Abstract
Schizophrenia is a disorder characterized by positive, negative, and cognitive symptoms. While positive symptoms can be effectively treated with typical antipsychotic medication, which generally affects the dopaminergic system, negative and cognitive symptoms, including attentional deficits and impulsive behavior, are less sensitive to standard treatments. It has further been well documented that schizophrenic patients use tobacco products at a rate much higher than the general population, and this persists despite treatment. It has been argued this behavior may be a form of self-medication, to alleviate some symptoms of schizophrenia. It has further been posited that prefrontal glutamatergic hypofunction may underlie some aspects of schizophrenia, and in accordance with this model, systemic phencyclidine has been used to model the disease. We employed a modified 5-choice serial reaction time test, a paradigm that is often used to investigate many of the treatment-resistant symptoms of schizophrenia including impulsivity, selective attention, and sustained attention/cognitive vigilance, to determine the medicinal effects of nicotine. We demonstrate that chronic oral, but not acute injections of, nicotine can selectively attenuate phencyclidine-induced increases in impulsivity without affecting other measures of attention. This suggests that nicotine use by schizophrenics may provide some relief of distinct symptoms that involve impulsive behaviors.
Keywords: Phencyclidine, Nicotine, Schizophrenia, Impulsivity
1 Introduction
Schizophrenia is a devastating mental illness with approximately 1.1% of the adult population demonstrating one-year prevalence [1]. It is characterized by positive symptoms, including delusions, hallucinations, and disordered thoughts and speech, negative symptoms, which can include anhedonia, speech and social deficits, and blunted affect, as well as cognitive and attentional deficits, and impulsivity [2-4]. While the positive symptoms of schizophrenia can be effectively treated, negative and cognitive symptoms are less responsive [5-12].
In addition to the multifaceted symptomatology of schizophrenia, it is also markedly comorbid with nicotine dependence: smoking rates among patients is approximately 80%, regardless of treatment status [13, 14], compared with general US adult use of 20%. A number of explanatory theories have been proposed. Shared genetic factors may underlie the likelihood of developing schizophrenia and nicotine addiction, and consistent with this, both conditions are more common among first-degree relatives [15, 16]. However, whether the same genes mediate susceptibility to both conditions remains unclear. Alternatively, smoking may increase the likelihood of developing schizophrenia, as the age of onset of smoking sometimes correlates with the age of onset of schizophrenia [17]. Again, such correlations do not imply a causative role of smoking in illness etiology. Another possibility is that schizophrenic patients may be compelled to smoke, whether to satisfy an oral fixation, relieve stress or anxiety, or simply to alleviate boredom [18]. Perhaps most intriguing, though, is the possibility that nicotine use is a form of self-medication in schizophrenic patients that works to alleviate some of the symptoms, especially those treatment resistant negative symptoms [19, 20]. This last hypothesis was investigated in the present study.
The disparity in treatment efficacy also may be due in part to alternative biological mechanisms underlying the disease itself. One theory of schizophrenia posits that dopamine hyperactivity, or more specifically, overactivation of dopamine D2-like receptors is involved. The most convincing support for this theory resides in the fact that the positive symptoms are alleviated by typical antipsychotic medications, which are D2 receptor antagonists [21, 22]. Moreover, positive symptoms can be mimicked by administration of psychostimulants, inducing a “stimulant psychosis” [23, 24]. Alternatively, there is evidence suggesting prefrontal glutamatergic hypofunction may cause some symptoms of schizophrenia (c.f., for review [25-27]), and specifically hypofunction of N-methyl-D-aspartate receptor (NMDAR)-mediated signaling [28]. Indeed, systemic administration of phencyclidine (PCP), a potent NMDAR antagonist has long been known to be psychotomimetic in humans [29, 30]. Moreover, it has been shown that PCP administration can induce negative symptoms, including impulsivity and deficits in attention and cognition, in both rodents and primates [31-35]. This NMDAR antagonist model of schizophrenia is therefore particularly relevant, as it reproduces both positive and negative symptoms [30, 36].
In animals, schizophrenia-like negative symptoms induced by PCP administration have been modeled using 5-choice serial reaction time tests (5CSRTT) to measure sustained attention and impulsivity [37-40]. It has also been demonstrated that acute and chronic nicotine can improve performance in this, and other attentional tasks [41-43], but whether nicotine would mitigate the detrimental effects of PCP in the 5CSRTT is unknown. Here, we exposed mice to nicotine, both chronic and acute, and tested its effects on schizophrenia-like symptoms induced by PCP in a modified 5CSRTT: only three options are available to the animal making it a 3-choice serial reaction time test (3CSRTT). Unlike those cited above and other studies modeling chronic nicotine exposure we used a single-bottle chronic oral nicotine administration paradigm. This gives the mice the ability to titrate their nicotine intake over time, and though consumption is “forced”, as their only source of water, this allows the determination of whether PCP exposure also affects intake. Using these methods, we tested the hypothesis that nicotine would attenuate the expression of negative symptoms in a NMDAR hypofunction model of schizophrenia.
2 Materials and Methods
2.1 Animals
Male C57BL/6 mice (Charles River, Kingston, NY) were delivered to our facility at seven weeks and allowed to acclimate to the facility for seven days prior to the beginning of behavioral testing. Mice were housed in groups of 4-5 for the duration of the study, and maintained on a 12:12-h light-dark cycle in a temperature- and humidity-controlled environment. Weight was maintained at 85-90% free feeding weight, and water was available ad libitum unless otherwise specified. All procedures adhered to policies in accordance with the Yale University Institutional Animal Care and Use Committee and the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals.
2.2 Drugs
Phencyclidine (PCP, Sigma, St. Louis, MO) was dissolved in 0.9% saline at a volume of 1.5 mg/ml, and injected intraparitoneally at a volume of 2 ml/kg. (−)-Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline at a concentration of 0.1 mg free base/ml, and injected subcutaneously at a volume of 2 ml/kg. For oral consumption, a solution of (−)-nicotine (Sigma, St. Louis, MO) was prepared at a concentration of 200 μg/ml in 2% saccharin sodium (w/v, Sigma, St. Louis, MO).
