Abstract
Objectives
To provide an overview of medication adherence, discuss the potential for smartphone medication adherence applications (adherence apps) to improve medication nonadherence, evaluate features of adherence apps across operating systems (OSs), and identify future opportunities and barriers facing adherence apps.
Practice description
Medication nonadherence is a common, complex, and costly problem that contributes to poor treatment outcomes and consumes health care resources. Nonadherence is difficult to measure precisely, and interventions to mitigate it have been largely unsuccessful.
Practice innovation
Using smartphone adherence apps represents a novel approach to improving adherence. This readily available technology offers many features that can be designed to help patients and health care providers improve medication-taking behavior.
Main outcome measures
Currently available apps were identified from the three main smartphone OSs (Apple, Android, and Blackberry). In addition, desirable features for adherence apps were identified and ranked by perceived importance to user desirability using a three-point rating system: 1, modest; 2, moderate; or 3, high. The 10 highest-rated apps were installed and subjected to user testing to assess app attributes using a standard medication regimen.
Results
160 adherence apps were identified and ranked. These apps were most prevalent for the Android OS. Adherence apps with advanced functionality were more prevalent on the Apple iPhone OS. Among all apps, MyMedSchedule, MyMeds, and RxmindMe rated the highest because of their basic medication reminder features coupled with their enhanced levels of functionality.
Conclusion
Despite being untested, medication apps represent a possible strategy that pharmacists can recommend to nonadherent patients and incorporate into their practice.
Keywords: Smartphones, nonadherence, applications
Medication adherence
Epidemiology of nonadherence
According to the International Society for Pharmacoeconomics and Outcome Research (ISPOR), adherence is “the extent to which a patient acts in accordance with the prescribed interval, and dose of a dosing regimen.”1 Medication nonadherence can affect patient health adversely, negatively impact a patient’s relationship with his/her care provider, skew results of clinical therapy trials, and increase health resource consumption.2,3 Medication nonadherence remains a common health care problem. Poor adherence causes approximately 33% to 69% of medication-related hospitalizations and accounts for $100 billion in annual health care costs.4 Irrespective of disease, medication complexity, or how adherence is measured, the average adherence rate to chronic medication therapy is approximately 50%.5 Adherence monitoring should be performed routinely to ensure therapeutic efficacy, avoid unnecessary dose and regimen changes, contain health care costs, and in certain cases, prevent resistance to therapy from emerging.6,7
Measurement of adherence
Methods to measure adherence, including patient self-reports, pill counts, refill rates, biological monitoring, and electronic monitoring, have limitations and are only proxy measures.6,8,9 Patient self-reports rely on memory and are prone to inaccuracies and recall bias.7 Pill counts are unreliable if patients fail to return bottles or dump pills before the count.4 Biological monitoring (e.g., sampling blood, urine) is either impractical, invasive, or intrusive and does not measure adherence unless the time and dose administered before sampling are verified. Refill rates or electronic monitoring cannot determine whether patients actually take the medication. Although the process of cap removal does not necessarily reflect dose ingestion, medication electronic monitoring systems are useful for calculating adherence rates for dose taking and dose timing and often are viewed as the best method to measure adherence.10–13 Nonetheless, despite their limitations, all of these methods are adequate for documenting nonadherence, but in general, only self-report methods can distinguish among the various types of nonadherence described below.
Types of nonadherence
The cause of medication nonadherence varies among patients and is broadly categorized as unintentional or intentional. Unintentional nonadherence involves intending to take a medication as instructed but failing to do so for some reason (e.g., forgetfulness, carelessness). Unintentional nonadherence is influenced by patient characteristics, treatment factors, and patient–provider issues.14,15 In contrast, intentional nonadherence involves making a reasoned decision not to take a medication as instructed based on perceptions, feelings, or beliefs.14–16 Intentional nonadherence reflects a rational decision-making process by the patient whereby the benefits of treatment are weighed against any adverse effects of the treatment.14,15 Broadly characterizing nonadherence may oversimplify the complexities involved with nonadherence, but it is practical and illustrates that mitigating nonadherence requires different interventions.6
Behavioral models of adherence
Most medication adherence models are based on several social cognition models, including the health belief model,17 social cognitive theory,18 and theory of planned behavior.19 These models are similar, and all assume that beliefs developed by individuals shape how they interpret information and experiences and ultimately influence their behavior.20 Accordingly, health behavior (e.g., medication taking) results from rational decisions based on all available information.15
Methods to improve medication adherence
Many methods to improve medication adherence have been studied. Most methods attempt to change patient behavior by using reminders, counseling, reinforcement, education, dosage simplification, or a combination of these methods.21,22 Generally, adherence interventions are categorized as behavioral, educational, or organizational based on modifying the patient’s environment or incentives, providing more information, or lifting barriers associated with medication complexity and communication with care providers.22
Traditional reminders
Intuitively, pill reminder systems (e.g., weekly pill boxes, packaged calendars, unit-of-use packaging), are helpful adherence aids, especially when nonadherence is unintentional.10,23 Current traditional reminder systems minimally involve the patient in the self-medication process and do not provide them access to their adherence data or other educational information. Although pill reminder systems have been tested and shown to be useful across many medications, these systems are cumbersome for complex regimens and only passively remind patients to take their medication.24,25 Electronic systems proactively deliver reminders by telephone, pager, and audiovisual devices but may be impractical for widespread use and more efficacious if combined with alternative behavioral strategies.26
Counseling and other behavioral interventions
Most studies on improving adherence involve behavioral interventions.27–29 Data suggest that patient education is one of the best methods for improving adherence, especially for those simultaneously managing more than six medications. Depending on the type of nonadherence and patient characteristics, using a combination of tailored interventions such as patient education, patient self-monitoring of specialized care, and stimuli to take medications have the greatest potential for improving adherence.22,28–30
Medication adherence and mobile devices
Smartphones are Internet-ready multipurpose devices that allow constant access to communication and information and perform many tasks. Most tasks are performed by specialized applications (apps) that consumers can easily download and use to assist them in a variety of functions. Using a smartphone app is a novel approach to improving adherence and patient behavior; it is constantly accessible, involves and educates the patient, and provides a repository for patient- and medication-specific information. A smartphone medication adherence–oriented app (adherence apps) can potentially consolidate all of the user’s medication-specific information and thereby provide a more streamlined process to educate the individual about his/her disease or care.
