ile-tRNA1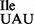 showed similar binding to the AUA codon with ribosomes from B. subtilis and E. coli. In contrast, binding of the mutant Ile-tRNA1
showed similar binding to the AUA codon with ribosomes from B. subtilis and E. coli. In contrast, binding of the mutant Ile-tRNA1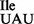 to the AUG codon was up about 3-fold with E. coli ribosomes (Supplementary Figure S6).
to the AUG codon was up about 3-fold with E. coli ribosomes (Supplementary Figure S6).
DISCUSSION
Characterization of the B. subtilis mutant tRNA1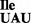
Strains of B. subtilis carrying a deletion in the gene encoding the essential tRNA2Ile modifying enzyme TilS can survive only in the presence of a mutant tRNA1Ile, whose anticodon wobble position has been changed from G34 to U34 (tRNA1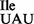 ). This anticodon mutation allows the tRNA1
). This anticodon mutation allows the tRNA1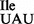 to act as a suppressor by reading the isoleucine codon AUA normally read by tRNA2
to act as a suppressor by reading the isoleucine codon AUA normally read by tRNA2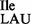 . Two major questions of interest were: (i) is U34 at the anticodon wobble position of the mutant tRNA modified, and (ii) besides binding to the AUA codon for isoleucine, does the mutant tRNA also bind to the AUG codon for methionine? Through the analysis of tRNA1
. Two major questions of interest were: (i) is U34 at the anticodon wobble position of the mutant tRNA modified, and (ii) besides binding to the AUA codon for isoleucine, does the mutant tRNA also bind to the AUG codon for methionine? Through the analysis of tRNA1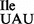 using RNA sequencing and mass spectrometry, we have shown here that unlike the U34 in the anticodon wobble position of bacterial, eukaryotic and archaeal tRNAs, which are almost always modified, the U34 in tRNA1
using RNA sequencing and mass spectrometry, we have shown here that unlike the U34 in the anticodon wobble position of bacterial, eukaryotic and archaeal tRNAs, which are almost always modified, the U34 in tRNA1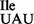 is not. Thus, the mutant tRNA1
is not. Thus, the mutant tRNA1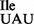 is lacking one or more of the determinants necessary for its modification by any of the U34 modifying enzymes in B. subtilis.
is lacking one or more of the determinants necessary for its modification by any of the U34 modifying enzymes in B. subtilis.
Interestingly, while the wild-type tRNA1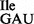 contains the modified nucleoside t6A at position 37 next to the anticodon, the mutant tRNA1
contains the modified nucleoside t6A at position 37 next to the anticodon, the mutant tRNA1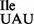 contains a mixture of A, t6A and m6A at this position. It is worth noting that several of the B. subtilis tRNAs, including tRNA2
contains a mixture of A, t6A and m6A at this position. It is worth noting that several of the B. subtilis tRNAs, including tRNA2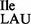 (36), contain m6A at position 37 (37). The presence of A, t6A and m6A at position 37 of tRNA1
(36), contain m6A at position 37 (37). The presence of A, t6A and m6A at position 37 of tRNA1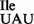 suggests that mutation of G34 to U34 has made the tRNA a poorer substrate for the t6A enzyme complex TsaB/C/D (38,39), so that A37 is either unmodified, modified to t6A or methylated to m6A by a tRNA adenine N6-methyltransferase, presumably a homolog of E. coli YfiC (40). In bacteria, archaea and eukaryotes, tRNAs that are substrates for the t6A enzyme usually contain G, C or a modified U in the anticodon wobble position (41). Therefore, why mutation of G34 to U34 should make the mutant tRNA1
suggests that mutation of G34 to U34 has made the tRNA a poorer substrate for the t6A enzyme complex TsaB/C/D (38,39), so that A37 is either unmodified, modified to t6A or methylated to m6A by a tRNA adenine N6-methyltransferase, presumably a homolog of E. coli YfiC (40). In bacteria, archaea and eukaryotes, tRNAs that are substrates for the t6A enzyme usually contain G, C or a modified U in the anticodon wobble position (41). Therefore, why mutation of G34 to U34 should make the mutant tRNA1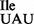 a poorer substrate for the t6A enzyme is not obvious, unless it is the lack of modification of U34.
a poorer substrate for the t6A enzyme is not obvious, unless it is the lack of modification of U34.
