Summary
Congenital human cytomegalovirus (HCMV) infection can lead to long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss. Unfortunately, CMVs are highly adapted to their specific species, precluding the evaluation of HCMV vaccines in animal models prior to clinical trials. Several species-specific CMVs have been characterized and developed in models of pathogenesis and vaccine-mediated protection against disease. These include the murine CMV (MCMV), the porcine CMV (PCMV), the rhesus macaque CMV (RhCMV), the rat CMV (RCMV), and the guinea pig CMV (GPCMV). Because of the propensity of the GPCMV to cross the placenta, infecting the fetus in utero, it has emerged as a model of particular interest in studying vaccine-mediated protection of the fetus. In this paper, a review of these various models, with particular emphasis on the value of the model in the testing and evaluation of vaccines against congenital CMV, is provided. Recent exciting developments and advances in these various models are summarized, and recommendations offered for high-priority areas for future study.
Keywords: Cytomegalovirus, Animal cytomegalovirus models, Guinea pig CMV, Rat CMV, Murine CMV, Rhesus CMV, Porcine CMV, Cytomegalovirus vaccine, Live, attenuated CMV vaccines, CMV pentameric complex, Guinea pig model, placenta, immune modulation
Maternal infection with human cytomegalovirus (HCMV) during pregnancy can produce severe disease in newborns, and can lead to long-term neurodevelopmental sequelae, particularly sensorineural deafness [1–3]. Development of a vaccine focused on protecting newborns from the sequelae of congenital HCMV infection is therefore a major public health priority, as identified by the National Vaccine Program Office [4] and the Institute of Medicine [5]. Discussions about the proposed deployment of a CMV vaccine have focused primarily on immunization of adolescent girls and women of child-bearing age, toward the goals of optimizing protection amongst women anticipating potential pregnancies in the near future and preventing congenital infection [6]. However, it is not clear what would constitute an optimal protective vaccine in this setting. Moreover, it has become increasingly clear in recent years that both CMV-naïve and CMV-immune women are at risk to acquire CMV infections during pregnancy, with subsequent transmission to the fetus [7]; hence, a targeted vaccination of CMV-seronegative women will not solve the problem of congenital CMV infection. Primary maternal infections carry a greater risk of transmission and severe sequelae for the neonate than do recurrent infections [8], but the burden associated with re-infection is nonetheless quite substantial, and the goal of improving protection of the fetus by “augmenting” immunity to CMV in a woman who is already CMV-seropositive is a very challenging concept for vaccine development.
The two general approaches to vaccination evaluated in clinical trials have been based on subunit vaccines, consisting of individual immunodominant antigens (administered as purified DNA or proteins, or as “vectored” vaccines) important in the cellular and/or humoral immune response, and live, attenuated vaccines (reviewed in [9]). CMV is a member of the herpesvirinae family [10]. Virus particles are enveloped and contain a nucleocapsid (consisting of viral genomic DNA with accessory proteins) and a proteinaceous tegument layer, lying between the nucleocapsid and the surrounding envelope. The double-stranded DNA genome of HCMV is approximately 230 kb in size, the largest among known human viruses, and consists of unique long (UL) and unique short (US) segments, each of which is flanked by inverted repeats sequences [11]. Most of the approximately 200 genes encode protein products. CMV gene nomenclature is complex: most genes are generally named by their position within the genome, although some also have additional descriptive names. For example, UL83 is the 83rd annotated gene in the unique long region of the genome; it encodes a 65 kDa phosphoprotein also known as pp65. Hence, the protein product is both known as ppUL83, and pp65. Several HCMV gene products have been recognized as key immunogens in the host response to infection. These include envelope glycoproteins such as UL55 (also known as glycoprotein B), UL75 (glycoprotein H), and others. Some proteins in the viral tegument are targets of cell-mediated immune responses, in particular the ppUL83 (pp65). As noted, many of these proteins are being employed as subunit vaccines in various clinical trials [9].
Success in HCMV vaccine programs has been limited to date – underscoring the need to continue to use preclinical models to study correlates of protection against CMV infection and disease. Subunit vaccines targeting the major envelope glycoprotein gB (gpUL55) have demonstrated varying degrees of efficacy against CMV infection and/or disease in high-risk populations, including young women, solid organ, and hematopoietic stem cell transplant patients [12–16]. However, it remains uncertain if a vaccine-induced antibody response to a single viral glycoprotein target would be sufficient for a vaccine designed to prevention infection of the fetus. Live, attenuated HCMV vaccines induce both antibody responses as well as broad-based cellular responses, including cytotoxic CD+ T-cell responses [9, 17], and hence may have theoretical benefits compared to subunit approaches. Safety considerations regarding theoretical long-term risks of a CMV live-virus approach, including atherosclerosis, immune senescence, reactivation from latency, and, potentially, even Alzheimer's disease have dampened enthusiasm for the live attenuated vaccine approach [18–20].
Given the striking species-specificity of CMVs, preclinical studies of HCMV vaccine are generally not feasible in animal models. HCMV-specific immunogens, including recombinant proteins, virions, dense bodies, and other vectored vaccines [21–30], have all be evaluated for immunogenicity in a number of animals, including mice, rabbits, hamsters, guinea pigs, and rhesus macaques. However, although immunogenicity studies are possible (and provide valuable information), efficacy evaluations of these vaccines cannot be conducted, since HCMV will not cause replicate or cause disease in animals. Hence, species-specific CMVs must be studied, in order to model infection and test potential vaccine strategies for HCMV. In this review, the current status of animal models for CMV vaccines is reviewed, with a particularly emphasis on studies of vaccines targeting protection against congenital infections. High-priority areas for future animal model research are proposed and outlined in the executive summary section of this article.
Basis for Species-Specificity of CMVs: Can it be Overcome?
The precise molecular/cellular basis for the species-specificity of betaherpesviruses, including CMV, remains unknown. Early experiments in multiple laboratories attempted to grow CMVs from one species in heterologous cell culture systems from different species. Typically these experiments show some evidence of uptake of heterologous virus, but a block exists preventing full productive replication. A study of human diploid WI-38 cells inoculated with murine CMV (MCMV) demonstrated cytopathic effect, including intranuclear inclusions, but infection was abortive [31]. Rabbit kidney cultures were found in one study to support some level of replication of MCMV [32], and rabbit lung cells have been reported to support low-level HCMV replication [33]. MCMV was also reported to replicate in guinea pig brain cells, and maintain infectivity (although not replicate) for 12 days in human cells [34]. A recombinant MCMV expressing the GFP gene demonstrated evidence of abortive infection in multiple mammalian cell types [35]. A comparative study of various human and non-human cell cultures to determine their sensitivity to the Davis strain of HCMV indicated that mink lung cell cultures appeared more sensitive than other cell cultures tested, in a shell-vial centrifugation assay, although it was not demonstrated that these cells supported productive viral infection [36]; moreover, additional studies suggested that the observed cytopathogenicity of CMV in mink cells might have been related to mycoplasma contamination of HCMV stocks [37]. In other studies, inoculation of HCMV into mixed cultures of guinea pig fibroblasts and epithelial cells demonstrated CPE following adsorption of virus by conventional microscopy, and uptake of virus particles both into the cytoplasm as well as in cytoplasmic vacuoles by electron microscopy [Figure 1]. These experiments demonstrated expression of HCMV antigens by immunofluorescence, but the infection was ultimately nonproductive [38]. The results of these various investigations suggested that the barrier to CMV cross-species infection was downstream of events related to binding and penetration of virions into the cell, and that some limited initial gene expression appears to occur prior to an undefined block in productive replication.
Figure 1.
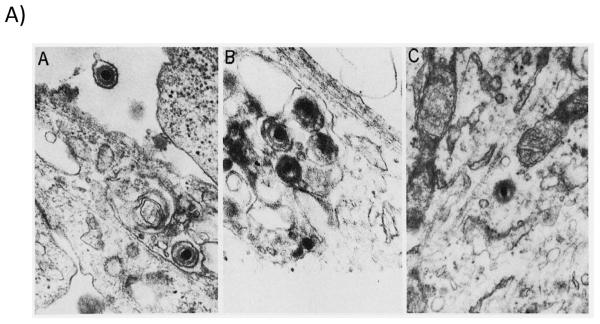
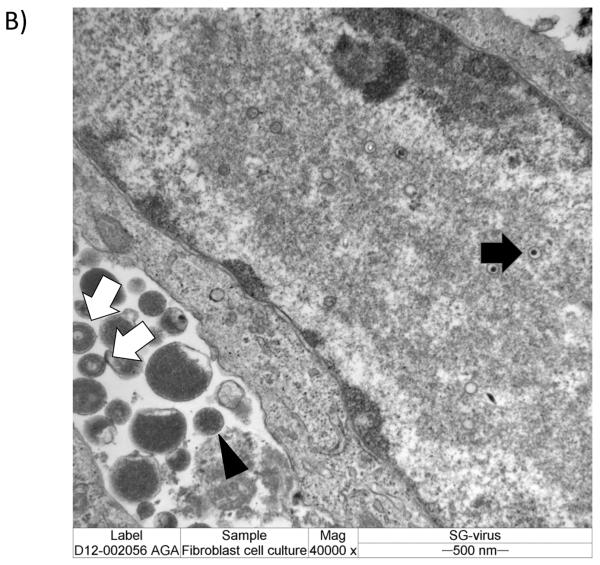
A) Nonproductive infection of guinea pig cells with human cytomegalovirus. Electron micrographs of thin sections of guinea pig cells (mixed fibroblasts and epithelial cells) infected with human cytomegalovirus, Towne strain. Reproduced from reference [38], with permission. Panel A, virus particles are noted near cell surface and cytoplasmic vacuoles within 15 minutes of infection. Panel B, viral nucleocapsids noted in vacuoles within 1 hour post-infection (x48,000). Panel C, fully unenveloped particle in cytoplasm 1 hour post-infection (x41,000). These and other data (see text) indicate that species-specific block to CMV replication is not due to binding and uptake of virus in cells of heterologous species. B) Productive infection of guinea pig cells with guinea pig cytomegalovirus. Experiment from our laboratory for comparison demonstrating productive infection of guinea pig fibroblast cell culture with salivary gland-adapted GPCMV (x40,000). Images obtained 48 hours post-infection. Intranuclear capsids are noted (black arrow) as well as extracellular virions (white arrows) and dense bodies (black arrowhead).
Recently there have been some insights into the molecular basis for species-specificity of CMV infection. Replication of MCMV in human cells was examined in detail several years ago by the late Gerd Maul at the Wistar Institute. Extending the work performed years earlier by other investigators, Maul demonstrated that MCMV can undergo all processes of its life cycle in human cells, but that there were several synergistic mechanisms of suppression of MCMV production, including hydrolysis of newly replicated viral DNA and very low capsid protein transcription [39]. These effects could be ameliorated by adding HCMV tegument proteins and IE 1 protein. Additional insights into the molecular basis for the species-specificity have also been gleaned from innovative work performed by the Brune laboratory. Preliminary work demonstrated that a key factor blocking MCMV replication in human cells was the induction of apoptosis, and that this block to replication could be partially overcome by the overexpression of members of the anti-apoptotic Bcl-2 protein family, including adenovirus E1B-19k [40]. Bcl-2 also allowed rat CMV (RCMV) to replicate and form plaques in human cells [40]. These studies were extended when these authors examined a spontaneously occurring MCMV mutant that had gained the ability to replicate rapidly and to high titers in human cells [41]. Compared to the wild-type virus, this mutant exhibited reduced apoptosis in cells, and sequence analysis of revealed mutations in the M112/M113-coding region of the genome, a region encoding a family of “early 1” (E1) proteins, involved in DNA replication. Another recent study reported by the Tang lab examined HCMV-infected mouse cells and MCMV-infected human cells, toward the goal of identifying intrinsic cellular defense mechanisms against cross-species infection at the post-entry level [42]. These studies demonstrated that the `knock-down' of ND10 components (PML, Daxx, and SP100) could result in significantly increased viral protein production, suggesting that ND10 and ND10 constituents might be important defensive factors against CMV cross-species infections. These various molecular mechanisms underlying the species-specificity of CMVs are summarized in Table 1.