Adherence apps can be downloaded for little to no cost, and their benefits may be realized by anyone taking prescription medications. However, these apps may prove most beneficial for patients with complex medication regimens or for caregivers of others or family members. The growing prevalence of smartphones in the United States and their constant, easy accessibility make adherence apps appealing to many because they cost little and can provide user-specific information.
The number of apps aimed at aiding the user in organizing and taking their medications is increasing across the dominant smartphone platforms. Among the currently marketed adherence apps, features include reminders that can be set for consumption and refills, doses that can be logged, data logs that can be accessed by patients or uploaded to care providers, and medication information (e.g., dosages, adverse effects, toxicities, specialized provider notes), all of which can be immediately accessible with the touch of a finger. In addition, these apps may also include calendar-based alarm reminders with specific dosage or functionality that integrates medication lists with specific drug information or combines pharmacy and primary care contact information or includes prescription drug discount cards. Efforts are under way to integrate smartphones with health-monitoring devices that transmit the output data directly to patients or physicians. Literature on the clinical application of the smartphone and use of apps in areas of health wellness (e.g., weight management) is growing, but empirical analyses of patient use of the smartphones with apps as an aid to facilitate adherence are lacking.31–36
Effectiveness of smartphone adherence apps
Although smartphone apps can potentially improve the effectiveness and reduce the costs of traditional medication adherence interventions, their efficacy is currently untested. Data demonstrate that electronic mobile devices using reminder systems through traditional means of telecommunication, like Short Message Service (SMS) text messaging, improve adherence and behavior and can be useful in measuring adherence in the short term.37 Investigators determined that sending photographs of medication capsules through cellular phones before ingestion provided more accurate time measures of adherence.38 One study found that teenagers with asthma who used a specialized system to create and schedule personal text message reminders gave the system high ratings for acceptability, ease of use, and usefulness; however, their asthma control was similar to baseline.39 In a study using a comprehensive, Web-based education system with Internet and cellular phone access to help control blood glucose levels in patients with diabetes, the frequency of accessing the eMOD (electronic Management of Diabetes) system through a cellular phone was significantly related to the change in glycosylated hemoglobin levels.40 Although a systematic review of Internet-based adherence interventions found promising results, it also found that the 13 studies lacked quality measurements of adherence.41 Various studies of the use of smartphones in the clinical setting have been performed,42 but studies empirically testing smartphone apps to improve adherence are lacking.
Currently available mobile app adherence technologies
Currently, a resource does not exist that compares the features of available apps. To develop such a resource, we searched the available medication adherence–oriented apps, provider websites, and app sources for Apple iTunes, Android Marketplace, and BlackBerry App World during August and September of 2012, which reflects a snapshot of the app market space for that time period. Search terms included adherence, compliance, dose, drug, med(s), medication(s), remind, reminder, Rx, take, therapy, treat, treatment. Apps also were identified using the alphabetical browse feature at each of the three sources. App descriptions and available screenshots were analyzed for content and app functionality.
To be included in our analysis, apps had to be described in English, medication related, capable of generating medication reminders, and available for the Apple (iPhone operating system [iOS]), Android, and/or Black- Berry operating systems (OSs). Apps were excluded if they were designed specifically for one medication type or a single disease. Lastly, those lacking a general description of functionality also were excluded.
