Abstract
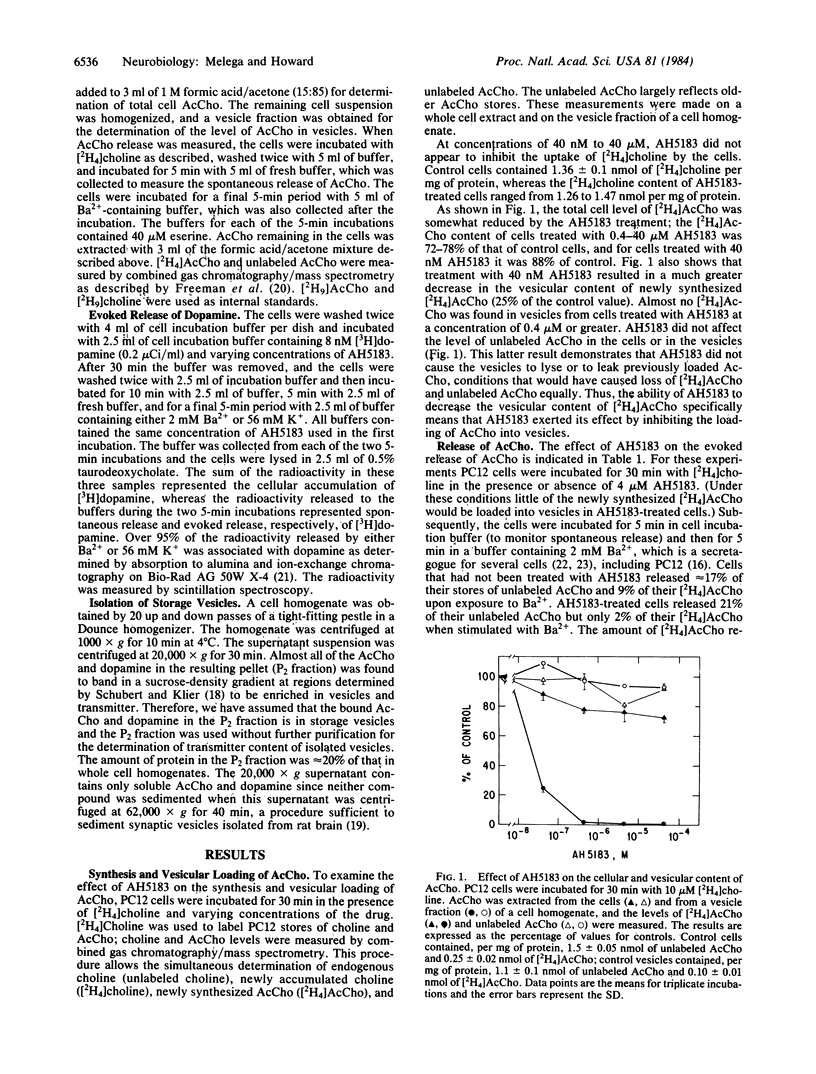
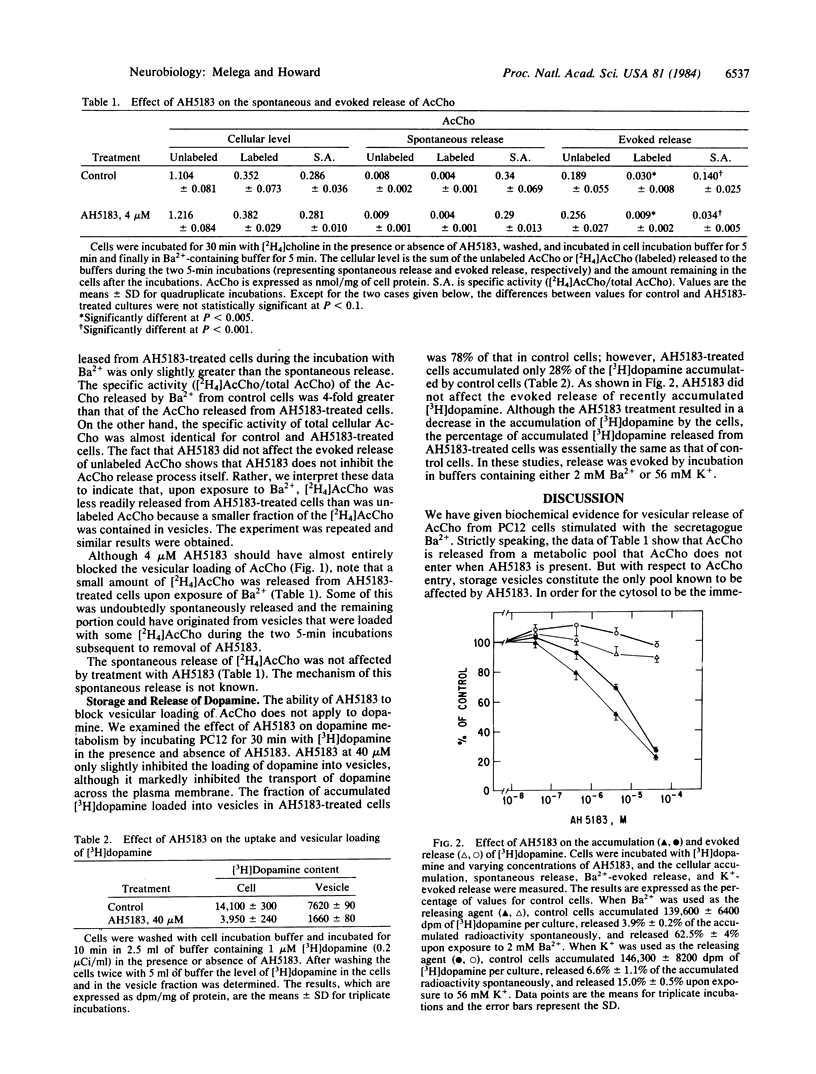
Treatment of PC12 cells with AH5183 at concentrations of 40 nM to 40 microM inhibited the loading of newly synthesized acetylcholine into storage vesicles, but it had little effect on choline uptake, acetylcholine synthesis, or the vesicular content of previously loaded acetylcholine. AH5183 at 4 microM inhibited the Ba2+-evoked release of newly synthesized acetylcholine but not of older stores of acetylcholine. These data indicate that the vesicles are the source of the acetylcholine released from stimulated cells. AH5183 had little effect on the vesicular loading of dopamine and on the evoked release of dopamine, but at concentrations of 4-40 microM it did inhibit dopamine uptake by the cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton T., Howard B. D. Inhibition of dopamine uptake by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a cause of parkinsonism. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1186–1190. doi: 10.1016/0006-291x(84)90901-x. [DOI] [PubMed] [Google Scholar]
- Freeman J. J., Choi R. L., Jenden D. J. Plasma choline: its turnover and exchange with brain choline. J Neurochem. 1975 Apr;24(4):729–734. [PubMed] [Google Scholar]
- Geffen L. B., Livett B. G. Synaptic vesicles in sympathetic neurons. Physiol Rev. 1971 Jan;51(1):98–157. doi: 10.1152/physrev.1971.51.1.98. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L., Axelrod J. Storage and synthesis of norepinephrine in the reserpine-treated rat brain. J Pharmacol Exp Ther. 1966 Mar;151(3):385–399. [PubMed] [Google Scholar]
- Greene L. A., Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977 Jul 1;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Synthesis, storage and release of acetylcholine by a noradrenergic pheochromocytoma cell line. Nature. 1977 Jul 28;268(5618):349–351. doi: 10.1038/268349a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner N., Viveros O. H. The secretory cycle in the adrenal medulla. Pharmacol Rev. 1972 Jun;24(2):385–398. [PubMed] [Google Scholar]