Table 1.
Molecular Basis for Species-Specificity of Cytomegaloviruses: Potential Mechanisms Blocking Trans-Species Infections
| CMV Used➔Heterologous Cell Types Infected | Mechanism of Suppression of Transpecies Infection | Factors Contributing to Overcoming Species-Specificity Barrier |
|---|---|---|
|
| ||
| MCMV ➔Human Foreskin Fibroblasts (HFFs) | • MCMV DNA replication but DNA Degradation | • HCMV IE1: mechanism involving increased amount and stability of MCMV DNA and enhanced activity of HCMV pp71 |
| • Impaired Encapsidation Due to Lack of Synthesis of Capsid Proteins | • HCMV pp71: possible mechanism of neutralization of human Daxx protein to relieve Daxx-mediated repression of MCMV replication? | |
| • Lack of Synthesis of “Late” Proteins | • Other HCMV tegument proteins – unknown mechanism? | |
|
| ||
| MCMV, RCMV➔Human Retinal Pigment Epithelial Cells (ARPE) | • Initiation of apoptosis identified as a key event in blocking cross-species infection | • Block to replication overcome by expression of anti-apoptotic Bcl-2 proteins |
| • Adenovirus E1b-19K (Bcl-2-like) | ||
| • HCMV pUL37 (vMIA; structurally and functionally distinct from Bcl-2 family) | ||
|
| ||
| MCMV➔Retinal Pigment Epithelial Cells; human embryonic lung fibroblasts (MRC-5); HFFs; human umbilical vein endothelial cells (HUVECs) | • Spontaneous isolation of MCMV mutant capable of replication in human cells | • M112/113 mutations decrease the proapoptotic effect of MCMV infection on human cells |
| • Mutations in M112/M113 appear responsible; this region encodes the E1 proteins, (four differentially spliced isoforms) | • Increase the formation of replication compartments and the efficiency of viral DNA replication | |
| • Presumed pro-apoptotic function | • The mutations may enhance the ability of MCMV to disrupt promyelocytic leukemia protein-associated nuclear bodies (PMLs) nuclear domains, in the process possibly relieving transcriptional repression | |
|
| ||
| MCMV➔MRC-5 cells; adenovirus-transformed human epithelial kidney cell 293 (HEK293) | • ND10 (nuclear domain 10) proteins P100, Daxx, and PML appeared to be strong repressors of cross-species infection | • Species-specificity barrier could not be overcome in these studies |
| HCMV➔murine NIH 3T3 cells | • Knock-down of ND10 enhanced cross-species protein synthesis but did not confer replication competence | |
|
| ||
| RhCMV➔MRC5; ARPE; HUVEC | • RhCMV deleted in UL128-131 homolog region unable to replicate in human cells | • Repair of RhCMV UL128-UL131 homologs confers cross-species infection of human cells by RhCMV |
| • UL36 homolog influenced epithelial replication (anti-apoptotic function?) | ||
Understanding the mechanisms preventing and/or facilitating cross-species CMV infections could one day potentially allow the testing of HCMV vaccines in animal models. These studies also may have practical implications for solid organ transplant recipients of porcine xenografts, given that HCMV was recent shown to produce infection of porcine endothelium [43, 44]. Even though there was no evidence of productive viral replication, HCMV IE antigens were expressed in infected endothelium, and it was postulated that infection could, by upregulation of E-selectin and vascular cell adhesion molecule-1, result in leukocyte recruitment and an increased risk of rejection. Human fibroblasts have also similarly been shown permissive for PCMV infection in cell culture [45], as have human endothelial cells [46].
Recent studies have also examined the tropism and replication of primate CMVs in human cells. These studies have focused primarily on Rhesus macaque cytomegalovirus (RhCMV). A strain of RhCMV strain, 68–1, was noted to spread slowly when grown in cultured rhesus epithelial cells; this strain was not fully wild-type in sequence in the UL128-131 locus that has been shown to be important in epithelial and endothelial tropism for HCMV infection [47–49]. Specifically, it was noted that this strain did not code for ORFs corresponding to homologs of HCMV UL128 and the second exon of HCMV UL130. Following repair of mutations of the UL128-UL131 locus of strain 68–1, as well as repair of a mutation in the UL36 ORF, repaired viruses replicated much more efficiently than virus in rhesus epithelial cells, and, remarkably, replicated efficiently in cultured human epithelial cells and endothelial cells [50]. These results suggest that these gene products, at least in primate CMVs, also play roles in dictating the species-specificity of CMV. Additional reports have examined the replication of HCMV in chimpanzee cells. This work demonstrated that primary skin fibroblast cells derived from chimpanzees supported the replication of HCMV, with infection virus recovered from supernatants that was only 10-fold reduced in titer compared to HCMV grown in HF cells [51].
Other studies have attempted to overcome the problem of species-specificity of CMV infection by examining human tissue explants maintained in compartments in animals (typically, mice) that could allow the inoculation and propagation of HCMV in human cells. These animals in turn could be evaluated with antiviral drugs or other therapeutic interventions specific for HCMV in a system one step closer to an in vivo model. These systems have included human fetal thymus/liver [52–54] or fetal retinal tissue implants [55, 56] maintained in SCID/hu mice. Another model involved the use of HCMV-infected human foreskin fibroblasts seeded onto a biodegradable gelatin matrix, Gelfoam [57]. Infected fibroblasts are then implanted subcutaneously into SCID mice, and antiviral drugs targeting HCMV can be administered to assess antiviral effect in an in vivo model. Although these models are to varying degrees useful in evaluation of antiviral drugs [58, 59], there is less information about exploiting the SCID/hu implant model for evaluation of HCMV vaccines and immunotherapies.
Another novel approach to overcoming species-specificity issues in the study of CMV vaccines in animal models was described by Khanna and colleagues [60, 61]. In these experiments, replication-deficient adenovirus vectors expressing the extracellular domain of HCMV gB along with other HCMV epitopes (corresponding to immunodominant peptides from the the IE-1, IE-2, gH, pp150, pp50, and pp65 gene products) were used to immunize HLA-2 transgenic mice. The chimeric vaccine consistently generated strong HCMV-specific CD8+ and CD4+ T-cell responses, as well as virus-neutralizing antibody. Intriguingly, immunization with the HCMV/adenoviral chimeric vaccine protected mice against a challenge (referred to by the authors as a “quasi-virus challenge”) with a recombinant vaccinia virus encoding various combinations of HCMV antigens. Protection was associated with the induction of antigen-specific cellular responses and antibody responses. Although not a true “HCMV challenge” in a heterologous animal model, these experiments nevertheless represent an innovative approach to overcoming the problem of species-specificity in CMV vaccine models, and provide some opportunity to validate and compare various subunit vaccine targets using clinically relevant virological endpoints.
Finally, another approach to dealing with the challenge of the species-specificity of CMVs in the context of vaccine studies is to “humanize” the specific animal CMV by engineering HCMV gene targets into the heterologous viral genome by BAC mutagenesis [62]. One such “swap” mutant approach was described by our lab recently, in which the guinea pig CMV (GPCMV) homolog of the HCMV UL97 gene, GP97, was replaced with its HCMV counterpart [63]. This experiment was of interest, since UL97 is the kinase responsible for phosphorylating the antiviral drug, ganciclovir, to its active form, and GPCMV is intrinsically resistant to ganciclovir. The recombinant virus expressed functional UL97 protein, and had improved susceptibility to ganciclovir, compared to wild-type GPCMV. Engineering GPCMV to express HCMV glycoprotein targets, such as gB, could allow the testing of HCMV vaccines in the guinea pig congenital infection model – in essence, another type of “quasi-virus challenge”. Animal model testing of species-specific CMV vaccines based on authentic homologous viral challenge are considered in the following section.
Animal Models to Test Vaccines for Prevention of Congenital CMV Infection
Although there has been, as noted, some progress in elucidating the basis of species-specificity of CMV infection, at the present time there are very limited options for the testing of vaccines against HCMV in any animal models of infection. The following section summarizes salient features of and recent developments in animal models of vertical CMV transmission, with a particular emphasis on how this information may be exploited for testing HCMV vaccines. Ideally, an animal model would have the features of convenience and low expense; clear-cut vertical transmission and fetal/neonatal disease endpoints that would allow assessment of vaccines specifically against congenital infection; a wide array of immunological reagents that would allow detailed characterization of immune correlates of protective immunity; a well-characterized genome allowing for the expression of relevant subunit vaccine candidates; and experimental endpoints in newborn animals that mimic those observed in infants. The extent to which the various animal CMVs fulfill these criteria is summarized in the following section. A summary table outlining these various models is also provided (Table 2).
Table 2.