2.3 Behavioral Testing
2.3.1 3CSRTT
All testing for the 3CSRTT was done in standard operant chambers (MedAssociates, St. Albans, VT), and the programs were controlled by Med-PC software. Operant chambers were individually housed in sound-attenuating chambers, and constructed of Plexiglas™ front walls, rear walls, and ceiling, with aluminum side walls. Floors consisted of stainless steel bars, 3mm in diameter, separated by 7mm. The interior of the operant chamber measured 16cm × 15cm × 13cm, and consisted of a side wall containing three nosepoke apertures, which could be illuminated, and could detect when the animal poked his nose into the port, breaking an infrared light beam. The other side wall had a central magazine port, with a stimulus light situated above or to the left of the opening. A 20-mg grain pellet (Bio-Serv, Frenchtown, NJ) could be delivered via a food hopper into this magazine, and head entries could be detected via the breaking of an infrared light beam. Boxes were also equipped with a houselight, located below the ceiling of the operant chamber above the nosepoke ports, and a ventilation fan, which provided for air circulation and ambient noise. Mice were trained in a 3CSRTT in a manner similar to that described previously in rodents (e.g., [44-46]). Briefly, mice were trained to perform a nosepoke into a lit aperture, while ignoring the two unlit apertures. For each individual trial during each session, one of the three nosepoke ports was randomly chosen by the computer program to be the correct, lit aperture.
2.3.1.1 Nosepoke Training
Nosepoke training was conducted daily for 14 days. Sessions lasted 40 minutes/day, and each the session began with the house light and magazine stimulus light lit. The first trial began when the mouse entered to magazine, terminating the magazine light. After a one second delay, referred to as an intertrial interval (ITI), one of the three nosepoke lights was illuminated. This nosepoke port remained lit until the animal performed a nosepoke in the lit aperture, at which point the light was terminated, a food pellet was delivered into the magazine, and the magazine stimulus light came on. Once the animal entered the magazine to receive his food reward, the magazine light was again illuminated, indicating the start of a new trial, to be triggered with a magazine entry. Incorrect responses, being a nosepoke into an unlit aperture or a response during the ITI, were recorded, but had no effect on the trial.
2.3.1.2 3CSRTT Training
Following nosepoke training, mice were trained in the 3CSRTT. This task was executed similarly to the nosepoke training, but with the following differences: The ITI was initially set at 2 sec, and the nosepoke aperture only remained lit initially for 60 seconds. In order to receive the food reward, the animal had to perform a correct response either during the 60-sec stimulus duration, or within 5 sec to the stimulus light terminating, referred to as the hold period. If the animal responded during the ITI, the house light was terminated for a two-sec time-out period, terminating the trial, and a new trial would then be initiated with a magazine entry. Such a response was designated as a premature response. If the animal performed a nosepoke into one of the unlit apertures, the house light was extinguished for a two sec time-out period, terminating the trial. Such action is referred to as an incorrect response. If the animal failed to make a response either during the time the stimulus light was on, or during the 5 sec hold period, the house light was terminated for a two sec time-out period, terminating the trial. This would be recorded as an omission trial. In the case of a premature, incorrect, or omitted response, no food reward was provided. Each session lasted for either 40 minutes, or until 200 trials were completed, whichever came first.
2.3.1.3 Behavioral Measures
Based on performance in the 3CSRTT task, data was characterized in four ways:
Percent Correct
This represents general performance in the task. Percent correct is calculated as (number of correct trials)/(total number of trials) × 100. This measure takes into account the results of all types of errors, while the other behavioral measures examine each type of error individually.
Accuracy
Accuracy is a measure of selective attention, or attention between restricted stimuli [47]. Accuracy is measured as (number of correct trials)/(number of correct trials + number of incorrect trials) × 100, and accounts only for errors of selection (i.e., incorrect responses).
Percent Omission
A measure of sustained attention, in which the animal is forced to simultaneously monitor multiple distinct sensory modalities, percent omission is measured as (number of omission trials)/(total number of trials) × 100.
Percent Premature
Used to measure impulsivity, percent premature is measured as (number of premature trials)/(total number of trials) × 100.
As the mouse learned the task, the parameters were changed to make the task progressively more difficult. The stimulus duration was lowered from 60 sec to 20 sec, 5 sec, then 2 sec. The ITI was then increased from 2 sec, to 4 sec, then 6 sec. The stimulus duration was then further lowered from 2 sec, to 1 sec, and finally to 0.5 sec. Mice had to achieve 70% correct under any given condition to be advanced.
2.3.1.4 Variable ITI Testing
During the testing phase of this study, mice had to perform the 3CSRTT with a fixed, 0.5 sec stimulus duration, but a variable ITI, which could be set at 5 sec, 7.5 sec, 10 sec, or 20 sec. The specific duration was randomly chosen by the computer program. Each session consisted of 60 trials.
2.3.1.5 Experimental Schedule
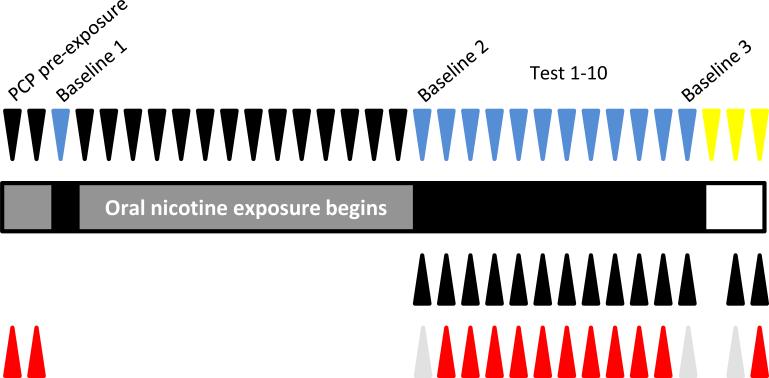
After mice had proceeded through the training phase of the 3CSRTT, they continued on daily 0.5 sec stimulus duration, 6 sec fixed ITI training sessions until they maintained a stable baseline of at least five days where their percent correct did not vary by more than 10% of the five-day rolling average. After this was achieved, mice were injected 30 minutes prior to the session with phencyclidine (3 mg/kg, i.p.), and tested under an identical fixed ITI schedule for two consecutive days. This PCP pre-exposure was done to assess the relative impact of acute PCP administration on performance in the 3CSRTT, such that mice could be divided into groups with approximately an equal response to PCP relative to baseline. The following day, mice were tested in the variable ITI testing session in the absence of any additional treatment. Data from this variable ITI day were recorded as Baseline 1. Following this, mice were divided into two groups – both received the saccharin solution as their only source of water in their home cage, and one of these groups also had nicotine (200 μg/ml) dissolved in the solution. The animals and bottles were weighed every other day to determine nicotine consumption, and fresh solution was provided. During the first 14 days of chronic oral nicotine exposure, mice were tested daily in 0.5 sec stimulus duration, 6 sec ITI training sessions. Mice were maintained on this fixed ITI training schedule in order to ensure no habituation to the variable ITI testing schedule over the course of chronic nicotine exposure prior to the ensuing testing phase. Following two weeks of chronic oral nicotine exposure, mice were injected with saline (2 ml/kg, i.p.) 30 minutes prior to a variable ITI testing session, and 20 minutes prior to the session injected with nicotine (0 or 0.2 mg/kg, s.c.), thus creating four groups, based on their chronic and acute exposure: saccharin only/saline, saccharin only/nicotine, nicotine/saline, and nicotine/nicotine. Data from this session were recorded as Baseline 2. The following day, mice were injected with PCP (3 mg/kg, i.p.) 30 minutes prior, and nicotine (0 or 0.2 mg/kg, s.c.) 20 minutes prior to the variable ITI test session. This schedule was repeated for 10 consecutive days, and data were recorded as Tests 1-10. One day following Test 10, mice were tested in a manner identical to Baseline 2, in order to determine any long-term effects of prior PCP exposure. Data from this day were recorded as Baseline 3. The experimental schedule is illustrated in Figure 1.