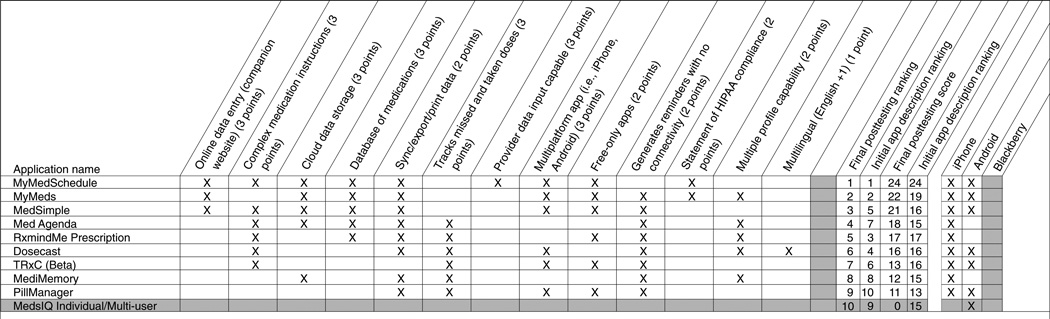
To identify the apps that might have the most utility for patients that could be recommended by pharmacy practitioners, the authors developed a list of desirable attributes of these apps by consensus of all of the authors to evaluate them for comparison. These attributes are described in Table 1, and whether the app possessed each attribute was assessed based on each app’s features described on their website or their respective product listing on their app source (e.g., iTunes). The relative desirability or usefulness of these features then were rated by the study authors using a three-point rating system (1, modest; 2, moderate; or 3, high) based on the perceived importance of each feature or characteristic (Table 1). This rating system was developed based on author consensus before evaluating the individual apps and only reflects the provider/researcher perspectives of the authors and does not directly assess the desirability of these features by patients. This rating system was used to score and ultimately rank the apps identified based on features claimed by manufacturers. After rating all of the apps, the 10 with the highest composite ratings were selected, then two of the authors installed and tested the apps and rated them by directly assessing the feature available against the claimed functionality. To test the apps on their corresponding OSs, a standardized medication regimen consisting of four medications was entered: lisinopril 10 mg daily, naproxen 500 mg twice daily, Neurontin 300 mg three times daily, and a prednisone 20-mg tapering regimen. Apps were evaluated for each manufacturer claim that met the authors’ scoring criteria, functionality of the reminder system, and ability to process reminders from the test medication regimen. Medication reminders were evaluated for each app for a period of at least 24 hours.
Table 1.
Attributes and description of adherence apps
| Attribute | Points | Description |
|---|---|---|
| Online data entry | 3 | App had companion website(s) that allow data and medication regimen entry from a computer. |
| Complex medication instructions | 3 | App had the capability to schedule medication instructions (including medication tapers; dose adminstrations that occurred nondaily, monthly, every X days; or medications with stop dates) that were considered complex. |
| Cloud data storage | 3 | App had the capability to back up and retrieve a medication regimen from a cloud storage system. |
| Database of medications | 3 | A medication database was available that allowed the user to enter, search, and select medications using features such as autopopulation. |
| Sync/export/print data | 3 | App had the capability to transmit, print, or export medication regimens and/or medication-taking behaviors for use by the patient or health care providers. |
| Tracks missed and taken doses | 3 | App had the capability to remind patients to take their medication and to record taken and missed doses that could potentially be used to calculate adherence rates. |
| Provider data input capable | 3 | App allows providers to input and maintain the patient’s medication regimen and “push” the regimen to the patient’s device. |
| Multiple platform app | 3 | App was available on more than one platform. (No apps were identified that were available on all three operating systems [iOS, Android, and Blackberry].) |
| Free-only apps | 2 | App was completely free (i.e., no fees for pro upgrades or charges to unlock additional features). |
| Generates reminders with no connectivity | 2 | App had the capability to generate medication reminders without the use of cellular (3G/4G/LTE) or wireless (Wi-Fi) connectivity. |
| Statement of HIPAA compliance | 2 | App had a statement from their manufacturer claiming HIPAA compliance. |
| Multiple profile capable | 2 | App had the capability to generate medication reminders for multiple individuals on different medications (i.e., enabled family use). |
| Multilingual | 1 | App was available in English plus any other language. |
Abbreviations used: HIPAA, Health Insurance Portability and Accountability Act; iOS, iPhone operating system.
Rating based on author consensus weighting of attributes by point scale.
Our search included 160 app descriptions in the three online marketplaces, yielding 147 unique apps after incorporating multiplatform functionality. There were 13 multiplatform apps, with 12 apps available on both iOS and Android platforms and only one app available for use on both the Android and Blackberry OSs. Because a portion of the apps are available across multiple platforms and the descriptions and feature may vary by platform, we evaluated 160 unique, platform-specific apps. Table 2 summarizes the frequency of the medication app features or characteristics by OS. We found that the Android OS had the most medication adherence apps available, followed by iOS and Blackberry. Although the ability to track missed doses and export data for provider analysis are important tools to assess adherence, these features were available on only 29.4% and 24.4% of the apps evaluated, respectively. Not surprisingly, notable differences existed among the OS platforms. More multilingual apps are available for the iOS (21.3%) compared with the Android (10.7%) and BlackBerry (0%) OSs. In addition, advanced features, including the ability to export medication regimen or medication-taking behavior data from the device or the ability to manage multiple patient profiles are more prevalent on apps using the iOS platform (36.1% and 32.8%, respectively) compared with the Android (19% and 15.5%, respectively) or Blackberry (6.7% and 20%, respectively) OSs. Lastly, Blackberry OS apps were the most expensive compared with apps using other OSs.
Table 2.