Comparison of Animal Models for Potential Study of Congenital CMV Infection
| Animal Model | Strengths | Challenges |
|---|---|---|
|
| ||
| Porcine CMV (PCMV) | • Congenital/placental Infections observed | • Genome sequence is currently incomplete |
| • Reinfection with transmission of virus in immune sows; could be useful model for re-infection | • Few immunological reagents for pig research | |
| • Fetal disease observed including lung, spleen and brain | • Expense of animals | |
| • PCR assays for viral genome detection are available | • No prior published reports of vaccine studies being performed in this model | |
| • Reports of PCMV cross-species infection of human cells; may have implications for xenotransplantation | • No clear endpoints that mimic congenital HCMV but not well studied | |
|
| ||
| Rat CMV (RCMV) | • Excellent model for CMV-associated vascular disease | • Relative paucity of reagents (monoclonal antibodies, immunological reagents for rat research) |
| • Several RCMVs sequenced; BAC clones available; role of RCMV genes in pathogenesis elucidated; molecular assays (PCR) available | • Intense immunosuppression required in some studies to observe disease | |
| • Recent observation of novel RCMV strain (ALL-03) capable of transplacental transmission | • No clear endpoints that mimic pathology in congenially infected infants; absence of brain infection | |
| • ALL-03 infects placenta, lung, liver, kidney, salivary gland, spleen, not brain | • No reports of vaccine studies in this model | |
| • Animals relatively inexpensive | ||
|
| ||
| Murine CMV (MCMV) | • Best-established animal model of CMV infection, immunology and pathogenesis | • Congenital/transplacental transmission unclear; conflicting reports in literature |
| • Vast array of knock-out mice, immunological reagents available | • In absence of transplacental transmission, vaccine studies specifically for prevention of congenital CMV infection have not been performed | |
| • Viral genomes sequenced, cloned as BACs for mutagenesis studies | • Neonatal models of pathogenesis are available and may be useful surrogate models for congenital HCMV infection; brain infection, cochlear infection models available following neonatal infection and could be exploited in future vaccine studies | |
| • Well-developed models for neuropathogenesis; relevant to HCMV | ||
| • Extensive literature on vaccine studies in this model; protein subunit, DNA vaccines, live attenuated vaccines, vectored vaccines | ||
| • Humoral and cellular targets elucidated; role of immune evasion genes well-defined; useful model to define roles of innate and adaptive immunity | ||
|
| ||
| Guinea Pig CMV (GPCMV) | • Best-established animal model of placental infection and congenital transmission | • Paucity of immunological reagents for guinea pig model |
| • Viral genomes sequenced, cloned as BACs for mutagenesis studies | • Relatively few reagents for GPCMV proteins; proteome not fully characterized | |
| • Congenital infection recapitulates pathology observed in infants; brain and cochlear infection; intrauterine growth retardation; vertical transmission with viremia and end-organ disease | • Endpoints of pup mortality/severe end-organ disease are extreme; congenital HCMV infection rarely causes mortality | |
| • Multiple studies of vaccines specifically targeting congenital transmission | • Long-term neurodevelopmental outcome studies following congenital GPCMV infection have not been performed | |
|
| ||
| Rhesus Macaque CMV (RhCMV) | • Viral genomes sequenced; BAC clones available for mutagenesis studies. | • Fetal pathogenesis requires direct inoculation of virus into developing fetus; bypasses placental route; relevance to congenital HCMV less clear using this route of inoculation |
| • Outstanding model for fetal pathogenesis including neuropathogenesis and cochlear infection | • RhCMV-seronegative animals difficult to procure | |
| • Model for other aspects of HCMV pathogenesis including re-infection, immunosenescence | • Expense of studies is considerable | |
| • Immunological reagents available | • Relatively few reagents for RhCMV (antibodies, viral mutants) although these are becoming increasingly available | |
| • Vaccine studies have been reported using a variety of subunit and vectored expression technologies | • No reports of vaccine studies to date specifically targeting congenital infection | |
| • Novel vaccine strategy of targeting viral immune modulation protein recently described | ||
Porcine Cytomegalovirus
Cytomegalic inclusion disease due to porcine cytomegalovirus (PCMV) was first recognized and described in 1964 [64], followed shortly thereafter by recognition of the causative agent by EM [65] and culture [66]. Many perinatal PCMV infections in piglets are probably acquired post-natally, from breast-feeding and other forms of close contact, since interventions such as early weaning of piglets and barrier rearing (interventions undertaken to try to increase the supply of PCMV-negative organs for xenotransplantation) does decrease the prevalence of PCMV infection [67, 68]. On the other hand, studies of pregnancy outcomes in pigs following intranasal exposure to PCMV have demonstrated transplacental infection as well, particularly if challenge is undertaken very early in pregnancy. In one study, five out of 22 embryos were infected when PCMV challenge occurred shortly after insemination. In infected embryos, virus was found in leptomeningeal cells, hepatic sinusoidal cells, peritoneal macrophages, periosteal cells and occasional alveolar cells, but less commonly in placenta [69]. A study of six seronegative sows infected with PCMV by intranasal route between 31 and 85 days of pregnancy followed by caesarean delivery at term demonstrated 18/60 mummified or stillborn fetuses. PCMV was isolated from 16 piglets from 4 of the 6 litters examined, most commonly from lung and liver but also from spleen, kidney, brain and nasal mucosa [70]. In another study, pregnant sows with PCMV antibody were challenged in pregnancy, and transmission occurred in spite of pre-existing immunity [71] – analogous to the issue of re-infection with vertical transmission that is being increasingly recognized as a concern in pregnant women [72–75]. In the reinfection study reported in sows, virus was detected in 8 of 24 fetuses, and in some fetuses virus could be identified in capillary endothelium and macrophages of the lung, as well as nasal mucosa, spleen and brain [71]. Interestingly, the majority of virus-positive fetuses in this study were reportedly normal in appearance, suggesting that these infections were asymptomatic. However, there is some evidence from the veterinary medicine literature that PCMV can be pathogenic in newborn piglets [76].
Although PCMV does transmit to the fetus and produce some interesting pathologies in the piglet, it has not been further exploited for development as a vaccine model. Genome information is incomplete, although PCR assays for detection of PCMV DNA have been developed [77, 78]. Recently, cloning and purification of a 270 nt region of the PCMV gB gene containing the putative immunodominant epitopes has allowed development of a PCMV ELISA assay [79]. If xenotransplantation with porcine organs continues to be developed as an intervention for humans with end-stage solid organ disease [68, 80], further examination of treatment or prevention of PCMV infections by either vaccination or antiviral therapy may become of practical medical importance [81].
Rat Cytomegalovirus (RCMV)
RCMV is an extensively used model that has been employed by many investigators to study CMV pathogenesis [82, 83]. The RCMV model has proven to be most useful in the context of viral challenge following immunosuppression of the rat, typically via total body irradiation [84], and has been used in this context to study a variety of antiviral therapies [85, 86]. RMCV also provides an excellent model for study of endovascular infection and disease [87], particularly in the setting of transplantation, especially cardiac allografts [88–90], but also in the setting of liver [91] and kidney [92] transplantation. RCMV can be used to model the phenomena of transplant vascular sclerosis (TVS; [93, 94]), a rapidly progressive and serious complication of heart transplantation in humans. Interesting, TVS is driven, in part, by the elaboration of RCMV-encoded chemokines (CKs), including the RCMV chemokine r129 [95], the homolog of the HCMV UL128 protein [96, 97].
To date, there are no reports of the use of the RCMV infection model for the study of CMV vaccines. Recent experiments reported by Loh and colleagues, however, have suggested that the RCMV model might be surprisingly useful for the study of congenital CMV infection and, conceivably, vaccines to prevent congenital transmission. In a study conducted at the Department of Veterinary Pathology and Microbiology at the Universiti Putra Malaysia, a novel strain of RCMV, designated ALL-03, was isolated from the placenta/uterus of a house rat (Rattus rattus diardii) and was found to: productively infect rat embryo fibroblasts; produce typical herpesvirus-like cytopathic effects; and demonstrate herpesviral inclusion bodies and virus related particles in the cytoplasm and nucleus of infected cells by EM [98]. Hyperimmune serum generated against another strain of RCMV, the Maastricht strain, was reactive against the ALL-03 strain, as assessed by neutralization test, immunoperoxidase assay, and immunofluorescence studies. Despite demonstrating typical morphological characteristics of a CMV, initial sequence analyses of the viral genome demonstrated that it was significantly different from that of the better-known Maastricht [99] and English [100] strains of RMCV. The finding that this virus infected the placenta and uterus of rats suggested that it might be vertically transmitted to the fetus. This was confirmed in a follow-up study by this group [101] that demonstrated, following experimental infection of immune compromised Sprague-Dawley rats, that virus challenge during pregnancy lead to infection of of uterus, placenta, and fetus, with virus identifiable in the lung, kidney, spleen, liver and salivary gland of congenitally infected pups. Inoculations were performed at day 10 of pregnancy (average gestational period, 22 days). In this study, virus could not be detected in the rat brain, potentially rendering this model less relevant to the problem of congenital HCMV infection. However, continued characterization of this strain of RCMV [102, 103] may be valuable, and could lead to the development of models of vaccine or antiviral therapies against congenital CMV infection in this model.
There are limited reagents available for the study of RCMV. There are PCR primers described for real-time detection of RCMV DNA [104]. A panel of monoclonal antibodies was developed by the Bruggeman lab several years ago [105], but do not appear to be commercially available. A BAC clone in E. coli of the Maastricht strain of RCMV has been developed and used to generate recombinant viruses [95].
Mouse Cytomegalovirus (MCMV)
The MCMV model has been the most powerful animal model for the study of CMV pathogenesis and immunity, due to the vast array of mouse immunological reagents; the plethora of transgenic mice; the well-characterized MCMV genome; the availability of recombinant MCMVs based on bacterial artificial chromosome (BAC) and other technologies; and the lower cost of mice compared to many other animals used in viral models. The MCMV model, particularly its usefulness in the study of latency, reactivation, and immune evasion, has been the subject of several excellent reviews [106–110]. With relevance to CMV vaccines, the MCMV model has been well-characterized, and targets of both the CD8+ T cell response [111–114] as well as the antibody response to infection have been evaluated in experimental models of vaccine-mediated protection. These studies have demonstrated protection against viral challenge following immunization with recombinant plasmids expressing key MCMV immunogens [115–121]; peptide-based vaccines [122, 123]; live, attenuated vaccines [124–129]; and vectored vaccines based on MCMV envelope glycoprotein homologs of gB and gH [113, 114]. Of particular interest has been the use of BAC technologies to selectively engineer live-virus vaccines deleted of viral immune evasion genes to improve immunogenicity and enhance safety [130–132]. In another variation of the BAC-based “designer vaccine” approach, a recombinant MCMV vaccine was engineered expressing the high-affinity NKG2D N cell ligand, RAE-1γ. This vaccine, by eliciting tight innate immune control via activation of NK cells, was highly attenuated, but induced strong and long-lasting protective immunity [133].
A chief limitation of the MCMV model for testing vaccines against congenital CMV infection is the probably inability of the virus to infect the fetus by transplacental route. This may relate to differences between the mouse placenta, which contains three trophoblastic layers, and the human placenta. Placental histology may contribute to the lack of transmission of MCMV across the mouse placenta [134, 135]. However, recently the dogma that the mouse fetus cannot be transplacentally infected has been challenged by studies reported by Woolf [136]. In these studies, timed-pregnant SCID mice were intraperitoneally injected with MCMV at embryonic stages E0–E7, and vertical MCMV transmission was noted at rates up to 53% when dams were challenged at day E4. Later time points of challenge were associated with reduced transmission. MCMV antigens were detected in PCR-positive fetuses, and transplacental MCMV transmission was associated with intrauterine growth retardation and microcephaly. The fact that these experiments were performed in SCID mice may have contributed to this result, since prior efforts to demonstrate vertical transmission of MCMV were uniformly unsuccessful [134, 135]. This model could conceivably be exploited to study vaccines against congenital MCMV infection, although there would be challenges intrinsic to the modeling of any active vaccination strategies in SCID mice.
Other models of neonatal MCMV disease exist. These have not been fully exploited for the testing of vaccines, although such studies may be of value: even though there may not be congenital transmission per se, maternal immunization, particularly with vaccines inducing humoral responses, could be studied as a strategy to improve the outcome following neonatal challenge. Studies performed by Tsuigui's group have pioneered the analysis of MCMV fetal neuropathogenesis using techniques that bypass the placenta to directly inoculate the fetus. Injection of MCMV into mid-term developing embryos is used to study brain abnormalities (neuronal migration defects, microcephaly, polymicrogyria), and eye abnormalities (microphthalmia), although unfortunately no vaccine studies have been pursued to date in this model [137–142]. Another intriguing study has demonstrated the effectiveness of antiviral immune globulin in limiting MCMV neuropathology [143]. In this study, treatment of MCMV infection in newborn mice with MCMV-immune serum or with MCMV-specific monoclonal antibodies reduced the titer of infectious virus detection limit, whereas brains of mice receiving control serum had significant viral load noted. In addition, histopathological and immunohistological analyses revealed more viral foci, mononuclear cell infiltrates, and glial nodules in the brains of untreated mice [143]. Another model developed by the Lokensgard laboratory has recently examined neonatal MCMV infection and cochlear pathology [144]. In this model MCMV was found to preferentially infect both cochlear perilymphatic epithelial cells and spiral ganglion neurons, and induce a chronic inflammatory response, including a prolonged increase in reactive oxygen species production by macrophages. An interesting report from a group at the Huazhong University of Science and Technology (Wuhan, China) examined fetal and newborn outcomes following placental inoculation of MCMV in BALB/c mice at 12.5 days gestation (average gestational period, 19–22 days). This group found that approximately 29% of newborn mice had MCMV brain infection, and these mice demonstrated not only microcephaly and reduced brain weight upon necropsy, but also had diminished performance in the Morris water maze compared to controls, with deficits in learning and memory capability [104]. This model could be very relevant to the HCMV-infected infant, since microcephaly and learning disabilities are commonly encountered. In addition, Jaskoll and colleagues have recently described an elegant in vitro model of MCMV cochlear pathogenesis [145]. All of these models of neonatal MCMV induced pathology are powerful, since they recapitulate the pathology observed in infants infected with congenital HCMV. Future studies of antiviral antibodies in many of these models could be considered, including those induced by maternal vaccination prior to delivery, and such data could be very instructive to HCMV vaccine development. Recent advances in real-time imaging of neonatal MCMV infection should also provide valuable new tools for addressing immunotherapies in the MCMV model [146].