Figure 1.
Experimental schedule. Downward facing arrowheads indicate the variety of behavioral testing. Black represents a fixed ITI training session, blue represents a variable ITI test session, and yellow represents locomotor activity testing. Upward facing arrowheads represent drug treatment. Upper row of black arrowheads represent nicotine (0.2 mg/kg, s.c.) or saline, given 20 minutes prior to testing. Lower row of arrowheads represents PCP (3 mg/kg, i.p.; red) or saline (grey) administered 30 minutes prior to testing.
2.3.2 Locomotor Testing
One day following the completion of 3CSRTT testing, mice were tested for spontaneous locomotor activity. The apparatus consisted of an empty cage, identical to the ones used to house the mice in the animal facility. Distance travelled was monitored with the Omnitech Digiscan Micromonitor system (Columbus, OH), and analyzed with Med-PC software. On the first day of locomotor testing, mice were habituated to the apparatus for 30 minutes in the absence of any additional injections. The following day, mice were injected with saline (2 ml/kg, i.p.) 30 minutes prior, and nicotine (0 or 0.2 mg/kg, s.c.) 20 minutes prior to testing. The next day, mice were injected with PCP (3 mg/kg, i.p.) 30 minutes prior and nicotine (0 or 0.2 mg/kg, s.c.) 20 minutes prior to testing. On these two testing days, activity was monitored for 60 minutes.
2.4 Statistics
For all behavioral analysis, including 3CSRTT and locomotor activity, we used a 3-way ANOVA, with factors of chronic oral treatment (nicotine vs. saccharin), acute treatment (nicotine vs. saline), and testing day, which was a repeated measure. When appropriate, we used 3-way ANOVA with chronic oral treatment as the only between-subjects factor, and ITI duration and testing day as within-subject repeated measures. We then used exploratory 2-way ANOVAs based on individual ITI durations to determine the specific nature of the effects observed in this analysis. To assess nicotine consumption, we used a one-way ANOVA, with measurement period as a repeated measure. Data were analyzed using SPSS statistical software (IBM, Armonk, NY) and significance was set at p < 0.05.
3 Results
3.1 Nicotine Consumption
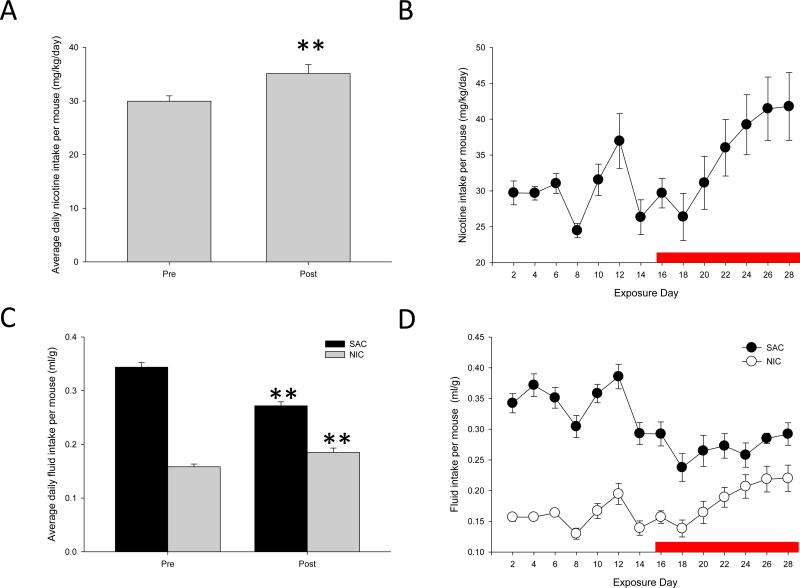
We measured the amount of nicotine consumed by the mice by weighing the mice and their bottles every other day, when providing fresh solution. Although, because the animals were group housed, individual variation in nicotine consumption could not be assessed, we were able to determine the total nicotine intake and total body weight among all the mice in a given cage, and could therefore determine the average daily nicotine consumption. Mice readily drank the sweetened nicotine solution, and according to previous studies, based on their average intake of between 30-35 mg/kg/day, could be predicted to maintain a steady-state blood nicotine concentration of approximately 60-70 ng/ml. This would be slightly above the range of what is typically seen in human smokers [48, 49]. When we compared the consumption of nicotine prior to the PCP exposure vs. during the course of PCP exposure, we found significantly higher consumption of nicotine during the course of exposure (F(1,27) = 10.169, p = 0.004; Fig 2A-B), suggesting that mice increased their nicotine intake in response to the effects of PCP administration. In contrast, fluid consumption among saccharin-exposed mice decreased over the course of PCP exposure (F(1,27) = 55.230, p < 0.001; Fig 2C-D).
Figure 2.