Aminoacylation of mutant B. subtilis tRNA1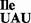
Mutant tRNA1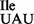 is a good substrate for aminoacylation in vivo and in vitro by B. subtilis IleRS. Suzuki et al. showed recently that a particular arginine residue in M. mobile IleRS is important for aminoacylation of M. mobile tRNA2
is a good substrate for aminoacylation in vivo and in vitro by B. subtilis IleRS. Suzuki et al. showed recently that a particular arginine residue in M. mobile IleRS is important for aminoacylation of M. mobile tRNA2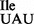 (42). It is, therefore, interesting to note that B. subtilis IleRS also has an arginine at the corresponding position, whereas E. coli IleRS has tryptophan.
(42). It is, therefore, interesting to note that B. subtilis IleRS also has an arginine at the corresponding position, whereas E. coli IleRS has tryptophan.
Codon recognition properties of the B. subtilis mutant tRNA1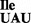
Binding experiments using B. subtilis ribosomes show that tRNA1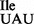 binds mostly to the AUA codon and only weakly to the AUU and AUG codons (Figure 6C, AUA >>> AUU > AUG). Thus, the mutant tRNA1
binds mostly to the AUA codon and only weakly to the AUU and AUG codons (Figure 6C, AUA >>> AUU > AUG). Thus, the mutant tRNA1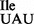 has an inherently low potential for misreading the methionine codon AUG and for inserting isoleucine in the place of methionine into proteins in vivo. This result explains the finding that the B. subtilis strain carrying the mutant tRNA1
has an inherently low potential for misreading the methionine codon AUG and for inserting isoleucine in the place of methionine into proteins in vivo. This result explains the finding that the B. subtilis strain carrying the mutant tRNA1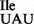 is viable and shows only a minimal level of mis-incorporation of isoleucine in the place of methionine in a reporter protein (17). The level of mis-incorporation of isoleucine in the place of methionine of 10−3/1 appears to be well tolerated in B. subtilis, since the suppressor strain JJS80 grows at a rate comparable to that of the wild-type strain (Figure 1B). The ribosome binding results in vitro also indicate that the low level read-through of AUG by the mutant tRNA1
is viable and shows only a minimal level of mis-incorporation of isoleucine in the place of methionine in a reporter protein (17). The level of mis-incorporation of isoleucine in the place of methionine of 10−3/1 appears to be well tolerated in B. subtilis, since the suppressor strain JJS80 grows at a rate comparable to that of the wild-type strain (Figure 1B). The ribosome binding results in vitro also indicate that the low level read-through of AUG by the mutant tRNA1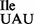 is more due to the inherently weak affinity of tRNA1
is more due to the inherently weak affinity of tRNA1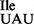 for the AUG codon than competition in vivo by the endogenous methionine tRNA.
for the AUG codon than competition in vivo by the endogenous methionine tRNA.
The weak affinity of tRNA1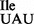 for the AUG codon on the ribosome is reminiscent of several tRNAs containing an unmodified U34 which read codons ending in A much better than to those ending in G (43–45). For example, transcripts of E. coli tRNA1Ser, which contain U34 instead of the modified U normally present in the tRNA1Ser, read the serine codon UCA just as well in an E. coli protein synthesis system but not UCG (46,47). Similarly, an anticodon stem loop fragment of human lysine tRNA carrying U34 binds to the lysine codon AAA but not to AAG on E. coli ribosomes (48). Yet another example comes from the analysis of human mitochondrial leucine tRNA, where replacement of 5-taurinomethyl modification of U normally present in the anticodon wobble position by U34 results in a severe deficiency in reading the leucine codon UUG without any effect on reading the UUA codon (49).
for the AUG codon on the ribosome is reminiscent of several tRNAs containing an unmodified U34 which read codons ending in A much better than to those ending in G (43–45). For example, transcripts of E. coli tRNA1Ser, which contain U34 instead of the modified U normally present in the tRNA1Ser, read the serine codon UCA just as well in an E. coli protein synthesis system but not UCG (46,47). Similarly, an anticodon stem loop fragment of human lysine tRNA carrying U34 binds to the lysine codon AAA but not to AAG on E. coli ribosomes (48). Yet another example comes from the analysis of human mitochondrial leucine tRNA, where replacement of 5-taurinomethyl modification of U normally present in the anticodon wobble position by U34 results in a severe deficiency in reading the leucine codon UUG without any effect on reading the UUA codon (49).