Guinea Pig Cytomegalovirus (GPCMV)
The chief value of the GPCMV model, compared to other small animal models, lies in the unequivocal ability of the virus to produce congenital infection following maternal inoculation with this virus. A number of aspects of guinea pig reproductive biology further contribute to the usefulness of this model. Guinea pig gestational periods are lengthy, compared to rodent models, ranging from 65–70 days [147]. Like the human plancenta (but in contrast to the mouse and rat placenta), the guinea pig placenta is hemomonochorial, containing a single trophoblast layer separating maternal and fetal circulation [148–150]. These and histologic and biological similarities to the human placenta add to the value of this model for vaccine testing. The ability of GPCMV to infect the guinea pig placenta was first described in the 1930s [151]. In the late 1970s, three groups independently described studies of fetal infection following infection of pregnant dams with GPCMV [152–154]. Notably, in one of these reports it was observed that “transplacental transmission of virus with invasion of the fetus was observed…in some mothers with preinoculation evidence of GPCMV antibody” [154], a finding that has significance in light of the increasing recognition of maternal re-infections in pregnancy leading to symptomatic congenital infections in infants [72–75]. Since these reports on transplacental transmission of GPCMV, numerous investigations have employed the guinea pig model for vaccine studies (Table 3). Depending upon the strain of guinea pig (inbred vs. outbred), source of virus (tissue culture-adapted or salivary gland-passaged), and timing of inoculation during pregnancy, a variety of experimental endpoints can be studied in this model [155]. These include mortality in dams, pup infection rate, pup mortality rate, maternal and pup weights, fetal resorption rate, total viral load in dams and pups (assessed by viral culture or PCR), and end-organ disease (including placenta), assessed histologically and/or by viral load analysis. Variability in the strain of animal, route of inoculation, and timing of inoculation has contributed to a range of levels of effectiveness of various vaccine strategies studied to date in this model: Table 4 compares the magnitude of the protective effect reported in these various studies. To date there has unfortunately not been any analysis of the impact of vaccines on functional outcomes in pups following maternal challenge. Of particular interest with respect to the problem of congenital CMV in infants, it has been noted that congenitally infected guinea pig pups can exhibit brain and visceral involvement, inner ear pathology, and sensorineural hearing loss, providing additional useful and highly relevant endpoints that should be emphasized for future vaccine and pathogenesis studies [156–160]. Figure 2 demonstrates both end-organ disease in a dam challenged with virulent, salivary gland-adapted GPCMV mid-gestation (pneumonia) and cochlear pathology in a pup challenged post-natally with GPCMV as examples of pathology induced by viral infection in this model.
Table 3.
Vaccines for Prevention of Congenital CMV in the GPCMV Model
| Vaccine Approach | Guinea Pig Strain | Findings |
|---|---|---|
|
| ||
| Live, attenuated vaccine isolated following 11–34 serial passages of ATCC virus in tissue culture [161, 166, 167] | Hartley | • Reduced incidence of maternal and fetal infection |
| • Vaccine virus did not reactivate or produce fetal infection in subsequent pregnancy | ||
| • Did vaccine lose pentameric complex genes on TC passage? | ||
|
| ||
| Strain 2 | • Protection against viremia, pneumonitis, and mortality in non-pregnant animals | |
| • Reduction in end-organ recovery by culture of SG challenge virus in vaccinated animals | ||
|
| ||
| Envelope vaccine (dense bodies and virions) with Freund's adjuvant [161] | Hartley | • Neutralizing antibody responses elicited |
| • Vaccinated animals demonstrated reduction in viremia following SG virus challenge | ||
| • Protection against congenital infection | ||
|
| ||
| Immunoaffinity purified envelope glycoprotein with Freund's adjuvant [170] | JY-9 | • Neutralizing antibody and cell-mediated responses elicited |
| • Reduced maternal DNAemia | ||
| • Protection against GPCMV-associated pup mortality and congenital infection | ||
|
| ||
| Lectin-purified envelope glycoprotein(s) with Freund's adjuvant [171] | Hartley | • Antibody responses to several glycoproteins including gB |
| • Neutralizing antibody responses | ||
| • Protection against GPCMV-associated pup mortality and congenital infection in live-born pups | ||
|
| ||
| DNA vaccines gB/GP83 and BAC DNA[175, 176] | Hartley | • Vaccination resulted in lower viral load in infected pups |
| • BAC DNA vaccine resulted in decreased pup mortality | ||
| • BAC DNA immunogenicity augmented by lipid adjuvant | ||
|
| ||
| Recombinant gB vaccine with Freund's, alum and MPL adjuvants [178] | Hartley | • Neutralizing antibody response adjuvant-dependent |
| • Decreased pup mortality and reduced viral load | ||
| • Magnitude of viral load reduction adjuvant-dependent | ||
| • Best protection against mortality seen with MPL | ||
|
| ||
| Adenovirus-vectored gB vaccine [181] | Hartley | • Single vaccination in early pregnancy |
| • Neutralizing and high-avidity antibody | ||
| • Reduced vertical transmission | ||
| • Protection at level of reduced placental spread | ||
|
| ||
| Single-cycle, RNA replicon-vectored vaccine (VRP) expressing GP83 [180] | Hartley | • Engineered using replication-deficient alphavirus |
| • Antibody, CD4 and CD8 responses elicited | ||
| • Reduced maternal and pup viral load | ||
| • Reduced pup mortality and improved weights | ||
|
| ||
| MHC class I deletion vaccine [168] | Hartley | • 3DX highly attenuated in vivo but not in vitro |
| • Vaccination with 3DX produced elevated cytokine levels and higher antibody titers than wild type (WT) virus while avidity and neutralizing titers were similar | ||
| • Decreased maternal viral loads and pup mortality noted in vaccinated animals following SG virus challenge | ||
Table 4.
Effectiveness of Vaccines Against Congenital Infection and Pup Mortality in the GPCMV Model
| Vaccine Approach | Pup Infection | Pup Mortality | ||
|---|---|---|---|---|
| Control | Vaccine | Control | Vaccine | |
| Live, attenuated vaccine 11 serial passages in cell culture [161, 166, 167] | 7/26 (27%) | 1/25 (4%) | 13/26 (50%) | 1/25 (4%) |
| Live, attenuated vaccine 33–34 serial passages in cell culture [167] | 6/35 (17%) | 0/42 (0%) | 8/35 (23%) | 6/42 (14%) |
| Envelope vaccine/Freund's adjuvant [161] | 7/26 (27%) | 0/16 (0%) | 13/26 (50%) | 5/16 (31%) |
| Immunoaffinity purified glycoprotein/Freund's adjuvant [170] | 12/23 (52%) | 4/22 (18%) | 10/23 (43%) | 1/22 (5%) |
| Lectin-purified envelope glycoprotein(s)/Freund's adjuvant [171] | 16/20 (80%) | 24/54 (44%) | 27/48 (57%) | 9/63 (14%) |
| GP83DNA vaccine [175] | 20/26 (77%) | 17/25 (68%) | 13/39 (33%) | 13/38 (34%) |
| gB DNA vaccine [175] | 20/26 (77%) | 11/27 (41%) | 13/39 (33%) | 14/41 (34%) |
| Recombinant gB/Freund's adjuvant (study 1) [178] | 5/10 (50%) | 8/36 (22%) | 31/41 (76%) | 6/42 (14%) |
| Recombinant gB/alum adjuvant [178] | 5/10 (50%) | 15/32 (47%) | 31/41 (76%) | 17/49 (35%) |
| GP83VRP vaccine [180] | 11/13 (85%) | 8/17 (47%) | 12/21 (57%) | 4/32 (13%) |
| BAC DNA vaccine [176] | NA | NA | 23/35 (66%) | 10/34 (29%) |
| Recombinant gB/Freund's adjuvant (study 2) [179] | 36/55 (65%) | 24/33 (72%) | 37/57 (65%) | 12/33 (36%) |
| Recombinant gB/MPL adjuvant [179] | 36/55 (65%) | 34/73 (46%) | 37/57 (65%) | 12/73 (16%) |
| MHC class I deletion vaccine[168] | NA | NA | 20/46 (43%) | 6/27 (22%) |
| Adenovirus-vectored gB vaccine [181] | 9/12 (75%) | 2/16 (13%) | NA | NA |
Figure 2.
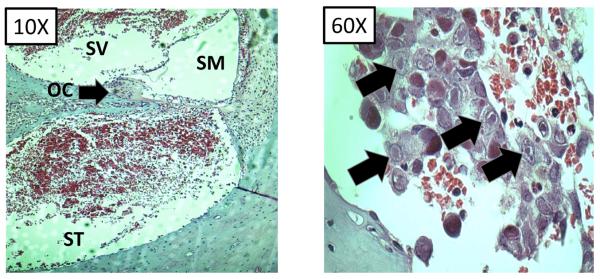
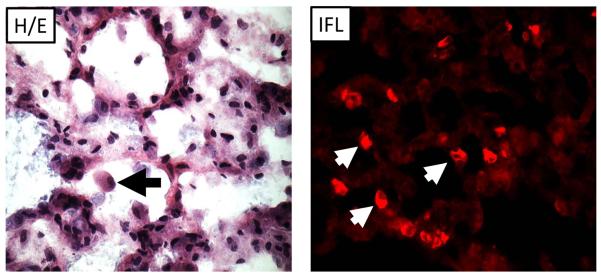
Pathology of GPCMV infection. A) Cochlear infection of guinea pig. Following GPCMV inoculation inflammatory infiltrates and viral inclusions. Left panel, cochlea (10X magnification) with organ of Corti (oc), scala media (sm), scala tympani (st) and scala vestibule (sv) labeled. Inflammatory infiltrates are most notable in perilymphatic compartment (st/sv). Right panel, 60x magnification of st demonstrating viral inclusions (black arrows). This pup had profound sensorineural hearing loss. B) GPCMV pneumonitis in pregnant dam (40x magnification). Left panel, hematoxylin/eosin stain (H/E) of lung from pregnant dam that died with GPCMV pneumonitis approximately 7 days following early third trimester challenge. Viral inclusion cell is noted (arrow). Right panel, immunofluorescence image (IFL) of lung using an anti-GPCMV gB antibody (IE321) generated in our laboratory demonstrating presence of gB in lung tissue (arrows).
The first vaccine study against congenital GPCMV reported in the literature was a study comparing a live, attenuated GPCMV vaccine, and a partially purified, soluble envelope vaccine, administered with Freund's adjuvant, in Hartley guinea pigs [161]. Live vaccine was prepared after 11 serial passages in tissue culture, whereas envelope vaccine was prepared by detergent treatment of GPCMV-derived dense bodies and virions, in a noninfectious vaccine enriched for viral envelope antigens. It is of interest that this vaccine preparation was derived from highly tissue culture-passaged GPCMV, which in retrospect may have selected for a the genome variant of the GPCMV 22122 strain deleted of the 1.6 kb region that encodes GP129, 131 and 133, homologs of the HCMV pentameric complex proteins UL128-131 [162–165]. This envelope vaccine preparation may have therefore been suboptimal in its ability to elicit neutralizing antibodies capable of blocking endocytic entry of virus into cells. In any case, after challenge with virulent GPCMV, animals inoculated with live virus vaccine were protected against acute viremia and death, and a reduced incidence of generalized maternal and fetal infection was observed. In contrast, envelope antigen-vaccinated animals showed acute viremia after similar challenge with virulent virus, but infection was less generalized than that in control animals, and GPCMV was not isolated from fetuses. These data provided the first suggestion in the GPCMV model that superior protection could be noted using live, attenuated vaccines, compared to adjuvanted subunit vaccines.