Frequency of medication app characteristics by operating system
| Attribute | iPhone No. (%) |
Android No. (%) |
Blackberry No. (%) |
Total No. (%) |
|---|---|---|---|---|
| Total apps | 61 (38.1) | 84 (52.5) | 15 (9.4) | 160 (100) |
| Online data entry | 2 (3.3) | 8 (9.5) | 1 (6.7) | 11 (6.9) |
| Complex medication instructions | 12 (19.7) | 7 (8.3) | 2 (13.3) | 21 (13.1) |
| Cloud data storage | 4 (6.6) | 5 (6) | 2 (13.3) | 11 (6.9) |
| Database of medications | 4 (6.6) | 6 (7.1) | 0 (0) | 10 (6.3) |
| Sync/export/print data | 22 (36.1) | 16 (19) | 1 (6.7) | 39 (24.4) |
| Tracks missed and taken doses | 23 (37.7) | 19 (22.6) | 5 (33.3) | 47 (29.4) |
| Provider data input capable | 1 (1.6) | 2 (2.4) | 0 (0) | 3 (1.9) |
| Multiple platform app | 11 (18) | 12 (14.3) | 2 (13.3) | 25 (15.6) |
| Free-only apps | 13 (21.3) | 48 (57.1) | 6 (40) | 67 (41.9) |
| Generates reminders with no connectivity | 53 (86.9) | 73 (86.9) | 13 (86.7) | 139 (86.9) |
| Statement of HIPAA compliance | 2 (3.3) | 3 (3.6) | 0 (0) | 5 (3.1) |
| Multiple profile capable | 20 (32.8) | 13 (15.5) | 3 (20) | 36 (22.5) |
| Multilingual (32 languages) | 13 (21.3) | 9 (10.7) | 0 (0) | 22 (13.8) |
| Average price ($) | 2.21 | 2.18 | 4.10 | 2.83 |
Abbreviation used: HIPAA, Health Insurance Portability and Accountability Act.
A total of 10 apps with the highest number of desirable features claimed by their developers were installed on their respective platforms for testing and evaluation of actual functionality. Each app that was available on multiple platforms (i.e., iOS, Android OS) was tested and evaluated on each available OS. Of the 10 apps that were installed and subjected to direct testing, 6 met or exceeded manufacturer claims listed on marketplace websites. The features available for these 10 apps are described in Figure 1. Overall, the majority of apps were intuitive, easy to use, met developer claims, and provided satisfactory medication reminders. Only one app, MediMemory, stood out as being more cumbersome and difficult to set up initially. The reminders generated by most of the apps resembled a SMS text message or a “push notification” style alert. Most apps provide a generic initial reminder, such as “you have a medication to take,” at which time you can open the app to see what medication is due. Some apps, including Med Agenda, Dosecast, and MedSimple display the drug, dose, and/or strength of the medication you are to take on your homescreen within the reminder itself, which may be a more effective reminder for patients who take multiple medications but also may pose patient privacy concerns. Apps that track doses taken or missed, including Med Agenda, RxmindMe, Dosecast, Med-sIQ, PillManager, and MediMemory, require a box to be checked to indicate that a dose has been taken or skipped with each reminder. Apps lacking dose-tracking capabilities generally provide simple alert-type reminders.
Figure 1.
Attributes and ratings of the 10 apps that were installed and subjected to user testing
Abbreviation used: HIPAA, Health Insurance Portability and Accountability Act.
Although the functionality of most apps matched the app descriptions, three apps possessed more features than described in their respective app description, resulting in a higher overall rating. MedsIQ could not be opened and used following installation on a variety of Android devices and was rated as a zero; we would not recommend use of this app. The rank of another app, MediMemory, decreased when it was unable to process complex medication instructions such as stop dates or medication tapers. TRxC, a multiplatform app, did not possess several of the claimed features upon testing, and its companion website could only be used if the patient’s provider entered an agreement with the app developer. PillManager’s scoring was reduced when multilingual functionality could not be found on the Android app.
Figure 1 can serve as a starting point for pharmacists to identify apps to possibly recommend to patients or use in their practice. Many of these apps, if used consistently, could be an asset to patients who struggle with their medication regimens. From a provider perspective, apps with functionality beyond a simple medication reminder system may offer significant advantages. Providers might find value in features such as the ability to input patients’ regimens and then “pushing” them to patients’ devices; the ability to process complex medication-taking instructions; multiple-platform functionality; the use of a cloud back-up system; the ability to export data, including administered and missed doses; and the incorporation of a medication database to assist in accurate data entry into the device.