The highly preferential reading of codons ending in A by a tRNA with an unmodified U34 is, however, not universal. Although the U34 in the anticodon wobble position is almost always modified in tRNAs that have been sequenced (37,41,50), there are several exceptions. For various reasons including genome compactions, parasitic lifestyles etc., fungal, insect and vertebrate mitochondria and most Mycoplasma species contain a fewer number of tRNAs to read all the codons of the genetic code (51–57). In these cases, a single tRNA containing an unmodified U34 is used to read all four codons of a four-codon box specifying the same amino acid, instead of using at least two tRNAs for the same purpose as most bacterial, eukaryotic and archaeal systems do. Thus, mitochondrial and Mycoplasma ribosomes and tRNAs have coevolved to allow an unmodified U34 to base pair with U, C, A or G on the ribosome.
A number of studies including work presented here suggest that the codon reading properties of a tRNA containing an unmodified U34 depend upon several factors including the nature of the ribosome, the tRNA sequence and the tRNA anticodon loop structure. A recent example of both the ribosome and tRNA playing important roles in the decoding properties of a tRNA comes from an AUA-reading tRNA2Ile isolated from M. mobile (42). As mentioned above, in contrast to the AUA codon-specific tRNA2Ile isolated from most bacteria or archaea which contain lysidine or agmatidine, the M. mobile tRNA2Ile contains an unmodified U (tRNA2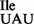 ). Suzuki et al. have shown that the M. mobile tRNA2
). Suzuki et al. have shown that the M. mobile tRNA2 binds to the isoleucine codon AUA but not to the methionine codon AUG on M. mobile ribosomes. However, the same tRNA binds to both AUA and AUG on E. coli ribosomes. Thus, the M. mobile ribosome and tRNA2
binds to the isoleucine codon AUA but not to the methionine codon AUG on M. mobile ribosomes. However, the same tRNA binds to both AUA and AUG on E. coli ribosomes. Thus, the M. mobile ribosome and tRNA2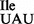 have coevolved in such a way that the M. mobile tRNA2
have coevolved in such a way that the M. mobile tRNA2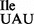 containing an unmodified U in the anticodon wobble position is restricted from binding to AUG, even though other M. mobile tRNAs containing an unmodified U34 read all four codons of a four-codon box. It would be interesting to see whether the M. mobile tRNA2
containing an unmodified U in the anticodon wobble position is restricted from binding to AUG, even though other M. mobile tRNAs containing an unmodified U34 read all four codons of a four-codon box. It would be interesting to see whether the M. mobile tRNA2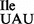 binds only to AUA or to both AUA and AUG codons on ribosomes isolated from other Mycoplasma species. The specific binding of M. mobile tRNA2
binds only to AUA or to both AUA and AUG codons on ribosomes isolated from other Mycoplasma species. The specific binding of M. mobile tRNA2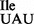 to AUA and not to AUG on M. mobile ribosomes highlights the importance of tRNA sequence and/or structure for the specificity of codon recognition. This may also apply to the mutant tRNA1
to AUA and not to AUG on M. mobile ribosomes highlights the importance of tRNA sequence and/or structure for the specificity of codon recognition. This may also apply to the mutant tRNA1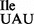 isolated from B. subtilis described in the present study, which binds preferentially to AUA, but only weakly to AUG on B. subtilis ribosomes. Similar to the M. mobile tRNA2
isolated from B. subtilis described in the present study, which binds preferentially to AUA, but only weakly to AUG on B. subtilis ribosomes. Similar to the M. mobile tRNA2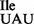 , the B. subtilis mutant tRNA1
, the B. subtilis mutant tRNA1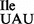 shows elevated binding to the AUG codon on E. coli ribosomes (Supplementary Figure S6), although the effect is not as pronounced as for the M. mobile tRNA (42).
shows elevated binding to the AUG codon on E. coli ribosomes (Supplementary Figure S6), although the effect is not as pronounced as for the M. mobile tRNA (42).
Perhaps, the strongest evidence that tRNA sequence and/or structure can affect the codon reading properties of U34 comes from studies of a mutant E. coli glycine tRNA, which contains an unmodified U34 in the anticodon wobble position. Lagerkvist et al. have shown that this tRNA reads the glycine codons GGA and GGG on E. coli ribosomes but not the other glycine codons GGU or GGC (58). Remarkably, however, a single change of U32 in the anticodon loop to C32, found in Mycoplasma mycoides glycine tRNA, now allowed the tRNA to read all four glycine codons on E. coli ribosomes (59). In a reciprocal experiment, it was shown that while the M. mycoides glycine tRNA containing U34 can read all four glycine codons on E. coli ribosomes, a mutant tRNA in which C32 is changed to U32 reads only GGA and GGG but not GGU or GGC. These results show that like the mitochondrial and the Mycoplasma systems mentioned above, E. coli ribosomes also have the potential for allowing an unmodified U34 in the anticodon wobble position to base pair with U, C, A or G. However, whether this happens or not depends on the tRNA sequence and/or the tRNA anticodon loop structure (60), with the presence of C32 in the anticodon loop being a critical determinant in the case of E. coli and M. mycoides glycine tRNAs. The importance of the anticodon context in tRNA on efficiency and accuracy of codon reading was pointed out several years ago (61) and has since then been well established (62,63).
Finally, in experiments parallel to the work described in here, we have mutated C34, a precursor of agmatidine, in the anticodon wobble position of Haloarcula marismortui tRNA2Ile to U34 and have purified the mutant tRNA and studied its codon recognition properties using H. marismortui ribosomes. We have found that U34 in the mutant tRNA is modified and that the modified tRNA binds not only to AUA but also to AUG and to AUU (AUA > AUG > AUU) (Mandal,D. and Köhrer,C. et al., unpublished data).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
US National Institutes of Health [GM17151 to U.L.R., GM58843 to P.A.L.]. Funding for open access charge: National Institutes of Health [GM17151 to U.L.R.].
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Annmarie McInnis for her usual cheerfulness and help in preparing this article. We also thank Céline Fabret (CNRS, Université Paris XI, Orsay, France) and Etienne Derwyn (INRA, Jouy-en-Josas, France) for providing the B. subtilis strains used in this work.
REFERENCES
- 1.Khorana HG. Nobel Lectures. Elsevier Publishing Company; 1968. Nucleic acid synthesis in the study of the genetic code. [Google Scholar]
- 2.Nirenberg M. Nobel Lectures. Elsevier Publishing Company; 1968. The genetic code. [Google Scholar]
- 3.Crick FH. Codon-anticodon pairing: the wobble hypothesis. J. Mol. Biol. 1966;19:548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- 4.Agris PF, Vendeix FAP, Graham WD. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Yokobori S, Kitamura A, Grosjean H, Bessho Y. Life without tRNAArg-adenosine deaminase TadA: evolutionary consequences of decoding the four CGN codons as arginine in Mycoplasmas and other Mollicutes. Nucleic Acids Res. 2013;41:6531–6543. doi: 10.1093/nar/gkt356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senger B, Auxilien S, Englisch U, Cramer F, Fasiolo F. The modified wobble base inosine in yeast tRNAIle is a positive determinant for aminoacylation by isoleucyl-tRNA synthetase. Biochemistry. 1997;36:8269–8275. doi: 10.1021/bi970206l. [DOI] [PubMed] [Google Scholar]
- 7.Munz P, Leupold U, Agris P, Kohli J. In vivo decoding rules in Schizosaccharomyces pombe are at variance with in vitro data. Nature. 1981;294:187–188. doi: 10.1038/294187a0. [DOI] [PubMed] [Google Scholar]