Another interest study reported by Bia and colleagues in 1984 examined “low-passage” GPCMV vaccine, which had been shown in the prior study to be effective in preventing GPCMV transmission, for the ability to undergo reactivation during pregnancy. In Hartley strain guinea pigs challenged by intraperitoneal route with low-passage vaccine, 27 (41%) of 66 throat swabs obtained during subsequent pregnancies were culture-positive for GPCMV, in contrast to 28 (24%) of 115 throat swabs obtained from nonpregnant controls. In contrast, high-passage GPCMV vaccine did not cause acute viremia, detectable generalized infection, or any evidence of GPCMV reactivation during subsequent pregnancies. In spite of its attenuation and apparent inability to establish latent infection in the salivary gland, these immune pregnant animals and their fetuses were protected against GPCMV when challenged with virulent salivary gland-adapted virus. Strain 2 guinea pigs were also protected against pneumonitis in a vaccine/challenge study in non-pregnant animals [166]. In retrospect, it seems likely that the experimental low-dose, high-passage GPCMV vaccine reported on by Bia's group in this study [167] – which failed to show any apparent vaccine reactivation during pregnancy – was attenuated due to selection of virus with deletions in the ~1.6 kb region of the viral genome identified by Inoue's group [162, 163] that is essential for conferring in vivo pathogenesis for GPCMV.
More recently, BAC-based mutagenesis has allowed further refinement of live, attenuated vaccines in the GPCMV model with targeted `knock-outs' of immune modulation genes. These viruses have been analyzed for protective efficacy in the congenital infection model. We reported on a vaccine study with a targeted deletion of three genes encoding MHC class I homologs (presumed NK evasins): gp147, gp148, and gp149. The resulting virus, 3DX, grew with wild-type kinetics in cell culture, but was attenuated in guinea pigs. In spite of attenuation, vaccination with 3DX produced elevated cytokine levels and higher antibody titers than wild-type virus, while avidity and neutralizing titers were similar. Protection, assessed by maternal viral loads and pup mortality following challenge with salivary gland-adapted virus during pregnancy, was comparable in animals vaccinated with 3DX and wild-type virus, and statistically significant compared to control (non-vaccinated) animals [168]. In the current issue of Future Virology, we report on another live, attenuated viral vaccine, vAM409, a vaccine with a targeted deletion in the pp65 (pUL83) homolog, GP83. This vaccine virus, although attenuated, provided protection against pup mortality in litters born to vaccinated dams compared to unvaccinated controls, with attendant reduction in congenital GPCMV infection. Further exploration of attenuated live-virus vaccines with targeted deletions in genes involved in immune modulation and pathogenesis is warranted in this model.
Since the early descriptions of detergent-treated crude envelope protein vaccines in the GPCMV model [161], subunit approaches to vaccination against congenital GPCMV infection utilized lectin-purified or immuno-affinity purified glycoprotein vaccines have continued to be studied. In a study in inbred guinea pigs, an immunoaffinity-purified glycoprotein was found to induce strong antibody and CMI responses when administered with Freund's adjuvant, and newborn pups were protected against congenital infection and disease [169, 170]. In a similar study, immunity conferred by immunization with a mixture of envelope glycoproteins resulted in substantial protection against pup mortality, with a reduction in pup mortality from 56% to 14% in the immunized group compared to controls (p<0.001), and reduced in utero transmission in surviving animals [171].
More recently, cloned recombinant technologies have been applied to the study of the GPCMV homologs of gB [172] and pUL83 (GP83) [173] in vaccination against congenital infection. When these ORFs were cloned in plasmid expression vectors [174] for DNA immunization studies, a four-dose series of plasmid vaccine administered epidermally by “gene gun” approach resulted in reduced pup mortality and a significant decrease in the incidence of congenital CMV infection for gB vaccine, but not GP83 [175]. However, both vaccines resulted in a reduced viral load in infected animals, compared to controls. The immunogenicity and efficacy of a DNA BAC vaccine, modified by transposon insertion into an essential gene, GP48 [176], was also studied. BAC plasmid DNA was adjuvanted by inclusion of the lipid adjuvant, DOTMA/DOPE. Following vaccination of Hartley guinea pigs with BAC DNA, third-trimester GPCMV challenge demonstrated that immunization resulted in reduced pup mortality, and a correlate of protection appeared to be reduced viral load in vaccinated dams.
Adjuvanted subunit studies with a recombinant, secreted form of gB expressed in insect cells, truncated at Pro692 [177] and purified by lectin column, have also been conducted in the GPCMV model [178]. One study compared a three-dose series (50 μg doses) of vaccine administered with either Freund's adjuvant or alum. ELISA titers and complement-dependent virus neutralizing titers were significantly higher in guinea pigs immunized with gB and Freund's compared to gB with alum. Among control dams (n=41 pups), pup mortality was 76% (31/41). In contrast, for pups born to gB-immunized dams, the overall pup mortality rate was reduced to 25% (23/91; p<0.0001). Differences were noted in mortality and congenital infection when the Freund's and alum groups were compared. The gB/Freund's adjuvant vaccine was also more effective than alum in reducing maternal viral load. A subsequent vaccine/challenge study examined GPCMV-gB vaccines formulated with the clinically-relevant adjuvant systems, AS01B and AS02V, or with Freund's adjuvant [179]. Efficacies against pup mortality were estimated at 64%, 84% and 44% for gB/AS01B, gB/AS02V and gB/FA, respectively. Efficacies DNAemia were estimated at 88%, 68% and 25% for the same vaccines, respectively, but were only significant for the gB/AS01B (p<0.001), and the gB/AS02V (p=0.002) adjuvants. These studies suggest that for optimum protection against congenital CMV conferred by a recombinant gB vaccine, adjuvant choice will be critical.
In addition to DNA vaccines and adjuvanted glycoprotein vaccines, subunit vaccine approaches to congenital GPCMV infection have been performed using vectored approaches. We reported on the use of a propagation-defective, single-cycle, RNA replicon vector system, derived from an attenuated strain of the alphavirus Venezuelan equine encephalitis virus, that was used to produce virus-like replicon particles (VRPs) expressing GP83 [180]. Vaccination with VRP-GP83 induced antibodies and CD4+ and CD8+ T cell responses, and dams vaccinated with VRP-GP83 had improved pregnancy outcomes (13% pup mortality), compared with dams vaccinated with a control VRP expressing influenza hemagglutinin (57% mortality; P<0.001, Fisher's exact test). Improved pregnancy outcome was also accompanied by reductions in maternal blood viral load. Another vectored approach was recently reported by Inoue's group [181], using a recombinant adenovirus approach expressing the GPCMV gB. This form of gB was also a truncated, secreted variant form of the gB molecule, truncated at Tyr674. Vaccination of dams with a single dose of a recombinant adenovirus-gB construct in early pregnancy resulted in reduced congenital GPCMV infection, from 75% in controls to 13% of fetuses immunized dams. Notably, placentas were infected less frequently in the gB-immunized animals, and there was reduced intrauterine growth retardation in fetuses in vaccinated dams. These authors postulated that antibodies against gB protect against spread of infection mainly at the placental interface, rather than from the placenta to the fetus, and that the development of strategies to block cell-to-cell viral spread in the placenta would represent the most effective strategy against congenital CMV infection.
In summary, vaccine studies in the GPCMV model (magnitude of protection noted in various vaccine studies is summarized for side-by-side comparisons in Table 4) have provided the following insights into vaccine-mediated protection against congenital infection: 1) there is strong evidence for the importance of both gB and GP83 in protective immunity against congenital CMV infection; 2) for subunit protein vaccines, the choice of adjuvant is critical; 3) the magnitude of the antibody response engendered, particularly the neutralizing antibody response, correlates with the extent of protection; 4) the magnitude of reduction of maternal DNAemia is an important correlate of protection of the fetus, and a threshold level of DNAemia exists that, above which, there are striking increases in pup mortality; 5) glycoprotein B vaccines to date have not provided “sterilizing immunity” of the maternal, placental, or fetal compartments; 6) reduction of cell-to-cell spread at the level of the placenta may be an important goal of vaccine-induced immunity; 7) live, attenuated vaccines confer superior protection to subunit vaccines. Given the regulatory issues implicit in licensure of a live, attenuated HCMV vaccine, greater value may be realized in future studies in the GPCMV model are focusing on subunit, and not live attenuated, vaccines. In particular, variables leading to optimization of antibody response, inclusion of cellular targets (such as GP83) in multicomponent vaccine studies, and examination of the protective immunity conferred by vaccination with proteins corresponding to the gH/gL/GP129–133 pentameric complex homologs of the HCMV gH/gL/UL128-131 complex are high priority areas for future studies.
Rhesus Macaque Cytomegalovirus (RhCMV)
The final animal model for the study of vaccines against congenital CMV infection is the rhesus macaque model. This model employs RhCMV, a closely related primate CMV that has a high degree of genome identity with the HCMV genome [182–186]. The rhesus macaque model is especially well suited for the study of HCMV pathogenesis, based on the strong developmental, immunological, anatomical, and biochemical similarities that humans and monkeys share by virtue of their close phylogenetic relationship. The RhCMV model has been the subject of several excellent reviews [187–189]. The RhCMV model also provides an excellent model for fetal neuropathogenesis due to CMV infection in the developing central nervous system. In a study of fetal rhesus monkeys inoculated intraperitoneally with RhCMV early in the second trimester, fetuses had evidence of severe CMV disease, including intrauterine growth restriction, ventriculomegaly, microcephaly, lissencephaly, and degenerative changes of cerebral parenchyma [190]. Histopathologic examination in this study revealed the presence of polymicrogyria, gliosis, leptomeningitis, periventricular calcifications, and inclusion-bearing cells. The demonstration that the developing macaque brain is susceptible to infection with RhCMV early in the second trimester and that intrauterine infection results in neuropathologic outcomes similar to those observed in infants provides a strong impetus for the continued development of this model to test vaccines against congenital CMV infection. The recent development of specific-pathogen-free colonies of macaques [191] is also of great potential value in helping to control for the issue of pre-existing maternal immunity in the context of vaccine studies.
There have not been, to date, any RhCMV vaccine studies reported in which the primary study endpoint was prevention of vertical transmission, or fetal/neonatal disease. A heterologous DNA prime/protein boost strategy was used in seronegative macaques, consisting of four DNA immunizations against pp65-2, gB, and immediate-early 1 (IE1), followed by two boosts with formalin-inactivated RhCMV virions; this approach was associated with strong cellular and humoral immune responses, and decreased viral replication at a site of primary inoculation [192]. A DNA vaccine study comparing combinations of plasmids expressing various combinations of RhCMV gB, pp65, and virally-encoded interleukin-10 homolog demonstrated a role for gB and pp65 vaccine in altering the course of acute and persistent RhCMV infection [193]. A study of viral shedding in saliva in macaques immunized with modified vaccinia virus Ankara (MVA) expressing gB, pp65, and IE1 demonstrated reduction in shedding in vaccinated animals compared to controls [194], and a prime-boost study in which animals were primed with DNA vaccination and boosted with MVA vaccine demonstrated reductions in viremia following viral challenge [195]. More recently, a MVA vaccine based on the RhCMV homologs of the gH/gL/UL128-131 pentameric complex demonstrated the induction of broadly neutralizing antibody responses and reduced plasma viral load in 8 vaccinated macaques [196].