Average daily fluid consumption. Fluid consumption was calculated by measuring the difference in weight of the water bottles since the previous measurement, and calculating consumption based on the total weight of all animals in the cage. A,C: Nicotine (A) and total fluid (C) consumption prior to and during PCP treatment. Pre: prior to PCP treatment. Post: during PCP treatment. B,D: Nicotine (B) and total fluid (D) consumption over the course of the experiment. Red line represents PCP exposure. ** represents p < 0.01 relative to days prior to PCP exposure. N = 4 cages/group.
3.2 3CSRTT
3.2.1 General Performance
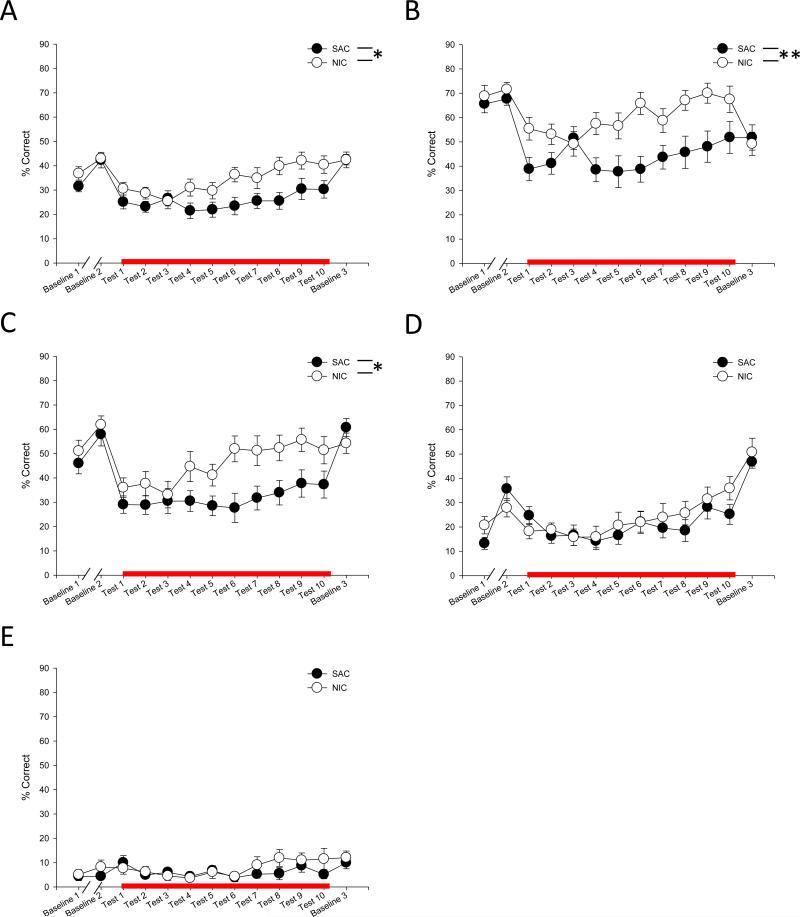
General performance in the 3CSRTT task was assessed by analyzing the percent of total trials that resulted in a correct choice and food delivery. We first analyzed these data based on the total number of trials per session, regardless of the specific ITI of the individual trials. We found that there was a main effect of chronic oral nicotine treatment that resulted in improved performance in the task (F(1,32) = 4.433, p = 0.043; Fig 3A). Because there was no significant main effect of acute s.c. nicotine given before the test (F(1,32) = 0.169, p = 0.684), we therefore collapsed these data based on whether the chronic oral treatment was nicotine or saccharin alone. We observed a significant chronic oral treatment × ITI duration effect (F(3,29) = 5.774, p = 0.003), so we then looked to see how performance in these two collapsed groups broke down based on specific ITI. We found again that chronic oral nicotine improved performance when the ITI duration was shortest, at 5 sec and 7.5 sec ITI, though that effect failed to reach significance at 10 sec and 20 sec ITI (5 sec: F(1,32) = 8.878, p = 0.005; 7.5 sec: F(1,32) = 4.775, p = 0.036; 10 sec: F(1,32) = 0.339, p = 0.564; 20 sec: F(1,32) = 0.725, p = 0.401; Fig 3B-E).
Figure 3.
General performance in the 3CSRTT. Percent correct trials were analyzed either after no treatment (Baseline 1), chronic nicotine treatment (Baseline 2, Baseline 3), or PCP and chronic nicotine treatment (Test 1-10). Groups treated with acute nicotine are not separately shown as acute nicotine treatment had no effect on behavior. Data is shown collapsed across all ITI durations (A), as well as with a 5 sec (B), 7.5 sec (C), 10 sec (D), or 20 sec ITI (E). NIC: chronic nicotine treatment. SAC: chronic saccharin treatment. Red line indicates days of PCP exposure. *, ** represents p < 0.05, 0.01 between chronic nicotine and saccharin treated groups, respectively.