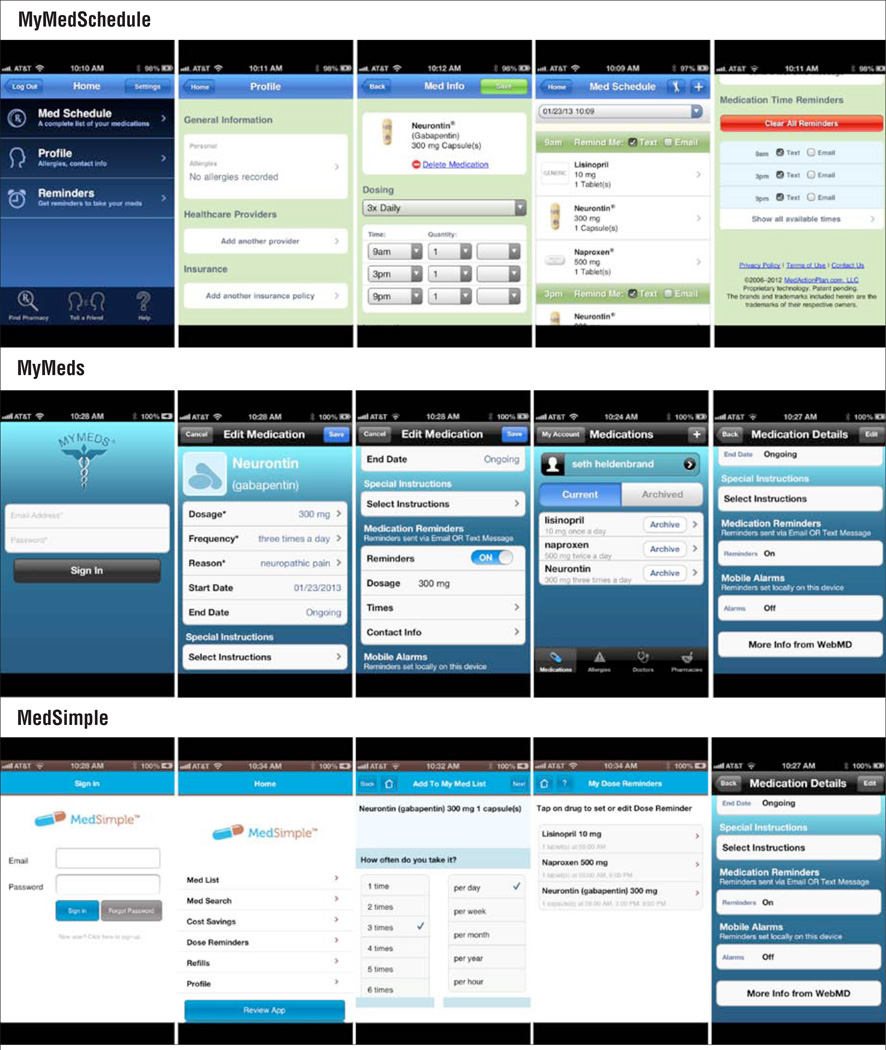
Providers caring for patients taking complex medication regimens may value having the ability to input patients’ prescribed regimens and then pushing them directly to patients’ devices. This feature removes the data entry burden from the patient and reduces the possibility of patient-generated medication regimen mistakes. Websites like www.medactionplan.com, the companion site for the app MyMedSchedule (Figure 2), offer regimen-building options that are useful for patients in specialty areas that often carry a high medication burden (e.g., organ transplant, human immunodeficiency virus [HIV], hematology, oncology). Health professionals can enter simple or complex medication regimens using extensive medications databases that can be pushed to patients’ mobile device with reminders enabled. Regimens also are stored on a Health Insurance Portability and Accountability Act (HIPAA)-compliant cloud server that enables patient profiles to be retrieved and modified with any new instructions and then resent to patients’ devices on subsequent encounters.
Figure 2.
MyMedSchedule, MyMeds, and MedSimple
Other apps feature companion websites for patient use. MyMeds (Figure 2) and its companion website www.my-meds.com enable patients to enter their regimens online (subscription fee of $5.99/year) rather than doing so only via their device. This feature offers the convenience of HIPAA-compliant cloud data storage that can be retrieved and modified from any Web-accessible computer and pushed back to patients’ mobile devices.
RxmindMe (Figure 2), MyMedSchedule, and MyMeds feature medication databases that enable patients or providers to save time and improve accuracy when entering regimens. However, only RxmindMe and MyMeds have the capability to track taken and missed doses and export that data to health professionals for review. When the patient properly uses the app, this feature can provide information to help health care providers assess medication adherence.
Simple medication reminder systems may be suitable for most patients needing assistance with their regimens, and all of the apps evaluated fit this criterion. However, health care providers are likely to appreciate a higher level of functionality and evaluative capabilities provided by MyMedSchedule, MyMeds, and RxmindMe. The basic medication reminder features coupled with their enhanced levels of functionality led to their being ranked as the top three apps.
Implications for pharmacy practice and future directions
There has been a proliferation of consumer- rather than clinician-oriented adherence apps. We evaluated more than 160 apps and suspect that many more will be available in the foreseeable future, until the market consolidates to the apps that garner a substantial market share and support a viable business model. As previously stated, currently no evidence exists regarding the efficacy or effectiveness of apps at actually improving adherence or clinically relevant outcomes. This lack of data underscores a critical need for research in this field. However, insights can be gained from data regarding the effectiveness of SMS text messaging, which uses similar prompts as those provided by mobile apps. Three of four studies demonstrate that SMS text messaging improved adherence in several chronic conditions.37 Given that many apps possess greater functionality that includes customizable audio and visual prompts capable of providing more robust reminders, the efficacy of apps may be equal to or greater than SMS text messaging.
Adherence apps can only be used by individuals who have access to a smartphone, which includes 55% of the U.S. adult population.43 Smartphone ownership is more likely for those with higher incomes and of younger age; however, between 14% and 42% of people 65 years or older own smartphones depending on income, suggesting that a sizable proportion of the older population has access to these devices. Clearly, these evolving technologies will not be available to all patients in a pharmacist’s practice but do represent a viable alternative for a large and growing proportion of the population. Another key limitation of the current market is that adherence apps are targeted primarily to consumers rather than health care professionals (e.g., pharmacists) to assist patients with adherence. Nearly all adherence apps require patients to enter and manage their prescription data, and most require manual data entry on the smartphone device by patients. Of the apps evaluated in this report, only five had a drug database that enabled autocompletion and/or allowed users to select prescription products. Further evidence that marketed adherence apps are more oriented toward consumers is that only three apps had a HIPAA policy statement, which would be an essential feature for providers to consider. Despite these limitations, adherence apps represent a low-cost strategy that could be incorporated into a variety of pharmacy services, including medication reconciliation and discharge planning in institutional settings and medication therapy management or other services in the community, ambulatory, or other outpatient practice settings. Some community chains already have incorporated adherence features into their brand apps; however, the extent to which these are being incorporated into pharmacists’ practices in these settings is unknown.
Interconnectivity of adherence apps
Currently, very few marketed adherence apps are interconnected with other information systems. Of the apps evaluated in this report, five offer cloud storage of prescription data, of which only one has a companion website. However, none of the apps evaluated is fully integrated with patient record systems (e.g., community pharmacy prescription databases, electronic medical records). Therefore, interoperability with existing prescription and medical records systems represents a vital frontier for future app development. For example, interfacing adherence apps with pharmacy prescription records could enable pharmacists to easily push patients’ drug regimens to smartphone apps, allowing seamless transmission of reminders to patients. More importantly, pharmacists and patients could collaborate to customize reminders for regimens where adherence has proven to be challenging. This interoperability would shift the current orientation of adherence apps to a more provider-focused technology and thereby provide pharmacists a potentially valuable tool to improve medication adherence.
In addition to connectivity to medical records systems, smartphone apps can be interfaced or synced to other devices with adherence capabilities. On the forefront of these technologies is Proteus Digital Health Feedback System (the Proteus system; www.proteusbiomed.com/technology), whereby solid oral dosage forms are combined with ingestible sensors that record basic physiologic parameters after ingestion and transmit the information to a dermal sensor or patch that can sync with smartphone devices. This unique system can record actual ingestion of individual doses and the precise timing of ingestion—the “holy grail” of adherence measurement. Adherence apps working in tandem with such a system could send customized reminders only when doses are actually missed. Although such an integrated system has obvious applicability to the clinical trial research setting, its cost and the inconvenience of wearing a monitoring patch likely limit its utility in routine clinical practice to therapies for which adherence is especially critical (e.g., management in tuberculosis and HIV infections). Several technologies for diabetes self- and automated monitoring have been developed and can be synced with smartphone devices. These integrated systems may hold similar promise to monitor and improve adherence in diabetes.44,45 Electronic pill boxes that are equipped to monitor box openings, wirelessly transmit a signal to servers when a scheduled opening is missed, and then send SMS text messages reminders have been shown to improve adherence.46
Tailoring the reminders
Currently, most adherence apps use a simple reminder and are primarily directed to mitigate unintentional nonadherence. These apps are a component of a multipurpose device; therefore, unlike previous reminder systems, patients do not have to be near or carry a separate device that enhances fidelity to the “reminder” device. However, the content of the adherence app messaging is at the nascent stage of development. Although the content of the reminders for the apps evaluated in this report was not formally assessed, few if any appeared to be tailored to patients based on proven theoretical behavioral models. A Cochrane review of randomized trials of adherence interventions showed that less than one-half improve both long-term adherence and clinical outcomes and that the most effective involved complex combinations and multiple strategies to improve adherence.29 Therefore, adherence apps will likely need to be incorporated into a multimodal strategy to result in sustained improvements in adherence. Because reminders primarily focus on unintentional nonadherence, identifying the reasons for nonadherence and developing a scale that assesses unintentional nonadherence47 would be a useful starting point toward effectively deploying app-based reminders. Tailoring messages to specific patient needs based on previous adherence behavior and incorporating theoretical behavioral models represent an area of future improvement for app developers. Behavioral models such as the transtheoretical model stages of change48 or motivational interviewing49 could be used to develop tailored messages with the potential to decrease unintentional and intentional nonadherence. Studies using smartphones combined with tailored interventions to improve dietary intake of fruits and vegetables are under way, and similar strategies should be tested to improve adherence.50 The potential for patient response to decrease with repeated reminders delivered by a smartphone (i.e., habituation) is a challenge to improving adherence to chronic therapy. Currently, whether and to what extent habituation will occur is unknown. Therefore, efforts to develop and study the impact of content that is constantly refreshed based on patient attributes, including feedback of their current adherence level, are necessary to determine the impact of these strategies on long-term adherence.
Prescription for apps
During the next decade, providers and payers may be looking to the app market to improve patient care and outcomes, as apps may be “medically prescribed” and paid for by health payers.51 For example, hospital systems in the New York City area are allowing physicians to prescribe health-oriented apps, and in the foreseeable future, payers may begin covering a diabetes care app that has been proven effective in mitigating nonadherence in a clinical trial setting. Medically prescribed and reimbursed apps will provide the financial incentive for app developers to build products, including adherence apps, that generate empirical evidence of improved patient outcomes. The Food and Drug Administration (FDA) has developed draft guidelines to regulate certain mobile apps that they are defining as “medical mobile apps,” and Congress recently cleared the pathway allowing FDA to regulate mobile devices.52 Although apps that are designated as medical mobile apps or are part of FDA-regulated devices have the most clear guidance, the regulatory framework for stand-alone apps such as adherence apps is less clear and whether payers will consider payment for unregulated apps is unknown.
Conclusion
Despite decades of research, medication nonadherence still represents a fundamental health care challenge. Adherence apps are inexpensive, scalable, accessible to anyone with smartphones, and do not require separate devices or packaging, which allows them to be easily implemented. Despite not being tested in trial settings, they could be considered a possible strategy for pharmacists to recommend to nonadherent patients and to incorporate into their practice. Although none of the evaluated apps possess every desirable attribute, three apps were identified that offer the widest range of features and would be the most appropriate to recommend to patients currently. Research is needed to determine whether and how effectively apps can improve adherence and therapeutic outcomes in acute and chronic conditions.
At a Glance
Synopsis
Medication adherence applications (apps) available on three main smartphone operating systems (OSs; Apple, Android, and Blackberry) were evaluated, and the authors gave MyMedSchedule, MyMeds, and RxmindMe the highest ratings based on their wide range of features and enhanced levels of functionality. Although they have not been tested in trial settings, adherence apps could be considered a possible strategy for pharmacists to recommend to nonadherent patients. The apps can be easily implemented because they are inexpensive, scalable, accessible to anyone with smartphones, and do not require separate devices or packaging.
Analysis
Using a smartphone app is a novel approach to improving adherence and patient behavior; it is constantly accessible, involves and educates the patient, and provides a repository for patient- and medication-specific information. Providers caring for patients taking complex medication regimens may value having the ability to input patients’ prescribed regimens and then “pushing” them directly to patients’ smartphones. Interoperability with existing prescription and medical records systems represents a vital frontier for future app development. This interoperability would shift the current orientation of adherence apps to a more provider-focused technology and thereby provide pharmacists a potentially valuable tool to improve medication adherence.
Acknowledgments
Funding: Dr. Martin’s work was partly supported by the Translational Research Institute, University of Arkansas for Medical Sciences (grant 1UL1RR029884).
Footnotes
Disclosure: The authors declare no conflicts of interest or financial interests in any product or service mentioned in this article, including grants, employment, gifts, stock holdings, or honoraria.
References
- 1.Cramer JA, Roy A, Burell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 3.Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11(6):1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 4.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 5.Iihara N, Tsukamoto T, Morita S, et al. Beliefs of chronically ill Japanese patients that lead to intentional non-adherence to medication. J Clin Pharm Ther. 2004;29(5):417–424. doi: 10.1111/j.1365-2710.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 6.Garfield S, Clifford S, Eliasson L, et al. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149. doi: 10.1186/1471-2288-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weingart SN, Brown E, Bach PB, et al. NCCN task force report: oral chemotherapy. J Natl Compr Cancer Netw. 2008;6(suppl 3):S1–S14. [PubMed] [Google Scholar]
- 8.Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261(22):3273–3277. [PubMed] [Google Scholar]
- 9.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 10.Rolnick S, Pawloski P, Bruzek R, et al. PS2-32: barriers and facilitators for medication adherence. Clin Med Res. 2011;9(3–4):157. [Google Scholar]
- 11.Spilker B. Methods of assessing and improving compliance in clinical trials. In: Cramer JA, Spilker B, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. pp. 37–56. [Google Scholar]
- 12.Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. J Acquir Immune Defic Syndr. 2002;31(suppl 3):S103–S106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- 13.Urquhart J. The electronic medication event monitor: lessons for pharmacotherapy. Clin Pharmacokinet. 1997;32(5):345–356. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25(4):355–372. doi: 10.1023/a:1015866415552. [DOI] [PubMed] [Google Scholar]
- 15.Lehane E, McCarthy G. Intentional and unintentional medication non-adherence: a comprehensive framework for clinical research and practice? A discussion paper. Int J Nurs Stud. 2007;44(8):1468–1477. doi: 10.1016/j.ijnurstu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Daleboudt G, Broadbent E, McQueen F, Kaptein AA. Intentional and unintentional treatment nonadherence in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63(3):342–350. doi: 10.1002/acr.20411. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15(2):175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 18.Bandura A. Social foundations of thought and action: a social cognitive theory. Upper Saddle River, NJ: Prentice Hall; 1986. [Google Scholar]
- 19.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 20.Weinman J, Petrie J, editors. Perceptions of health & illness. Amsterdam: Harwood Academic; 1997. [Google Scholar]
- 21.Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet. 1996;348(9024):383–386. doi: 10.1016/s0140-6736(96)01073-2. [DOI] [PubMed] [Google Scholar]
- 22.Graves MM, Roberts MC, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. J Pediatr Psychol. 2010;35(4):368–382. doi: 10.1093/jpepsy/jsp072. [DOI] [PubMed] [Google Scholar]
- 23.Harbig P, Barat I, Damsgaard EM. Suitability of an electronic reminder device for measuring drug adherence in elderly patients with complex medication. J Telemed Telecare. 2012;18(6):352–356. doi: 10.1258/jtt.2012.120120. [DOI] [PubMed] [Google Scholar]