Other recent studies have highlighted the rhesus macaque model's usefulness in studying the immunopathogenesis of CMV infection, particularly the role of viral immune modulation genes in immunity and infection. As noted, since the genome sequences of rhCMV [182–186] and the related cynomolgus macaque cytomegalovirus [197] are known, the high degree of genetic relatedness of these CMVs to HCMV should make future vaccine studies of particularly high relevance to human health. Of particular interest are studies reported by the Picker laboratory examining the results of mutation of the RhCMV homologs of HCMV UL128-131 on the CD8+ cell repertoire in RhCMV infected macaques. In this report, it was shown that the absence of Rh157.5, Rh157.4, and Rh157.6 (homologs of human CMV UL128, UL130, and UL131 genes) allowed CD8+ T cells to be generated against unusual, diverse and highly “promiscuous” epitopes [198]. The ability to engineer a live-virus vaccine capable of achieving distinct and more diverse patterns of CD8+ T cell epitope recognition may prove useful in both the development of new HCMV vaccines, as well as in the use of HCMV itself as a vaccine “vector” for other infectious diseases. Another innovative, recently reported RhCMV vaccine study targeted the viral homolog of the anti-inflammatory cytokine, viral IL-10 [199], (encoded by the RhCMV genome) as a potential vaccine target. RhCMV-seronegative macaques were immunized with engineered, nonfunctional RhCMV IL-10 variants, which were constructed to abolish binding to the cellular IL-10 receptor. Vaccine recipients developed antibodies that neutralized RhCMV IL-10 function with no cross-neutralization of cellular IL-10. Following viral challenge, vaccine recipients demonstrated reduced replication at the inoculation site reduced Rh CMV shedding in body fluids compared to controls [200]. Neutralization of viral immunomodulation genes represents a novel vaccine paradigm for HCMV vaccines. Future studies examining the role of this and other subunit vaccines in protection of the macaque fetus against congenital RhCMV infection with attendant neuropathogenic intrauterine infection are eagerly awaited.
Future Perspective
As summarized in Table 3, there have been now a number of vaccine studies for congenital CMV infection performed in the most tractable animal model, the guinea pig model. Using GPCMV, a number of live-attenuated and protein subunit vaccine approaches have demonstrated varying degrees of effectiveness. For subunit vaccines, the choice of adjuvant is critical. Notably, even with optimal and potent adjuvants, sterilizing immunity is not observed with gB based vaccines. The recent recognition and characterization of the GPCMV homologs of the pentameric complex proteins (HCMV gH/gL/UL128-131) opens up the exciting opportunity to examine the comparative efficacy of vaccines based on this complex head-to-head with gB vaccines. In addition, cell–mediated targets, such as GP83 (the pUL83 homolog), have been validated as useful vaccines in the GPCMV model. It will be important to more fully characterize the depth and breadth of the guinea pig response to other cellular targets to optimize this model. Finally, the rat and mouse models may be more useful for the study of vaccines against fetal CMV infection than has been previously recognized, and these models deserve further development. The most relevant model, the rhesus macaque, also deserves additional study, since the neurological and neurodevelopmental sequelae of infection can be best studied in this relevant primate system.
Executive Summary Points.
Basis for Species-Specificity of CMVs
Congenital CMV infection is a major public health problem and CMV vaccines are a major priority, but must be tested in preclinical models of congenital infection.
CMVs unfortunately only infect animals of their respective species; i.e., there is a mouse CMV, a human CMV, a rat CMV, but these viruses won't infect across species.
The species-specificity of CMVs is poorly understood, but recent insights from MCMV suggest a role of the M112/113 region of the viral genome as well as constituents of the PML, Daxx, and SP100 proteins in dictating species-specificity. Insights into the molecular basis of species-specificity suggests the very real possibility of directly studying HCMV in animal models in the future.
Animal Models to Test Vaccines for Prevention of Congenital CMV Infection
Transplacental infection is an important pre-requisite for a model of study of vaccines for congenital infection, but RCMV and MCMV have been underappreciated as potential models of congenital infection.
Glycoprotein vaccines for congenital CMV require optimized adjuvants.
Live, attenuated vaccines using molecular mutagenesis techniques should not be dismissed as potential HCMV vaccines, and the safety and efficacy of such approaches should be examined in animal models.
Future studies should emphasize the pentameric complex, diverse CTL targets, improved adjuvant systems, and more nuanced outcome measures, such as hearing loss and neurological injury, in animal models.
The recognition of the role of deletion/modification of immune modulation genes in rhCMV (particularly Rh157.5, Rh157.4, and Rh157.6) in directing cell-mediated responses, including CD8+ T-cell responses, to unusual and diverse epitopes, could offer new vaccine design strategies. These concepts should be further developed, given the close similarity in the molecular biology and pathophysiology of RhCMV and HCMV.
References
* Of interest
** Of considerable interest
- 1.Whitley RJ. Congenital cytomegalovirus infection: epidemiology and treatment. Adv Exp Med Biol. 2004;549:155–160. doi: 10.1007/978-1-4419-8993-2_21. [DOI] [PubMed] [Google Scholar]
- 2.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90(6):862–866. [PubMed] [Google Scholar]
- 3.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths P, Plotkin S, Mocarski E, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31(Suppl 2):B197–203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause PR, Bialek SR, Boppana SB, et al. Priorities for CMV vaccine development. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(2):e11–13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 8.Nyholm JL, Schleiss MR. Prevention of maternal cytomegalovirus infection: current status and future prospects. Int J Womens Health. 2010;2:23–35. doi: 10.2147/ijwh.s5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung H, Schleiss MR. Update on the current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2010;9(11):1303–1314. doi: 10.1586/erv.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schleiss MR. Persistent and recurring viral infections: the human herpesviruses. Curr Probl Pediatr Adolesc Health Care. 2009;39(1):7–23. doi: 10.1016/j.cppeds.2008.10.003. [DOI] [PubMed] [Google Scholar]
- *11.Boeckh M, Geballe AP. Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest. 2011;121(5):1673–1680. doi: 10.1172/JCI45449. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Excellent review of the biology and clinical significance of cytomegalovirus
- **12.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **13.Griffiths PD, Stanton A, Mccarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Both the Pass and Griffiths references are of considerable interest to the CMV vaccine field insofar as they demonstrate, for the first time, protection conferred by a recombinant subunit vaccine based on CMV gB
- 14.Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. The Journal of Infectious Diseases. 2011;203(11):1534–1541. doi: 10.1093/infdis/jir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–299. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 16.Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. Reanalysis of TransVax immunogenicity. Lancet Infect Dis. 2013;13(1):18. doi: 10.1016/S1473-3099(12)70296-7. [DOI] [PubMed] [Google Scholar]
- 17.Heineman TC, Schleiss M, Bernstein DI, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. The Journal of infectious diseases. 2006;193(10):1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 18.Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. The Journal of Infectious Diseases. 2013;208(4):564–572. doi: 10.1093/infdis/jit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. Journal of virology. 2005;79(6):3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259(3):219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]; ** Excellent review of potential long-term consequences of CMV infection in populations other than pregnant women and transplant patients.
- 21.Gonczol E, Berensci K, Pincus S, et al. Preclinical evaluation of an ALVAC (canarypox)--human cytomegalovirus glycoprotein B vaccine candidate. Vaccine. 1995;13(12):1080–1085. doi: 10.1016/0264-410x(95)00048-6. [DOI] [PubMed] [Google Scholar]
- 22.Gonczol E, Detaisne C, Hirka G, et al. High expression of human cytomegalovirus (HCMV)-gB protein in cells infected with a vaccinia-gB recombinant: the importance of the gB protein in HCMV immunity. Vaccine. 1991;9(9):631–637. doi: 10.1016/0264-410x(91)90187-b. [DOI] [PubMed] [Google Scholar]
- 23.Marshall GS, Ricciardi RP, Rando RF, et al. An adenovirus recombinant that expresses the human cytomegalovirus major envelope glycoprotein and induces neutralizing antibodies. The Journal of infectious diseases. 1990;162(5):1177–1181. doi: 10.1093/infdis/162.5.1177. [DOI] [PubMed] [Google Scholar]
- 24.Selinsky C, Luke C, Wloch M, et al. A DNA-based vaccine for the prevention of human cytomegalovirus-associated diseases. Hum Vaccin. 2005;1(1):16–23. doi: 10.4161/hv.1.1.1335. [DOI] [PubMed] [Google Scholar]
- 25.Temperton NJ, Quenelle DC, Lawson KM, et al. Enhancement of humoral immune responses to a human cytomegalovirus DNA vaccine: adjuvant effects of aluminum phosphate and CpG oligodeoxynucleotides. Journal of medical virology. 2003;70(1):86–90. doi: 10.1002/jmv.10357. [DOI] [PubMed] [Google Scholar]
- 26.Pepperl S, Munster J, Mach M, Harris JR, Plachter B. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. Journal of virology. 2000;74(13):6132–6146. doi: 10.1128/jvi.74.13.6132-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartikka J, Bozoukova V, Morrow J, et al. Preclinical evaluation of the immunogenicity and safety of plasmid DNA-based prophylactic vaccines for human cytomegalovirus. Hum Vaccin Immunother. 2012;8(11):1595–1606. doi: 10.4161/hv.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu TM, Wang D, Freed DC, et al. Restoration of viral epithelial tropism improves immunogenicity in rabbits and rhesus macaques for a whole virion vaccine of human cytomegalovirus. Vaccine. 2012;30(52):7469–7474. doi: 10.1016/j.vaccine.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 29.Shedlock DJ, Talbott KT, Wu SJ, et al. Vaccination with synthetic constructs expressing cytomegalovirus immunogens is highly T cell immunogenic in mice. Hum Vaccin Immunother. 2012;8(11):1668–1681. doi: 10.4161/hv.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasari V, Smith C, Zhong J, Scott G, Rawlinson W, Khanna R. Recombinant glycoprotein B vaccine formulation with Toll-like receptor 9 agonist and immune-stimulating complex induces specific immunity against multiple strains of cytomegalovirus. The Journal of general virology. 2011;92(Pt 5):1021–1031. doi: 10.1099/vir.0.029413-0. [DOI] [PubMed] [Google Scholar]
- 31.Kim KS, Carp RI. Abortive infection of human diploid cells by murine cytomegalovirus. Infection and immunity. 1972;6(5):793–797. doi: 10.1128/iai.6.5.793-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plummer G, Goodheart CR. Growth of murine cytomegalovirus in a heterologous cell system and its enhancement by 5-iodo-2'-deoxyuridine. Infection and immunity. 1974;10(1):251–256. doi: 10.1128/iai.10.1.251-256.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart H, Norval M. Association of human cytomegalovirus (HCMV) with mink and rabbit lung cells. Archives of virology. 1981;67(3):203–215. doi: 10.1007/BF01318131. [DOI] [PubMed] [Google Scholar]