3.2.2 Impulsivity
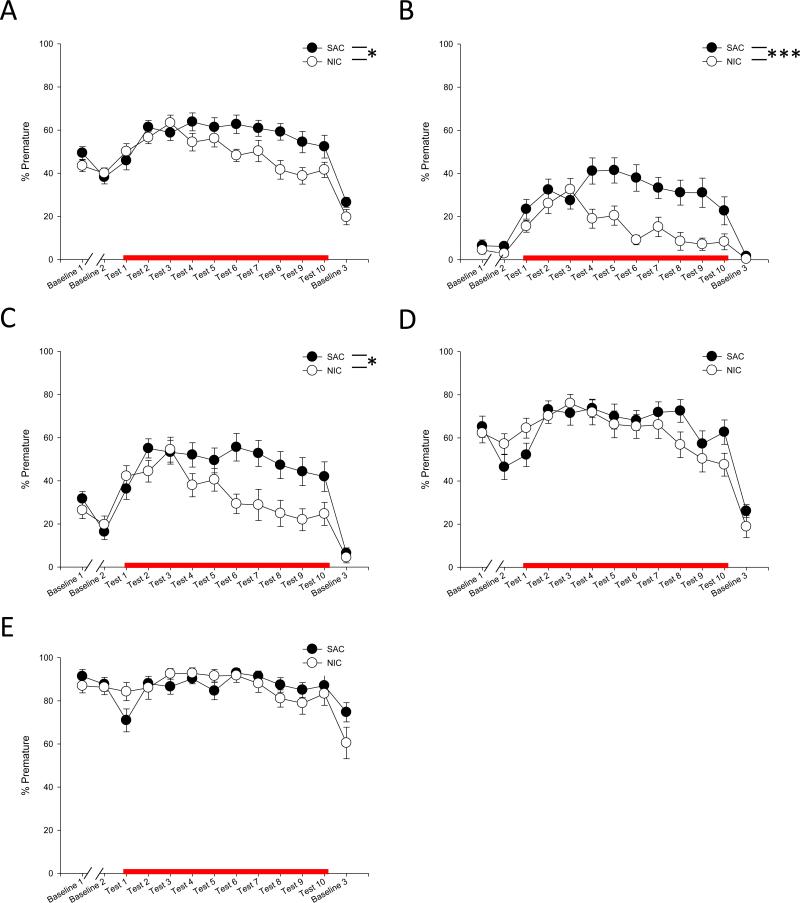
Figure 4 shows the effect of nicotine on impulsivity, as measured by the percentage of trials that resulted in a premature response. When collapsed for all ITI durations, a main effect of chronic oral treatment was evident, but no effect of acute s.c. treatment, so we again collapsed these data based on chronic treatment with nicotine or saccharin alone (F(1,32) = 5.570, p = 0.025; Fig 4A). We again observed a significant chronic oral treatment × ITI duration effect (F(3,29) = 5.127, p = 0.006), so we performed exploratory 2-way ANOVAs for each ITI duration to determine the nature of this effect. When each ITI was analyzed separately, a main effect for chronic treatment was present when the task was least likely to result in a premature response, at 5 sec and 7.5 sec ITI, but absent when the task required a longer inhibition of responding, with a 10 sec or 20 sec ITI (5 sec: F(1,32) = 15.945, p < 0.0001; 7.5 sec: F(1,32) = 5.947, p = 0.020; 10 sec: F(1,32) = 0.715, p = 0.404; 20 sec: F(1,32) = 0.405, p = 0.529; Fig 4B-E). There was no difference between the groups during any of the baseline testing days, suggesting chronic oral, but not acute s.c. nicotine, attenuates PCP-induced increases in impulsivity without affecting impulsivity in a drug-free state: These data are in contrast to previous reports showing an acute nicotine-induced increase in impulsive behavior [42, 50-52].
Figure 4.
Impulsivity in the 3CSRTT. Percentage of premature responses were analyzed either after no treatment (Baseline 1), chronic nicotine treatment (Baseline 2, Baseline 3), or PCP and chronic nicotine treatment (Test 1-10). Groups treated with acute nicotine are not separately shown as acute nicotine treatment had no effect on behavior. Data is shown collapsed across all ITI durations (A), as well as with a 5 sec (B), 7.5 sec (C), 10 sec (D), or 20 sec ITI (E). NIC: chronic nicotine treatment. SAC: chronic saccharin treatment. Red line indicates days of PCP exposure. *, *** represents p < 0.05, 0.0001 between chronic nicotine and saccharin treated groups, respectively.
3.2.3 Selective/Sustained Attention
We also analyzed selective attention, by assessing the accuracy of responding, as well as sustained attention/cognitive vigilance, by looking at the percentage of trials that resulted in no response. We saw no effect of either acute s.c. or chronic oral nicotine on either accuracy (acute treatment: F(1,32) = 0.002, p = 0.961, chronic oral treatment: F(1,32) = 0.908, p = 0.348, data not shown) or omission trials (acute treatment: F(1,32) = 0.077, p = 0.783, chronic oral treatment: F(1,32) = 0.115, p = 0.737, data not shown). However, this could be due to the fact that as the task only varied in the ITI duration, this made errors of premature responding more likely than other types of errors.
3.3 Locomotor Activity
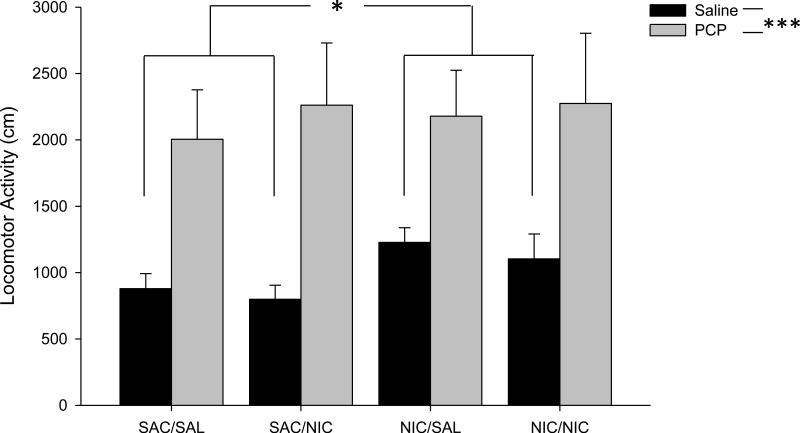
In order to determine if any difference in behavior in the 3CSRTT could be explained by changes in locomotor activity, mice were tested for activity 20 minutes following acute nicotine (0 or 0.2 mg/kg, s.c.) and 30 minutes after vehicle or PCP (3 mg/kg, i.p.). All animals were treated with both vehicle and PCP on separate days to serve as internal controls for PCP exposure. Although activity was monitored for 60 minutes, we limited our analysis to the first 20 minutes of testing, as our behavioral tests were most often completed within that time frame. As we were attempting to only assess locomotor differences during the testing phase of the 3CSRTT, restricting our analysis to this time point seemed most appropriate. Although we found a significant increase in locomotion after PCP injection relative to vehicle, nicotine did not alter this effect (PCP: F(1,32) = 32.940, p < 0.0001; PCP × Chronic Nicotine: F(1,32) = 0.322, p = 0.574; PCP × Acute Nicotine: F(1,32) = 0.462, p = 0.502; PCP × Chronic Nicotine × Acute Nicotine: F(1,32) = 0.020, p = 0.887; Fig 5). However, we did see increased activity after saline injection in mice that underwent chronic oral nicotine exposure (F(1,34) = 6.241, p = 0.017), but this difference disappeared after PCP exposure. As we did not see any effect of chronic oral nicotine in the 3CSRTT during in the absence of PCP administration, this locomotor effect does not appear to be relevant to our behavioral analysis.
Figure 5.
Locomotor activity. Mice were independently assayed for locomotor activity 30 minutes after both saline and PCP treatment. Activity was assayed for 20 minutes per session. Groups also received additional acute saline or nicotine treatment 20 minutes prior to testing. The four groups shown are as follows: SAC/SAL: chronic saccharin only and acute saline treatment. SAC/NIC: chronic saccharin only and acute nicotine treatment. NIC/SAL: chronic nicotine and acute saline treatment. NIC/NIC: chronic nicotine and acute nicotine treatment. *, *** represents p < 0.05, 0.0001, respectively.
4 Discussion
Here, we demonstrate that chronic oral nicotine exposure significantly attenuates PCP-induced impulsivity, without affecting measures of selective or sustained attention. While it is possible that chronic nicotine affected impulsive behavior in this task indirectly, perhaps through effects on social behaviors or anxiety, we find this explanation unlikely, as animals chronically exposed to nicotine display tolerance to such consequences of nicotine exposure [53]. Further, while the acute 0.2 mg/kg dose of s.c. nicotine had no effect on any of these behaviors, the amelioration of PCP-induced impulsivity by chronic oral nicotine was robust and ITI dependent. While chronic nicotine administered through a subcutaneous osmotic minipump has been previously reported not to reduce PCP-induced disruptions in 5CSRTT performance [41], this could be due to dose, route of administration or more likely task details. Specifically, we used a task with an unpredictable ITI duration, thus challenging the animal to adapt its’ responding over the course of the session, while the above study used a fixed duration. Indeed, in pilot studies, we did not see positive effects of nicotine on PCP-induced impairments on tasks with fixed ITIs. Notably, predictability would allow the animal to learn a timed-response that potentially would mask the beneficial effects of nicotine. Conversely, a variable ITI could engender a greater degree of impulsivity in the experimental animals, such that the effects of chronic nicotine exposure become apparent.
Interestingly, in our study, we found the nicotine only improved performance in the task when the ITI durations were shortest, and thus the task was least likely to result in premature responding. When challenged with a 10 sec or 20 sec ITI, any effect of nicotine disappeared. Of course, at these longer ITIs it is likely that ceiling effects would obscure any potential beneficial effects of nicotine. The inclusion of these longer ITIs in the test sessions allow us to determine the limits of the effectiveness of nicotine in promoting resilience to the PCP-induced increases in impulsivity. These rather modest yet specific effects of oral chronic nicotine exposure therefore might be maximally effective in tasks that require a moderate degree of cognitive engagement.
Of particular significance was the finding that the difference in impulsivity between nicotine- and saccharin-exposed mice observed in this study did not manifest itself until after several days of PCP exposure. While saccharin- and nicotine-exposed mice showed no significant difference in the total amount of impulsive behavior during the first five days of PCP exposure (58.2% vs. 56.0% premature, respectively), the beneficial effects of nicotine in this paradigm became quite apparent over the last five days of testing (57.9% vs. 44.0% premature). It has been demonstrated that repeated PCP administration can induce long-term changes in NMDAR subunit expression [54-56], and such regulation can be attenuated with atypical antipsychotic treatment [57]. Therefore, it may be possible that these long-term changes establish a state in which the effects of nicotine become more pronounced. Indeed, it has been shown that chronic nicotine can have subtle effects on NMDAR expression in the brain [58], and nicotine can assist in NMDAR-mediated synaptic plasticity [59-61]. Further studies are necessary to determine how chronic nicotine affects PCP-induced changes in NMDAR subunit expression, and how nicotine affects synaptic plasticity after repeated PCP exposure.
One potential reason that the chronic oral, but not acute s.c. nicotine, effectively attenuates PCP-induced impulsive behavior may be due to the long-term changes caused by prolonged nicotine exposure. It has long been known that chronic nicotine induces an upregulation of specific nicotinic acetylcholine receptor (nAChR) subunits in a regionally specific manner [62-66]. Moreover, it has been shown that the expression of specific nAChR subunits can influence impulsive behavior in laboratory animals [67, 68]. Furthermore, it has been demonstrated that schizophrenic patients show decreased expression of nAChRs in the brain (c.f., [69]), and activation of high-affinity β2-containing nAChRs improves attentional performance in animal models [70-72]. Thus, it would be important to determine whether chronic nicotine-induced upregulation of nAChR subunits in the brain, especially in cortical regions involved in impulsive behavior, correlate with resilience to the effects of PCP exposure.
Many studies have found marked effects of acute nicotine under a variety of conditions [42, 73], and though it appeared that our dose of acute nicotine did not counter any of the behavioral deficits induced by PCP in our task, we also saw no difference in behavior after acute nicotine injection in the absence of PCP administration, i.e., at Baselines 2 and 3. Although mice exposed to chronic oral nicotine and then challenged with an acute injection may show no improvement in performance because of the biphasic effects of nicotine, and its aversive properties at higher doses [74-76], it remains unclear why nicotine naïve mice, when challenged with an acute dose, showed no change in their performance. However, given the design of the current experiment, the effects of nicotine on accuracy and percent of omission trials would be minimized, as the variable ITI biases the paradigm towards the evoking of premature responding. This effect seems to persist even when the ITI is shortest, suggesting the unpredictability of the ITI is sufficient to bias this task toward errors of impulsivity over other attentional and cognitive errors. Indeed, nicotine has been shown to improve performance on more stimulus-dependent tasks [43, 77, 78], and thus it is likely that altering our task to include a variable stimulus duration, while maintaining a fixed ITI, could highlight some additional effects of acute or chronic nicotine on attentional processes.
One additional finding to highlight was the increase in nicotine consumption during the course of PCP administration. While this effect was modest, we noticed a steady increase in consumption over the ten days of PCP exposure. This must be viewed with some caution, though, as the nicotine solution was the only source of water available to the animals, and thus this does not represent a truly voluntary consumption. Notably, the fluid consumption of the control group actually decreased throughout the course of study, a trend that was reversed by the presence of nicotine. We hypothesize that this increase in nicotine intake in PCP-exposed mice could represent either the development of tolerance to the aversive qualities of the nicotine solution, an increase in its rewarding properties, or a medicinal response to the PCP exposure. It is widely known that schizophrenic smokers smoke at a higher rate than smokers in the general population [79, 80], so this increase in intake selectively in PCP exposed animals could be evidence for self medication-like behavior. However, further work must be done to determine if this is, in fact, a change in voluntary intake, using a 2-bottle choice, or self-administration paradigm, as well as to determine whether intake returns to baseline levels after the termination of PCP exposure. Moreover, it must be noted that because the animals were group-housed, and fluid consumption was measured every other day, we cannot determine the nicotine intake for each individual mouse, generating the possibility that this increase in consumption may only exist among a subset of the nicotine exposed mice, nor can we determine a more temporally precise account of the change in nicotine consumption. Further studies are necessary to more carefully examine this phenomenon.