- 8.Curran JF. Decoding with the A:I wobble pair is inefficient. Nucleic Acids Res. 1995;23:683–688. doi: 10.1093/nar/23.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada F, Nishimura S. Purification and characterization of AUA specific isoleucine transfer ribonucleic acid from Escherichia coli B. Biochemistry. 1974;13:300–307. doi: 10.1021/bi00699a011. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988;336:179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- 11.Ikeuchi Y, Kimura S, Numata T, Nakamura D, Yokogawa T, Ogata T, Wada T, Suzuki T. Agmatine-conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nat. Chem. Biol. 2010;6:277–282. doi: 10.1038/nchembio.323. [DOI] [PubMed] [Google Scholar]
- 12.Köhrer C, Srinivasan G, Mandal D, Mallick B, Ghosh Z, Chakrabarti J, RajBhandary UL. Identification and characterization of a tRNA decoding the rare AUA codon in Haloarcula marismortui. RNA. 2008;14:117–126. doi: 10.1261/rna.795508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal D, Köhrer C, Su D, Russell SP, Krivos K, Castleberry CM, Blum P, Limbach PA, Soll D, RajBhandary UL. Agmatidine, a modified cytidine in the anticodon of archaeal tRNAIle, base pairs with adenosine but not with guanosine. Proc. Natl Acad. Sci. USA. 2010;107:2872–2877. doi: 10.1073/pnas.0914869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosjean H, Björk GR. Enzymatic conversion of cytidine to lysidine in anticodon of bacterial tRNAIle - an alternative way of RNA editing. Trends Biochem. Sci. 2004;29:165–168. doi: 10.1016/j.tibs.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Miyauchi K. Discovery and characterization of tRNAIle lysidine synthetase (TilS) FEBS Lett. 2010;584:272–277. doi: 10.1016/j.febslet.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 16.Phillips G, de Crecy-Lagard V. Biosynthesis and function of tRNA modifications in Archaea. Curr. Opin. Microbiol. 2011;14:335–341. doi: 10.1016/j.mib.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Fabret C, Dervyn E, Dalmais B, Guillot A, Marck C, Grosjean H, Noirot P. Life without the essential bacterial tRNAIle2-lysidine synthetase TilS: a case of tRNA gene recruitment in Bacillus subtilis. Mol. Microbiol. 2011;80:1062–1074. doi: 10.1111/j.1365-2958.2011.07630.x. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Suzuki T. Chaplet column chromatography: isolation of a large set of individual RNAs in a single step. Methods Enzymol. 2007;425:231–239. doi: 10.1016/S0076-6879(07)25010-4. [DOI] [PubMed] [Google Scholar]
- 19.Köhrer C, RajBhandary UL. The many applications of acid urea polyacrylamide gel electrophoresis to studies of tRNAs and aminoacyl-tRNA synthetases. Methods. 2008;44:129–138. doi: 10.1016/j.ymeth.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moazed D, Noller HF. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989;57:585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- 21.Triana-Alonso FJ, Spahn CM, Burkhardt N, Rohrdanz B, Nierhaus KH. Experimental prerequisites for determination of tRNA binding to ribosomes from Escherichia coli. Methods Enzymol. 2000;317:261–276. doi: 10.1016/s0076-6879(00)17019-3. [DOI] [PubMed] [Google Scholar]
- 22.Silberklang M, Gillum AM, RajBhandary UL. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- 23.Stanley J, Vassilenko S. A different approach to RNA sequencing. Nature. 1978;274:87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- 24.Gupta RC, Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979;6:3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchino Y, Kato M, Sugisaki H, Nishimura S. Nucleotide sequence of starfish initiator tRNA. Nucleic Acids Res. 1979;6:3459–3469. doi: 10.1093/nar/6.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RajBhandary UL. Recent developments in methods for RNA sequencing using in vitro 32P-labeling. Fed. Proc. 1980;39:2815–2821. [PubMed] [Google Scholar]