- 34.Barabas G, Wroblewska Z, Gilden DH. Growth of murine cytomegalovirus in murine and heterologous brain cell cultures. Brief report. Arch Virol. 1980;65(2):193–200. doi: 10.1007/BF01317331. [DOI] [PubMed] [Google Scholar]
- 35.Van Den Pol AN, Vieira J, Spencer DD, Santarelli JG. Mouse cytomegalovirus in developing brain tissue: analysis of 11 species with GFP-expressing recombinant virus. J Comp Neurol. 2000;427(4):559–580. doi: 10.1002/1096-9861(20001127)427:4<559::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Mackenzie D, Mclaren LC. Increased sensitivity for rapid detection of cytomegalovirus by shell vial centrifugation assay using mink lung cell cultures. Journal of virological methods. 1989;26(2):183–188. doi: 10.1016/0166-0934(89)90147-x. [DOI] [PubMed] [Google Scholar]
- 37.Darai G, Flugel RM, Zoller L, et al. The plaque-forming factor for mink lung cells present in cytomegalovirus and herpes-zoster virus stocks identified as Mycoplasma hyorhinis. The Journal of general virology. 1981;55(Pt 1):201–205. doi: 10.1099/0022-1317-55-1-201. [DOI] [PubMed] [Google Scholar]
- 38.Fioretti A, Furukawa T, Santoli D, Plotkin SA. Nonproductive infection of guinea pig cells with human cytomegalovirus. J Virol. 1973;11(6):998–1003. doi: 10.1128/jvi.11.6.998-1003.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Tang Q, Maul GG. Mouse cytomegalovirus crosses the species barrier with help from a few human cytomegalovirus proteins. Journal of virology. 2006;80(15):7510–7521. doi: 10.1128/JVI.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Jurak I, Brune W. Induction of apoptosis limits cytomegalovirus cross-species infection. The EMBO journal. 2006;25(11):2634–2642. doi: 10.1038/sj.emboj.7601133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Schumacher U, Handke W, Jurak I, Brune W. Mutations in the M112/M113-coding region facilitate murine cytomegalovirus replication in human cells. Journal of virology. 2010;84(16):7994–8006. doi: 10.1128/JVI.02624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Cosme RC, Martinez FP, Tang Q. Functional interaction of nuclear domain 10 and its components with cytomegalovirus after infections: cross-species host cells versus native cells. PLoS One. 2011;6(4):e19187. doi: 10.1371/journal.pone.0019187. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** References 39–42 are all of considerable interest; they provide data that helps explain the species-specificity of CMV infection, an enigma that has challenged vaccine development (since HCMV cannot be directly studied in animal models)
- 43.Ghielmetti M, Millard AL, Haeberli L, et al. Human CMV infection of porcine endothelial cells increases adhesion receptor expression and human leukocyte recruitment. Transplantation. 2009;87(12):1792–1800. doi: 10.1097/TP.0b013e3181a75a41. [DOI] [PubMed] [Google Scholar]
- 44.Millard AL, Haberli L, Sinzger C, et al. Efficiency of porcine endothelial cell infection with human cytomegalovirus depends on both virus tropism and endothelial cell vascular origin. Xenotransplantation. 2010;17(4):274–287. doi: 10.1111/j.1399-3089.2010.00594.x. [DOI] [PubMed] [Google Scholar]
- 45.JL. Dudani AK, Tackaberry ES. Human fibroblasts are permissive for porcine cytomegalovirus in vitro. Transplantation. 2008;86(1):155–162. doi: 10.1097/TP.0b013e31817d4823. [DOI] [PubMed] [Google Scholar]
- 46.Degre M, Ranneberg-Nilsen T, Beck S, Rollag H, Fiane AE. Human cytomegalovirus productively infects porcine endothelial cells in vitro. Transplantation. 2001;72(7):1334–1337. doi: 10.1097/00007890-200110150-00028. [DOI] [PubMed] [Google Scholar]
- 47.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. Journal of virology. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryckman BJ, Rainish BL, Chase MC, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. Journal of virology. 2008;82(1):60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL131–128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. Journal of virology. 2004;78(18):10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lilja AE, Shenk T. Efficient replication of rhesus cytomegalovirus variants in multiple rhesus and human cell types. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19950–19955. doi: 10.1073/pnas.0811063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perot K, Walker CM, Spaete RR. Primary chimpanzee skin fibroblast cells are fully permissive for human cytomegalovirus replication. The Journal of general virology. 1992;73(Pt 12):3281–3284. doi: 10.1099/0022-1317-73-12-3281. [DOI] [PubMed] [Google Scholar]
- 52.Mocarski ES, Bonyhadi M, Salimi S, Mccune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc Natl Acad Sci U S A. 1993;90(1):104–108. doi: 10.1073/pnas.90.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Taylor SL, Leisenfelder SA, et al. Human cytomegalovirus genes in the 15-kilobase region are required for viral replication in implanted human tissues in SCID mice. Journal of virology. 2005;79(4):2115–2123. doi: 10.1128/JVI.79.4.2115-2123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JM, Kaneshima H, Mocarski ES. Dramatic interstrain differences in the replication of human cytomegalovirus in SCID-hu mice. J Infect Dis. 1995;171(6):1599–1603. doi: 10.1093/infdis/171.6.1599. [DOI] [PubMed] [Google Scholar]
- 55.Bidanset DJ, Rybak RJ, Hartline CB, Kern ER. Replication of human cytomegalovirus in severe combined immunodeficient mice implanted with human retinal tissue. The Journal of Infectious Diseases. 2001;184(2):192–195. doi: 10.1086/322015. [DOI] [PubMed] [Google Scholar]
- 56.Prichard MN, Quenelle DC, Bidanset DJ, et al. Human cytomegalovirus UL27 is not required for viral replication in human tissue implanted in SCID mice. Virology journal. 2006;3:18. doi: 10.1186/1743-422X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo FJ, Cardin RD, Bernstein DI. A model of human cytomegalovirus infection in severe combined immunodeficient mice. Antiviral research. 2007;76(2):104–110. doi: 10.1016/j.antiviral.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **58.Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother. 2001;12(Suppl 1):149–156. [PubMed] [Google Scholar]
- **59.Kern ER. Pivotal role of animal models in the development of new therapies for cytomegalovirus infections. Antiviral Res. 2006;71(2–3):164–171. doi: 10.1016/j.antiviral.2006.05.018. [DOI] [PubMed] [Google Scholar]; **** Excellent review of the use of animal models for study of CMV infection with an emphasis on antiviral therapies
- 60.Zhong J, Khanna R. Ad-gBCMVpoly: A novel chimeric vaccine strategy for human cytomegalovirus-associated diseases. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2009;46(Suppl 4):S68–72. doi: 10.1016/j.jcv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Zhong J, Rist M, Cooper L, Smith C, Khanna R. Induction of pluripotent protective immunity following immunisation with a chimeric vaccine against human cytomegalovirus. PLoS One. 2008;3(9):e3256. doi: 10.1371/journal.pone.0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brune W, Messerle M, Koszinowski UH. Forward with BACs: new tools for herpesvirus genomics. Trends Genet. 2000;16(6):254–259. doi: 10.1016/s0168-9525(00)02015-1. [DOI] [PubMed] [Google Scholar]
- 63.Mcgregor A, Choi KY, Cui X, Mcvoy MA, Schleiss MR. Expression of the human cytomegalovirus UL97 gene in a chimeric guinea pig cytomegalovirus (GPCMV) results in viable virus with increased susceptibility to ganciclovir and maribavir. Antiviral Res. 2008;78(3):250–259. doi: 10.1016/j.antiviral.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corner AH, Mitchell D, Julian RJ, Meads EB. A Generalized Disease in Piglets Associated with the Presence of Cytomegalic Inclusions. J Comp Pathol. 1964;74:192–199. doi: 10.1016/s0368-1742(64)80024-2. [DOI] [PubMed] [Google Scholar]
- 65.Duncan JR, Ramsey FK, Switzer WP. Electron microscopy of cytomegalic inclusion disease of swine (inclusion body rhinitis) Am J Vet Res. 1965;26(113):939–947. [PubMed] [Google Scholar]
- 66.L'ecuyer C, Corner AH. Propagation of porcine cytomegalic inclusion disease virus in cell cultures. Preliminary report. Can J Comp Med Vet Sci. 1966;30(12):321–326. [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller NJ, Kuwaki K, Knosalla C, et al. Early weaning of piglets fails to exclude porcine lymphotropic herpesvirus. Xenotransplantation. 2005;12(1):59–62. doi: 10.1111/j.1399-3089.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 68.Clark DA, Fryer JF, Tucker AW, et al. Porcine cytomegalovirus in pigs being bred for xenograft organs: progress towards control. Xenotransplantation. 2003;10(2):142–148. doi: 10.1034/j.1399-3089.2003.01128.x. [DOI] [PubMed] [Google Scholar]
- 69.Edington N, Wrathall AE, Done JT. Porcine cytomegalovirus (PCMV) in early gestation. Vet Microbiol. 1988;17(2):117–128. doi: 10.1016/0378-1135(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 70.Edington N, Watt RG, Plowright W. Experimental transplacental transmission of porcine cytomegalovirus. J Hyg (Lond) 1977;78(2):243–251. doi: 10.1017/s0022172400056138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edington N, Broad S, Wrathall AE, Done JT. Superinfection with porcine cytomegalovirus initiating transplacental infection. Vet Microbiol. 1988;16(2):189–193. doi: 10.1016/0378-1135(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. American journal of obstetrics and gynecology. 2010;202(3):297, e291–298. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(4):522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *74.Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. The Journal of Infectious Diseases. 2010;201(3):386–389. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Important paper regarding the phenomena of re-infection in women of childbearing age, an increasingly recognized risk leading to congenital CMV infections
- 75.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344(18):1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 76.Sekiguchi M, Shibahara T, Miyazaki A, et al. In situ hybridization and immunohistochemistry for the detection of porcine cytomegalovirus. Journal of virological methods. 2012;179(1):272–275. doi: 10.1016/j.jviromet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Widen BF, Lowings JP, Belak S, Banks M. Development of a PCR system for porcine cytomegalovirus detection and determination of the putative partial sequence of its DNA polymerase gene. Epidemiol Infect. 1999;123(1):177–180. doi: 10.1017/s0950268899002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fryer JF, Griffiths PD, Fishman JA, Emery VC, Clark DA. Quantitation of porcine cytomegalovirus in pig tissues by PCR. Journal of clinical microbiology. 2001;39(3):1155–1156. doi: 10.1128/JCM.39.3.1155-1156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, Zhu L, Shi X, et al. Indirect-blocking ELISA for detecting antibodies against glycoprotein B (gB) of porcine cytomegalovirus (PCMV) Journal of virological methods. 2012;186(1–2):30–35. doi: 10.1016/j.jviromet.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 80.Clark DA, Fryer JF, Emery VC, Griffiths PD. Clinical xenotransplantation. Lancet. 2003;362(9393):1421. doi: 10.1016/S0140-6736(03)14660-0. [DOI] [PubMed] [Google Scholar]
- 81.Fryer JF, Griffiths PD, Emery VC, Clark DA. Susceptibility of porcine cytomegalovirus to antiviral drugs. J Antimicrob Chemother. 2004;53(6):975–980. doi: 10.1093/jac/dkh231. [DOI] [PubMed] [Google Scholar]
- 82.Bruggeman CA, Meijer H, Bosman F, Van Boven CP. Biology of rat cytomegalovirus infection. Intervirology. 1985;24(1):1–9. doi: 10.1159/000149612. [DOI] [PubMed] [Google Scholar]