In summary, we found that chronic oral, but not acute nicotine, could significantly attenuate increases in impulsivity induced by repeated PCP administration. Moreover, PCP administration seemed to induce an increase in oral nicotine consumption. These data help shed some light on the behavioral consequences of nicotine self-medication by schizophrenic patients, and though more work is necessary to determine the molecular substrates of these effects, our study gives some insight as to the putative and specific beneficial effects evoked by chronic nicotine exposure. The development of a tobacco-free medication to help supplement current pharmacotherapies without the dire health consequences inherent in smoking might help mitigate impulsive behavior in schizophrenics.
Highlights.
- Phencyclidine (PCP) administration increases impulsive behavior in mice.
- Chronic nicotine attenuates PCP-induced impulsivity.
- Nicotine has no effect on PCP-induced deficits in selective or sustained attention.
- Oral nicotine intake increases during phencyclidine exposure.
- These findings suggest a target for treatment of negative symptoms of schizophrenia.
Acknowledgments
Support: We thank members of the Division of Molecular Psychiatry, and funding from NARSAD (JRT), DA011717 (JRT) and the CT Department of Mental Health. Daniel Scott was supported by a NIMH T32-14276 (Duman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regier DA, Narrow WE, Rae DS, Manderscheid RW, Locke BZ, Goodwin FK. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 3.Bartko JJ, Strauss JS, Carpenter WT., Jr. The diagnosis and understanding of schizophrenia. Part II. Expanded perspectives for describing and comparing schizophrenic patients. Schizophr Bull. 1974:50–60. doi: 10.1093/schbul/1.11.50. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter WT, Jr., Strauss JS, Bartko JJ. The diagnosis and understanding of schizophrenia. Part I. Use of signs and symptoms for the identification of schizophrenic patients. Schizophr Bull. 1974:37–49. doi: 10.1093/schbul/1.11.37. [DOI] [PubMed] [Google Scholar]
- 5.Szymanski SR, Cannon TD, Gallacher F, Erwin RJ, Gur RE. Course of treatment response in first-episode and chronic schizophrenia. Am J Psychiatry. 1996;153:519–25. doi: 10.1176/ajp.153.4.519. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter WT, Jr., Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophr Bull. 1985;11:440–52. doi: 10.1093/schbul/11.3.440. [DOI] [PubMed] [Google Scholar]
- 7.Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–6. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 8.Smith RC, Chua JW, Lipetsker B, Bhattacharyya A. Efficacy of risperidone in reducing positive and negative symptoms in medication-refractory schizophrenia: an open prospective study. J Clin Psychiatry. 1996;57:460–6. doi: 10.4088/jcp.v57n1004. [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT., Jr. Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology. 2000;22:303–10. doi: 10.1016/S0893-133X(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 10.Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006;32:234–7. doi: 10.1093/schbul/sbj055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837–55. doi: 10.2165/11314280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Conn PJ, Tamminga C, Schoepp DD, Lindsley C. Schizophrenia: moving beyond monoamine antagonists. Mol Interv. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- 13.Keltner NL, Grant JS. Smoke, smoke, smoke that cigarette. Perspect Psychiatr Care. 2006;42:256–61. doi: 10.1111/j.1744-6163.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 14.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Ferchiou A, Szoke A, Laguerre A, Meary A, Leboyer M, Schurhoff F. Exploring the relationships between tobacco smoking and schizophrenia in first-degree relatives. Psychiatry Res. 2012;200:674–8. doi: 10.1016/j.psychres.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 16.de Leon J. Smoking and vulnerability for schizophrenia. Schizophr Bull. 1996;22:405–9. doi: 10.1093/schbul/22.3.405. [DOI] [PubMed] [Google Scholar]
- 17.Kelly C, McCreadie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 1999;156:1751–7. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- 18.McCloughen A. The association between schizophrenia and cigarette smoking: a review of the literature and implications for mental health nursing practice. Int J Ment Health Nurs. 2003;12:119–29. doi: 10.1046/j.1440-0979.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 19.Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- 20.Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–34. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–9. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 22.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–3. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–33. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 24.Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 25.Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–25. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- 26.Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 28.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–9. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- 30.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–8. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 31.Jentsch JD, Roth RH, Taylor JR. Object retrieval/detour deficits in monkeys produced by prior subchronic phencyclidine administration: evidence for cognitive impulsivity. Biol Psychiatry. 2000;48:415–24. doi: 10.1016/s0006-3223(00)00926-4. [DOI] [PubMed] [Google Scholar]
- 32.Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 33.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–32. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, et al. PNU-120596, a positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine- induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. 2012;26:1265–70. doi: 10.1177/0269881111431747. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen CS, Goetghebeur P, Dias R. Chronic infusion of PCP via osmotic mini-pumps: a new rodent model of cognitive deficit in schizophrenia characterized by impaired attentional set-shifting (ID/ED) performance. J Neurosci Methods. 2009;185:66–9. doi: 10.1016/j.jneumeth.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Stillman RC. Phencyclidine: an overview. NIDA Res Monogr. 1978:1–17. [PubMed] [Google Scholar]
- 37.Le Pen G, Grottick AJ, Higgins GA, Moreau JL. Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology. 2003;28:1799–809. doi: 10.1038/sj.npp.1300208. [DOI] [PubMed] [Google Scholar]
- 38.Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson DM, McVie A, Morris BJ, Pratt JA. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: influence of clozapine. Psychopharmacology (Berl) 2011;213:681–95. doi: 10.1007/s00213-010-2020-7. [DOI] [PubMed] [Google Scholar]
- 40.Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, et al. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology (Berl) 2011;217:255–69. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 41.Amitai N, Markou A. Chronic nicotine improves cognitive performance in a test of attention but does not attenuate cognitive disruption induced by repeated phencyclidine administration. Psychopharmacology (Berl) 2009;202:275–86. doi: 10.1007/s00213-008-1246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolerman IP, Naylor CG, Mesdaghinia A, Morris HV. The duration of nicotine-induced attentional enhancement in the five-choice serial reaction time task: lack of long-lasting cognitive improvement. Behav Pharmacol. 2009;20:742–54. doi: 10.1097/FBP.0b013e328333b290. [DOI] [PubMed] [Google Scholar]
- 43.Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J Neurosci. 2005;25:5225–9. doi: 10.1523/JNEUROSCI.0719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torregrossa MM, Xie M, Taylor JR. Chronic corticosterone exposure during adolescence reduces impulsive action but increases impulsive choice and sensitivity to yohimbine in male Sprague-Dawley rats. Neuropsychopharmacology. 2012;37:1656–70. doi: 10.1038/npp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nairn AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology (Berl) 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krueger DD, Howell JL, Oo H, Olausson P, Taylor JR, Nairn AC. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behav Pharmacol. 2009;20:695–704. doi: 10.1097/FBP.0b013e328333a2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins GA, Breysse N. Rodent model of attention: the 5-choice serial reaction time task. Curr Protoc Pharmacol. 2008 doi: 10.1002/0471141755.ph0549s41. Chapter 5:Unit5 49. [DOI] [PubMed] [Google Scholar]
- 48.Rowell FJ, Seymour RA, Rawlins MD. Pharmacokinetics of intravenous and oral dihydrocodeine and its acid metabolites. Eur J Clin Pharmacol. 1983;25:419–24. doi: 10.1007/BF01037958. [DOI] [PubMed] [Google Scholar]
- 49.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 50.Stolerman IP, Mirza NR, Hahn B, Shoaib M. Nicotine in an animal model of attention. Eur J Pharmacol. 2000;393:147–54. doi: 10.1016/s0014-2999(99)00886-9. [DOI] [PubMed] [Google Scholar]
- 51.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–37. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 52.Bizarro L, Patel S, Murtagh C, Stolerman IP. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- 53.Irvine EE, Cheeta S, File SE. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav Pharmacol. 1999;10:691–7. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Owczarek S, Hou J, Secher T, Kristiansen LV. Phencyclidine treatment increases NR2A and NR2B N-methyl-D-aspartate receptor subunit expression in rats. Neuroreport. 2011;22:935–8. doi: 10.1097/WNR.0b013e32834d2ef7. [DOI] [PubMed] [Google Scholar]
- 55.Sircar R, Follesa P, Ticku MK. Postnatal phencyclidine treatment differentially regulates N-methyl-D-aspartate receptor subunit mRNA expression in developing rat cerebral cortex. Brain Res Mol Brain Res. 1996;40:214–20. doi: 10.1016/0169-328x(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 56.Anastasio NC, Johnson KM. Differential regulation of the NMDA receptor by acute and sub-chronic phencyclidine administration in the developing rat. J Neurochem. 2008;104:1210–8. doi: 10.1111/j.1471-4159.2007.05047.x. [DOI] [PubMed] [Google Scholar]
- 57.Anastasio NC, Johnson KM. Atypical anti-schizophrenic drugs prevent changes in cortical N-methyl-D-aspartate receptors and behavior following sub-chronic phencyclidine administration in developing rat pups. Pharmacol Biochem Behav. 2008;90:569–77. doi: 10.1016/j.pbb.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rezvani AH, Tizabi Y, Getachew B, Hauser SR, Caldwell DP, Hunter C, et al. Chronic nicotine and dizocilpine effects on nicotinic and NMDA glutamatergic receptor regulation: interactions with clozapine actions and attentional performance in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1030–40. doi: 10.1016/j.pnpbp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res. 2002;946:148–52. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]
- 60.Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–20. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 62.Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ. Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J Neurochem. 2011;119:153–64. doi: 10.1111/j.1471-4159.2011.07408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kassiou M, Eberl S, Meikle SR, Birrell A, Constable C, Fulham MJ, et al. In vivo imaging of nicotinic receptor upregulation following chronic (−)-nicotine treatment in baboon using SPECT. Nucl Med Biol. 2001;28:165–75. doi: 10.1016/s0969-8051(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 64.Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–53. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- 65.Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, Brown RW, Cooper J, et al. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. J Pharmacol Exp Ther. 2011;337:187–200. doi: 10.1124/jpet.110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Even N, Cardona A, Soudant M, Corringer PJ, Changeux JP, Cloez-Tayarani I. Regional differential effects of chronic nicotine on brain alpha 4-containing and alpha 6-containing receptors. Neuroreport. 2008;19:1545–50. doi: 10.1097/WNR.0b013e3283112703. [DOI] [PubMed] [Google Scholar]
- 67.Vinals X, Molas S, Gallego X, Fernandez-Montes RD, Robledo P, Dierssen M, et al. Overexpression of alpha3/alpha5/beta4 nicotinic receptor subunits modifies impulsive-like behavior. Drug Alcohol Depend. 2012;122:247–52. doi: 10.1016/j.drugalcdep.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 68.Ohmura Y, Tsutsui-Kimura I, Yoshioka M. Impulsive behavior and nicotinic acetylcholine receptors. J Pharmacol Sci. 2012;118:413–22. doi: 10.1254/jphs.11r06cr. [DOI] [PubMed] [Google Scholar]
- 69.Lewis AS, Picciotto MR. High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–82. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- 71.Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–67. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb Perspect Med. 2013;3:a012112. doi: 10.1101/cshperspect.a012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leach PT, Cordero KA, Gould TJ. The effects of acute nicotine, chronic nicotine, and withdrawal from chronic nicotine on performance of a cued appetitive response. Behav Neurosci. 2013;127:303–10. doi: 10.1037/a0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–41. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- 75.Risinger FO, Oakes RA. Nicotine-induced conditioned place preference and conditioned place aversion in mice. Pharmacol Biochem Behav. 1995;51:457–61. doi: 10.1016/0091-3057(95)00007-j. [DOI] [PubMed] [Google Scholar]
- 76.Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–7. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- 77.Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013;240:119–33. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–87. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 79.Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- 80.Williams JM, Gandhi KK, Lu SE, Kumar S, Steinberg ML, Cottler B, et al. Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug Alcohol Depend. 2011;118:313–9. doi: 10.1016/j.drugalcdep.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]