- 27.Silberklang M, Gillum AM, RajBhandary UL. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977;4:4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menezes S, Gaston KW, Krivos KL, Apolinario EE, Reich NO, Sowers KR, Limbach PA, Perona JJ. Formation of m2G6 in Methanocaldococcus jannaschii tRNA catalyzed by the novel methyltransferase Trm14. Nucleic Acids Res. 2011;39:7641–7655. doi: 10.1093/nar/gkr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 30.Matthaei JH, Voigt HP, Heller G, Neth R, Schöch G, Kübler H, Amelunxen F, Sander G, Parmeggiani A. Specific interactions of ribosomes in decoding. Cold Spring Harb. Symp. Quant. Biol. 1966;31:25–38. doi: 10.1101/sqb.1966.031.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Simoncsits A, Brownlee GG, Brown RS, Rubin JR, Guilley H. New rapid gel sequencing method for RNA. Nature. 1977;269:833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- 32.Donis-Keller H, Maxam AM, Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977;4:2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockard RE, Alzner-Deweerd B, Heckman JE, MacGee J, Tabor MW, RajBhandary UL. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978;5:37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura S. Chromatographic mobilities of modified nucleotides. In: Schimmel PR, Söll D, Abelson JN, editors. tRNA: Structure, Properties, and Recognition. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1979. pp. 551–552. [Google Scholar]
- 35.Penswick JR, Holley RW. Specific cleavage of the yeast alanine RNA into two large fragments. Proc. Natl Acad. Sci. USA. 1965;53:543–548. doi: 10.1073/pnas.53.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsugi J, Murao K, Ishikura H. Characterization of a B. subtilis minor isoleucine tRNA deduced from tDNA having a methionine anticodon CAT. J. Biochem. 1996;119:811–816. doi: 10.1093/oxfordjournals.jbchem.a021312. [DOI] [PubMed] [Google Scholar]
- 37.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutsch C, El Yacoubi B, de Crecy-Lagard V, Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J. Biol. Chem. 2012;287:13666–13673. doi: 10.1074/jbc.M112.344028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lauhon CT. Mechanism of N6-threonylcarbamoyladenonsine (t6A) biosynthesis: isolation and characterization of the intermediate threonylcarbamoyl-AMP. Biochemistry. 2012;51:8950–8963. doi: 10.1021/bi301233d. [DOI] [PubMed] [Google Scholar]
- 40.Golovina AY, Sergiev PV, Golovin AV, Serebryakova MV, Demina I, Govorun VM, Dontsova OA. The yfiC gene of E. coli encodes an adenine-N6 methyltransferase that specifically modifies A37 of tRNA1Val(cmo5UAC) RNA. 2009;15:1134–1141. doi: 10.1261/rna.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Björk GR. Modified nucleosides at positions 34 and 37 of tRNAs and their predicted coding capacities. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. Washington, DC: American Society for Microbiology; 1998. pp. 577–581. [Google Scholar]
- 42.Taniguchi T, Miyauchi K, Nakane D, Miyata M, Muto A, Nishimura S, Suzuki T. Decoding system for the AUA codon by tRNAIle with the UAU anticodon in Mycoplasma mobile. Nucleic Acids Res. 2013;41:2621–2631. doi: 10.1093/nar/gks1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustilo EM, Vendeix FA, Agris PF. Trna’s modifications bring order to gene expression. Curr. Opin. Microbiol. 2008;11:134–140. doi: 10.1016/j.mib.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasvall SJ, Chen P, Björk GR. The wobble hypothesis revisited: uridine-5-oxyacetic acid is critical for reading of G-ending codons. RNA. 2007;13:2151–2164. doi: 10.1261/rna.731007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson MJ, Esberg A, Huang B, Björk GR, Bystrom AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell Biol. 2008;28:3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takai K, Takaku H, Yokoyama S. Codon-reading specificity of an unmodified form of Escherichia coli tRNA1Ser in cell-free protein synthesis. Nucleic Acids Res. 1996;24:2894–2899. doi: 10.1093/nar/24.15.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takai K, Okumura S, Hosono K, Yokoyama S, Takaku H. A single uridine modification at the wobble position of an artificial tRNA enhances wobbling in an Escherichia coli cell-free translation system. FEBS Lett. 1999;447:1–4. doi: 10.1016/s0014-5793(99)00255-0. [DOI] [PubMed] [Google Scholar]