- 83.Bruggeman CA, Debie WM, Grauls G, Majoor G, Van Boven CP. Infection of laboratory rats with a new cytomegalo-like virus. Archives of virology. 1983;76(3):189–199. doi: 10.1007/BF01311103. [DOI] [PubMed] [Google Scholar]
- 84.Stals FS, Bosman F, Van Boven CP, Bruggeman CA. An animal model for therapeutic intervention studies of CMV infection in the immunocompromised host. Archives of virology. 1990;114(1–2):91–107. doi: 10.1007/BF01311014. [DOI] [PubMed] [Google Scholar]
- 85.Stals FS, Wagenaar SS, Bruggeman CA. Generalized cytomegalovirus (CMV) infection and CMV-induced pneumonitis in the rat: combined effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and specific antibody treatment. Antiviral research. 1994;25(2):147–160. doi: 10.1016/0166-3542(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 86.Stals FS, Wagenaar SS, Kloover JS, Vanagt WY, Bruggeman CA. Combinations of ganciclovir and antibody for experimental CMV infections. Antiviral research. 1996;29(1):61–64. doi: 10.1016/0166-3542(95)00918-3. [DOI] [PubMed] [Google Scholar]
- 87.Span AH, Grauls G, Bosman F, Van Boven CP, Bruggeman CA. Cytomegalovirus infection induces vascular injury in the rat. Atherosclerosis. 1992;93(1–2):41–52. doi: 10.1016/0021-9150(92)90198-p. [DOI] [PubMed] [Google Scholar]
- 88.Lemstrom K, Sihvola R, Bruggeman C, Hayry P, Koskinen P. Cytomegalovirus infection-enhanced cardiac allograft vasculopathy is abolished by DHPG prophylaxis in the rat. Circulation. 1997;95(12):2614–2616. doi: 10.1161/01.cir.95.12.2614. [DOI] [PubMed] [Google Scholar]
- 89.Koskinen PK, Kallio EA, Tikkanen JM, Sihvola RK, Hayry PJ, Lemstrom KB. Cytomegalovirus infection and cardiac allograft vasculopathy. Transpl Infect Dis. 1999;1(2):115–126. doi: 10.1034/j.1399-3062.1999.010205.x. [DOI] [PubMed] [Google Scholar]
- 90.Streblow DN, Van Cleef KW, Kreklywich CN, et al. Rat cytomegalovirus gene expression in cardiac allograft recipients is tissue specific and does not parallel the profiles detected in vitro. Journal of virology. 2007;81(8):3816–3826. doi: 10.1128/JVI.02425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martelius T, Krogerus L, Hockerstedt K, Bruggeman C, Lautenschlager I. Cytomegalovirus infection is associated with increased inflammation and severe bile duct damage in rat liver allografts. Hepatology. 1998;27(4):996–1002. doi: 10.1002/hep.510270415. [DOI] [PubMed] [Google Scholar]
- 92.Koskinen PK, Yilmaz S, Kallio E, Bruggeman CA, Hayry PJ, Lemstrom K. Rat cytomegalovirus infection and chronic kidney allograft rejection. Transpl Int. 1996;9(Suppl 1):S3–4. doi: 10.1007/978-3-662-00818-8_1. [DOI] [PubMed] [Google Scholar]
- 93.Streblow DN, Kreklywich CN, Smith P, et al. Rat cytomegalovirus-accelerated transplant vascular sclerosis is reduced with mutation of the chemokine-receptor R33. Am J Transplant. 2005;5(3):436–442. doi: 10.1111/j.1600-6143.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- 94.Streblow DN, Dumortier J, Moses AV, Orloff SL, Nelson JA. Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Current topics in microbiology and immunology. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *95.Vomaske J, Denton M, Kreklywich C, et al. Cytomegalovirus CC chemokine promotes immune cell migration. Journal of virology. 2012;86(21):11833–11844. doi: 10.1128/JVI.00452-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *96.Murrell I, Tomasec P, Wilkie GS, Dargan DJ, Davison AJ, Stanton RJ. Impact of Sequence Variation in the UL128 Locus on Production of Human Cytomegalovirus in Fibroblast and Epithelial Cells. Journal of virology. 2013 doi: 10.1128/JVI.01546-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *97.Akter P, Cunningham C, Mcsharry BP, et al. Two novel spliced genes in human cytomegalovirus. The Journal of general virology. 2003;84(Pt 5):1117–1122. doi: 10.1099/vir.0.18952-0. [DOI] [PubMed] [Google Scholar]; * References 95–97 are a series of interesting papers describing the biology of HCMV UL128, a CC chemokine homolog and constituent of the pentameric complex of HCMV proteins involved in endothelial/epithelial cell entry
- 98.Loh HS, Mohd-Azmi ML, Lai KY, Sheikh-Omar AR, Zamri-Saad M. Characterization of a novel rat cytomegalovirus (RCMV) infecting placenta-uterus of Rattus rattus diardii. Archives of virology. 2003;148(12):2353–2367. doi: 10.1007/s00705-003-0173-y. [DOI] [PubMed] [Google Scholar]
- 99.Vink C, Beuken E, Bruggeman CA. Complete DNA sequence of the rat cytomegalovirus genome. Journal of virology. 2000;74(16):7656–7665. doi: 10.1128/jvi.74.16.7656-7665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ettinger J, Geyer H, Nitsche A, et al. Complete genome sequence of the english isolate of rat cytomegalovirus (Murid herpesvirus 8) Journal of virology. 2012;86(24):13838. doi: 10.1128/JVI.02614-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loh HS, Mohd-Lila MA, Abdul-Rahman SO, Kiew LJ. Pathogenesis and vertical transmission of a transplacental rat cytomegalovirus. Virology journal. 2006;3:42. doi: 10.1186/1743-422X-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Camalxaman SN, Zeenathul NA, Quah YW, et al. Establishment of rat brain endothelial cells susceptible to rat cytomegalovirus ALL-03 infection. In Vitro Cell Dev Biol Anim. 2013;49(3):238–244. doi: 10.1007/s11626-012-9553-5. [DOI] [PubMed] [Google Scholar]
- 103.Loh HS, Mohd-Azmi ML. Development of a quantitative real-time RT-PCR for kinetic analysis of immediate-early transcripts of rat cytomegalovirus. Acta virologica. 2009;53(4):261–269. doi: 10.4149/av_2009_04_261. [DOI] [PubMed] [Google Scholar]
- 104.Chen J, Feng Y, Chen L, et al. Long-term impact of intrauterine MCMV infection on development of offspring nervous system. Journal of Huazhong University of Science and Technology. 2011;31(3):371–375. doi: 10.1007/s11596-011-0383-6. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban. [DOI] [PubMed] [Google Scholar]
- 105.Bruning JH, Debie WH, Dormans PH, Meijer H, Bruggeman CA. The development and characterization of monoclonal antibodies against rat cytomegalovirus induced antigens. Archives of virology. 1987;94(1–2):55–70. doi: 10.1007/BF01313725. [DOI] [PubMed] [Google Scholar]
- 106.Reddehase MJ, Podlech J, Grzimek NK. Mouse models of cytomegalovirus latency: overview. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2002;25(Suppl 2):S23–36. doi: 10.1016/s1386-6532(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 107.Reddehase MJ, Simon CO, Seckert CK, Lemmermann N, Grzimek NK. Murine model of cytomegalovirus latency and reactivation. Current topics in microbiology and immunology. 2008;325:315–331. doi: 10.1007/978-3-540-77349-8_18. [DOI] [PubMed] [Google Scholar]
- 108.Kavanagh DG, Hill AB. Evasion of cytotoxic T lymphocytes by murine cytomegalovirus. Semin Immunol. 2001;13(1):19–26. doi: 10.1006/smim.2001.0292. [DOI] [PubMed] [Google Scholar]
- 109.Lemmermann NA, Bohm V, Holtappels R, Reddehase MJ. In vivo impact of cytomegalovirus evasion of CD8 T-cell immunity: facts and thoughts based on murine models. Virus research. 2011;157(2):161–174. doi: 10.1016/j.virusres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 110.Doom CM, Hill AB. MHC class I immune evasion in MCMV infection. Medical microbiology and immunology. 2008;197(2):191–204. doi: 10.1007/s00430-008-0089-y. [DOI] [PubMed] [Google Scholar]
- *111.Munks MW, Gold MC, Zajac AL, et al. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. Journal of immunology. 2006;176(6):3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]; *Excellent paper demonstrating the diversity of the T cell response to MCMV; important implications for vaccine studies in this model
- *112.Reddehase MJ. The immunogenicity of human and murine cytomegaloviruses. Curr Opin Immunol. 2000;12(4):390–396. doi: 10.1016/s0952-7915(00)00106-0. [DOI] [PubMed] [Google Scholar]; * Excellent overview of the comparative immune responses to HCMV and MCMV in their respective hosts
- 113.Shanley JD, Wu CA. Mucosal immunization with a replication-deficient adenovirus vector expressing murine cytomegalovirus glycoprotein B induces mucosal and systemic immunity. Vaccine. 2003;21(19–20):2632–2642. doi: 10.1016/s0264-410x(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 114.Shanley JD, Wu CA. Intranasal immunization with a replication-deficient adenovirus vector expressing glycoprotein H of murine cytomegalovirus induces mucosal and systemic immunity. Vaccine. 2005;23(8):996–1003. doi: 10.1016/j.vaccine.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 115.Morello CS, Kelley LA, Munks MW, Hill AB, Spector DH. DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. Journal of virology. 2007;81(14):7766–7775. doi: 10.1128/JVI.00633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morello CS, Ye M, Hung S, Kelley LA, Spector DH. Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge. Journal of virology. 2005;79(1):159–175. doi: 10.1128/JVI.79.1.159-175.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ye M, Morello CS, Spector DH. Multiple epitopes in the murine cytomegalovirus early gene product M84 are efficiently presented in infected primary macrophages and contribute to strong CD8+-T-lymphocyte responses and protection following DNA immunization. Journal of virology. 2004;78(20):11233–11245. doi: 10.1128/JVI.78.20.11233-11245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morello CS, Ye M, Spector DH. Development of a vaccine against murine cytomegalovirus (MCMV), consisting of plasmid DNA and formalin-inactivated MCMV, that provides long-term, complete protection against viral replication. Journal of virology. 2002;76(10):4822–4835. doi: 10.1128/JVI.76.10.4822-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ye M, Morello CS, Spector DH. Strong CD8 T-cell responses following coimmunization with plasmids expressing the dominant pp89 and subdominant M84 antigens of murine cytomegalovirus correlate with long-term protection against subsequent viral challenge. Journal of virology. 2002;76(5):2100–2112. doi: 10.1128/jvi.76.5.2100-2112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morello CS, Cranmer LD, Spector DH. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65) Journal of virology. 2000;74(8):3696–3708. doi: 10.1128/jvi.74.8.3696-3708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H, Yao Y, Huang C, Chen Q, Chen J, Chen Z. Immunization with cytomegalovirus envelope glycoprotein M and glycoprotein N DNA vaccines can provide mice with complete protection against a lethal murine cytomegalovirus challenge. Virol Sin. 2013;28(3):174–182. doi: 10.1007/s12250-013-3330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lyons PA, Allan JE, Carrello C, Shellam GR, Scalzo AA. Effect of natural sequence variation at the H-2Ld-restricted CD8+ T cell epitope of the murine cytomegalovirus ie1-encoded pp89 on T cell recognition. The Journal of general virology. 1996;77(Pt 10):2615–2623. doi: 10.1099/0022-1317-77-10-2615. [DOI] [PubMed] [Google Scholar]
- 123.Gopal IN, Quinn A, Henry SC, Hamilton JD, Staats HF, Frothingham R. Nasal peptide vaccination elicits CD8 responses and reduces viral burden after challenge with virulent murine cytomegalovirus. Microbiol Immunol. 2005;49(2):113–119. doi: 10.1111/j.1348-0421.2005.tb03710.x. [DOI] [PubMed] [Google Scholar]
- 124.Sweet C, Ball K, Morley PJ, Guilfoyle K, Kirby M. Mutations in the temperature-sensitive murine cytomegalovirus (MCMV) mutants tsm5 and tsm30: a study of genes involved in immune evasion, DNA packaging and processing, and DNA replication. Journal of medical virology. 2007;79(3):285–299. doi: 10.1002/jmv.20797. [DOI] [PubMed] [Google Scholar]
- 125.Morley PJ, Ertl P, Sweet C. Immunisation of Balb/c mice with severely attenuated murine cytomegalovirus mutants induces protective cellular and humoral immunity. Journal of medical virology. 2002;67(2):187–199. doi: 10.1002/jmv.2207. [DOI] [PubMed] [Google Scholar]
- 126.Gill TA, Morley PJ, Sweet C. Replication-defective mutants of mouse cytomegalovirus protect against wild-type virus challenge. