Abstract
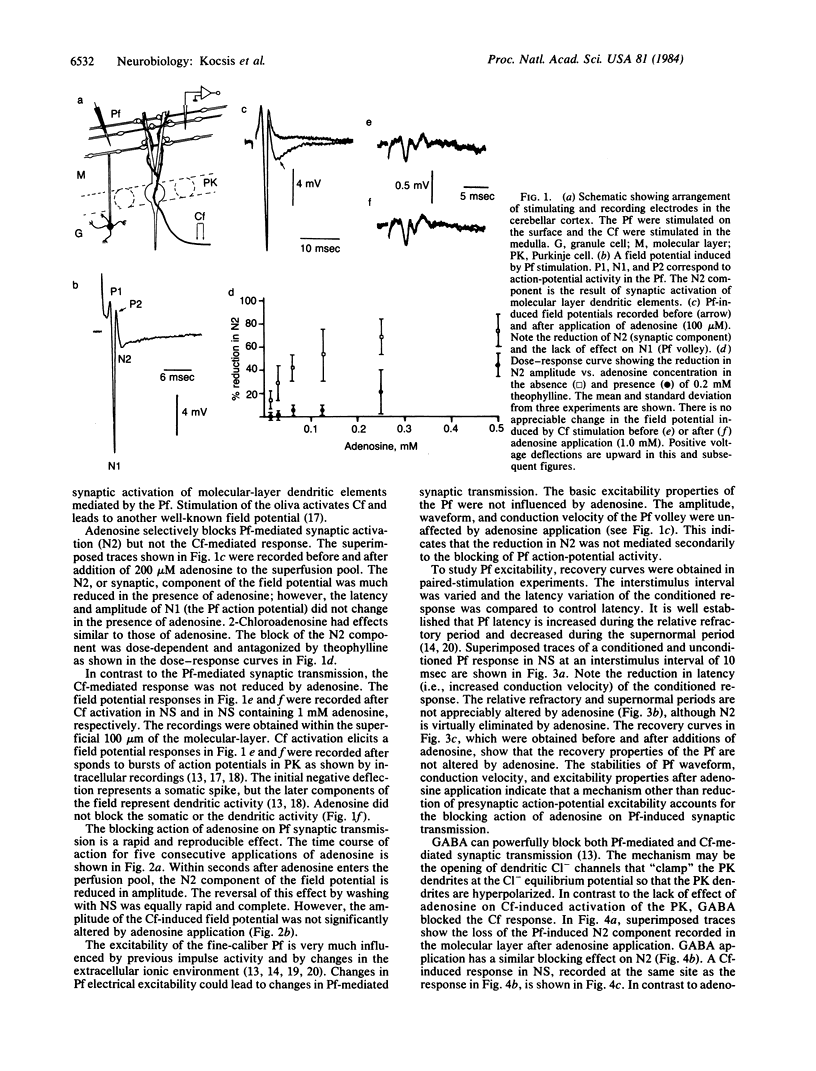
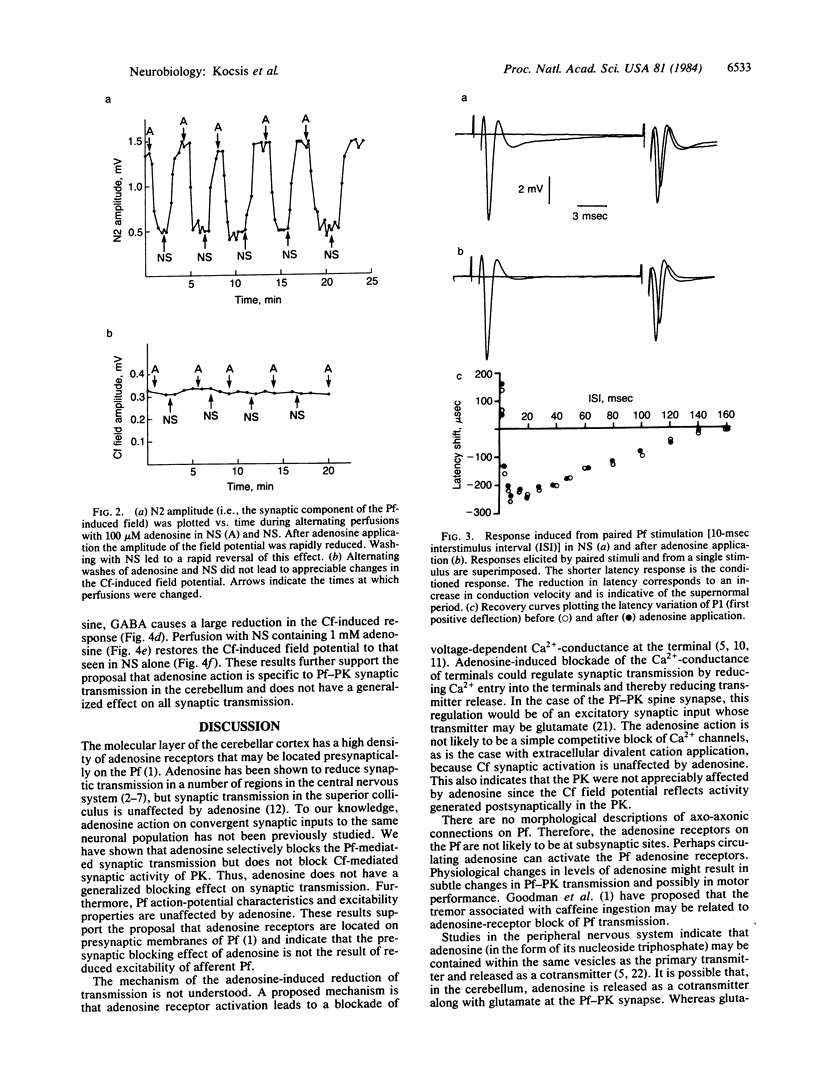
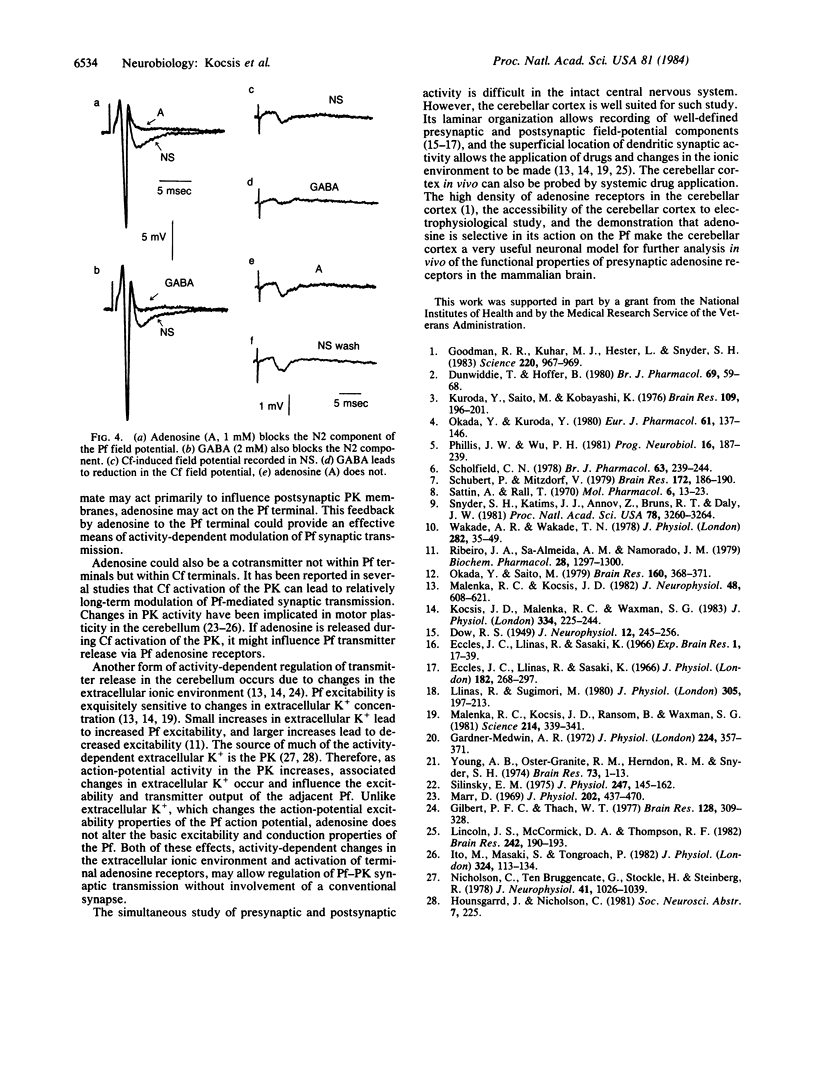
Electrophysiological techniques were used to study the efficacy of adenosine in modulating synaptic transmission mediated from convergent parallel- and climbing-fiber inputs to Purkinje cells. Our results indicate that adenosine application leads to selective blocking of parallel fiber-mediated synaptic activity but not of climbing fiber activity. Adenosine does not alter the action-potential excitability properties of the parallel fibers. However, application of gamma-aminobutyric acid (GABA), which directly affects Purkinje cell dendritic membranes [Malenka, R. C. & Kocsis, J. D. (1982) J. Neurophysiol. 48, 608-621], leads to reduction of both parallel- and climbing-fiber synaptic activity. These results support the proposals that adenosine receptors in the cerebellar cortex are selectively localized on the nonmyelinated parallel fibers and that the blocking action of adenosine is the result of a mechanism other than direct alteration of axon excitability.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunwiddie T. V., Hoffer B. J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980 May;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. An extreme supernormal period in cerebellar parallel fibres. J Physiol. 1972 Apr;222(2):357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. F., Thach W. T. Purkinje cell activity during motor learning. Brain Res. 1977 Jun 10;128(2):309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Kuhar M. J., Hester L., Snyder S. H. Adenosine receptors: autoradiographic evidence for their location on axon terminals of excitatory neurons. Science. 1983 May 27;220(4600):967–969. doi: 10.1126/science.6302841. [DOI] [PubMed] [Google Scholar]
- Ito M., Sakurai M., Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982 Mar;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis J. D., Malenka R. C., Waxman S. G. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol. 1983 Jan;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y., Saito M., Kobayashi K. Concomitant changes in cyclic AMP level and postsynaptic potentials of olfactory cortex slices induced by adenosine derivatives. Brain Res. 1976 Jun 4;109(1):196–201. doi: 10.1016/0006-8993(76)90393-0. [DOI] [PubMed] [Google Scholar]
- Lincoln J. S., McCormick D. A., Thompson R. F. Ipsilateral cerebellar lesions prevent learning of the classically conditioned nictitating membrane/eyelid response. Brain Res. 1982 Jun 17;242(1):190–193. doi: 10.1016/0006-8993(82)90510-8. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Kocsis J. D. Effects of GABA on stimulus-evoked changes in [K+]o and parallel fiber excitability. J Neurophysiol. 1982 Sep;48(3):608–621. doi: 10.1152/jn.1982.48.3.608. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kocsis J. D., Ransom B. R., Waxman S. G. Modulation of parallel fiber excitability by postsynaptically mediated changes in extracellular potassium. Science. 1981 Oct 16;214(4518):339–341. doi: 10.1126/science.7280695. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969 Jun;202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C., ten Bruggencate G., Stöckle H., Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978 Jul;41(4):1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Okada Y., Kuroda Y. Inhibitory action of adenosine and adenosine analogs on neurotransmission in the olfactory cortex slice of guinea pig - structure-activity relationships. Eur J Pharmacol. 1980 Jan 25;61(2):137–146. doi: 10.1016/0014-2999(80)90156-9. [DOI] [PubMed] [Google Scholar]