- 24.Mahtani KR, Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2011;9:CD005025. doi: 10.1002/14651858.CD005025.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Zedler BK, Kakad P, Colilla S, et al. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33(1):62–73. doi: 10.1016/j.clinthera.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Fenerty SD, West C, Davis SA, et al. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127–135. doi: 10.2147/PPA.S26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bain-Brickley D, Butler LM, Kennedy GE, Rutherford GW. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev. 2011;12:CD009513. doi: 10.1002/14651858.CD009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conn VS, Hafdahl AR, Cooper PS, et al. Interventions to improve medication adherence among older adults: meta-analysis of adherence outcomes among randomized controlled trials. Gerontologist. 2009;49(4):447–462. doi: 10.1093/geront/gnp037. [DOI] [PubMed] [Google Scholar]
- 29.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Williams A, Manias E, Walker R. Interventions to improve medication adherence in people with multiple chronic conditions: a systematic review. J Adv Nurs. 2008;63(2):132–143. doi: 10.1111/j.1365-2648.2008.04656.x. [DOI] [PubMed] [Google Scholar]
- 31.Delpier T, Giordana S, Wedin BM. Decreasing sugar-sweetened beverage consumption in the rural adolescent population. J Pediatr Health Care. doi: 10.1016/j.pedhc.2012.07.002. [published online ahead of print, August 26, 2012] [DOI] [PubMed] [Google Scholar]
- 32.Gamble KH. Beyond phones: with the proper infrastructure, smartphones can help improve clinician satisfaction and increase EMR use. Healthc Inform. 2009;26(8):23–44. 26. [PubMed] [Google Scholar]
- 33.Kharrazi H, Chisholm R, Vannasdale D, Thompson B. Mobile personal health records: an evaluation of features and functionality. Int J Med Inform. 2012;81(9):579–593. doi: 10.1016/j.ijmedinf.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Lee DS, Jeon BG, Ihm C, et al. A simple and smart telemedicine device for developing regions: a pocket-sized colorimetric reader. Lab Chip. 2011;11(1):120–126. doi: 10.1039/c0lc00209g. [DOI] [PubMed] [Google Scholar]
- 35.Sposaro F, Tyson G. iFall: an Android application for fall monitoring and response; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 6119–6122. [DOI] [PubMed] [Google Scholar]
- 36.Wohlers EM, Sirard JR, Barden CM, Moon JK. Smart phones are useful for food intake and physical activity surveys; Conf Proc IEEE Eng Med Biol Soc; 2009. pp. 5183–5186. [DOI] [PubMed] [Google Scholar]
- 37.Vervloet M, Linn AJ, van Weert JC, et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galloway GP, Coyle JR, Guillen JE, et al. A simple, novel method for assessing medication adherence: capsule photographs taken with cellular telephones. J Addict Med. 2011;5(3):170–174. doi: 10.1097/ADM.0b013e3181fcb5fd. [DOI] [PubMed] [Google Scholar]
- 39.Britto MT, Munafo JK, Schoettker PJ, et al. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clin Pediatr (Phila) 2012;51(2):114–121. doi: 10.1177/0009922811412950. [DOI] [PubMed] [Google Scholar]
- 40.Noh JH, Cho YJ, Nam HW, et al. Web-based comprehensive information system for self-management of diabetes mellitus. Diabetes Technol Ther. 2010;12(5):333–337. doi: 10.1089/dia.2009.0122. [DOI] [PubMed] [Google Scholar]
- 41.Linn AJ, Vervloet M, van Dijk L, et al. Effects of eHealth interventions on medication adherence: a systematic review of the literature. J Med Internet Res. 2011;13(4):e103. doi: 10.2196/jmir.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu R, Rossos P, Quan S, et al. An evaluation of the use of smartphones to communicate between clinicians: a mixed-methods study. J Med Internet Res. 2011;13(3):e59. doi: 10.2196/jmir.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blodget H. Actually, the US smartphone revolution has entered the late innings. [Accessed December 20, 2012]; www.businessinsider.com/us-smartphone-market-2012-9.
- 44.O’Grady MJ, Retterath AJ, Keenan DB, et al. The use of an automated, portable glucose control system for overnight glucose control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35(11):2182–2187. doi: 10.2337/dc12-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cafazzo JA, Casselman M, Hamming N, et al. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;14(3):e70. doi: 10.2196/jmir.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vervloet M, van Dijk L, Santen-Reestman J, et al. SMS reminders improve adherence to oral medication in type 2 diabetes patients who are real time electronically monitored. Int J Med Inform. 2012;81(9):594–604. doi: 10.1016/j.ijmedinf.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Gadkari AS, McHorney CA. Unintentional non-adherence to chronic prescription medications: How unintentional is it really? BMC Health Serv Res. 2012;12:98. doi: 10.1186/1472-6963-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ficke DL, Farris KB. Use of the transtheoretical model in the medication use process. Ann Pharmacother. 2005;39(7–8):1325–1330. doi: 10.1345/aph.1G122. [DOI] [PubMed] [Google Scholar]
- 49.Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerr DA, Pollard CM, Howat P, et al. Connecting Health and Technology (CHAT): protocol of a randomized controlled trial to improve nutrition behaviours using mobile devices and tailored text messaging in young adults. BMC Public Health. 2012;12:477. doi: 10.1186/1471-2458-12-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brustein J. Coming next: using an app as prescribed. [Accessed October 19, 2012]; www.nytimes.com/2012/08/20/technology/coming-next-doctors-prescribing-apps-to-patients.html?pagewanted=all&_r=0.
- 52.Department of Health & Human Services; Food and Drug Administration. Draft guidance for industry and Food and Drug Administration staff; mobile medical applications. [Accessed October 19, 2012]; availability. www.regulations.gov/#!documentDetail;D=FDA-2011-D-0530-0001.