- Kopin I. J. Biochemical aspects of release of norepinephrine and other amines from sympathetic nerve endings. Pharmacol Rev. 1966 Mar;18(1):513–523. [PubMed] [Google Scholar]
- Langston J. W., Ballard P. A., Jr Parkinson's disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med. 1983 Aug 4;309(5):310–310. doi: 10.1056/nejm198308043090511. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Marshall I. G. Studies on the blocking action of 2-(4-phenyl piperidino) cyclohexanol (AH5183). Br J Pharmacol. 1970 May;38(3):503–516. doi: 10.1111/j.1476-5381.1970.tb10592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega W. P., Howard B. D. Choline and acetylcholine metabolism in PC12 secretory cells. Biochemistry. 1981 Jul 21;20(15):4477–4483. doi: 10.1021/bi00518a036. [DOI] [PubMed] [Google Scholar]
- Morris S. J. The structure and stoichiometry of electric ray synaptic vesicles. Neuroscience. 1980;5(9):1509–1516. doi: 10.1016/0306-4522(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Rebois R. V., Reynolds E. E., Toll L., Howard B. D. Storage of dopamine and acetylcholine in granules of PC12, a clonal pheochromocytoma cell line. Biochemistry. 1980 Mar 18;19(6):1240–1248. doi: 10.1021/bi00547a031. [DOI] [PubMed] [Google Scholar]
- Schubert D., Klier F. G. Storage and release of acetylcholine by a clonal cell line. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5184–5188. doi: 10.1073/pnas.74.11.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L., Howard B. D. Evidence that an ATPase and a protonmotive force function in the transport of acetylcholine into storage vesicles. J Biol Chem. 1980 Mar 10;255(5):1787–1789. [PubMed] [Google Scholar]