- 48.Yarian C, Marszalek M, Sochacka E, Malkiewicz A, Guenther R, Miskiewicz A, Agris PF. Modified nucleoside dependent Watson-Crick and wobble codon binding by tRNALysUUU species. Biochemistry. 2000;39:13390–13395. doi: 10.1021/bi001302g. [DOI] [PubMed] [Google Scholar]
- 49.Kirino Y, Yasukawa T, Ohta S, Akira S, Ishihara K, Watanabe K, Suzuki T. Codon-specific translational defect caused by a wobble modification deficiency in mutant tRNA from a human mitochondrial disease. Proc. Natl Acad. Sci. USA. 2004;101:15070–15075. doi: 10.1073/pnas.0405173101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosjean H, de Crecy-Lagard V, Marck C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010;584:252–264. doi: 10.1016/j.febslet.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 51.Barrell BG, Anderson S, Bankier AT, de Bruijn MH, Chen E, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, et al. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc. Natl Acad. Sci. USA. 1980;77:3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonitz SG, Berlani R, Coruzzi G, Li M, Macino G, Nobrega FG, Nobrega MP, Thalenfeld BE, Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc. Natl Acad. Sci. USA. 1980;77:3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heckman JE, Sarnoff J, Alzner-DeWeerd B, Yin S, RajBhandary UL. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc. Natl Acad. Sci. USA. 1980;77:3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andachi Y, Yamao F, Muto A, Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J. Mol. Biol. 1989;209:37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- 55.Osawa S, Jukes TH, Watanabe K, Muto A. Recent evidence for evolution of the genetic code. Microbiol. Rev. 1992;56:229–264. doi: 10.1128/mr.56.1.229-264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inagaki Y, Kojima A, Bessho Y, Hori H, Ohama T, Osawa S. Translation of synonymous codons in family boxes by Mycoplasma capricolum tRNAs with unmodified uridine or adenosine at the first anticodon position. J. Mol. Biol. 1995;251:486–492. doi: 10.1006/jmbi.1995.0450. [DOI] [PubMed] [Google Scholar]
- 57.de Crecy-Lagard V, Marck C, Brochier-Armanet C, Grosjean H. Comparative RNomics and modomics in Mollicutes: prediction of gene function and evolutionary implications. IUBMB Life. 2007;59:634–658. doi: 10.1080/15216540701604632. [DOI] [PubMed] [Google Scholar]
- 58.Lustig F, Boren T, Guindy YS, Elias P, Samuelsson T, Gehrke CW, Kuo KC, Lagerkvist U. Codon discrimination and anticodon structural context. Proc. Natl Acad. Sci. USA. 1989;86:6873–6877. doi: 10.1073/pnas.86.18.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lustig F, Boren T, Claesson C, Simonsson C, Barciszewska M, Lagerkvist U. The nucleotide in position 32 of the tRNA anticodon loop determines ability of anticodon UCC to discriminate among glycine codons. Proc. Natl Acad. Sci. USA. 1993;90:3343–3347. doi: 10.1073/pnas.90.8.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olejniczak M, Uhlenbeck OC. tRNA residues that have coevolved with their anticodon to ensure uniform and accurate codon recognition. Biochimie. 2006;88:943–950. doi: 10.1016/j.biochi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Yarus M. Translational efficiency of transfer RNA's: uses of an extended anticodon. Science. 1982;218:646–652. doi: 10.1126/science.6753149. [DOI] [PubMed] [Google Scholar]
- 62.Raftery LA, Egan JB, Cline SW, Yarus M. Defined set of cloned termination suppressors: in vivo activity of isogenetic UAG, UAA, and UGA suppressor tRNAs. J. Bacteriol. 1984;158:849–859. doi: 10.1128/jb.158.3.849-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmeing TM, Voorhees RM, Kelley AC, Ramakrishnan V. How mutations in tRNA distant from the anticodon affect the fidelity of decoding. Nat. Struct. Mol. Biol. 2011;18:432–436. doi: 10.1038/nsmb.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]