- Okada Y., Saito M. Inhibitory action adenosine, 5-HT (serotonin) and GABA (gamma-aminobutyric acid) on the postsynaptic potential (PSP) or slices from olfactory cortex and superior colliculus in correlation to the level of cyclic AMP. Brain Res. 1979 Jan 12;160(2):368–371. doi: 10.1016/0006-8993(79)90434-7. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sá-Almeida A. M., Namorado J. M. Adenosine and adenosine triphosphate decrease 45Ca uptake by synaptosomes stimulated by potassium. Biochem Pharmacol. 1979 Apr 15;28(8):1297–1300. doi: 10.1016/0006-2952(79)90428-3. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Scholfield C. N. Depression of evoked potentials in brain slices by adenosine compounds. Br J Pharmacol. 1978 Jun;63(2):239–244. doi: 10.1111/j.1476-5381.1978.tb09752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert P., Mitzdorf U. Analysis and quantitative evaluation of the depressive effect of adenosine on evoked potentials in hippocampal slices. Brain Res. 1979 Aug 17;172(1):186–190. doi: 10.1016/0006-8993(79)90910-7. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Katims J. J., Annau Z., Bruns R. F., Daly J. W. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981 May;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Oster-Granite M. L., Herndon R. M., Snyder S. H. Glutamic acid: selective depletion by viral induced granule cell loss in hamster cerebellum. Brain Res. 1974 Jun 14;73(1):1–13. doi: 10.1016/0006-8993(74)91002-6. [DOI] [PubMed] [Google Scholar]


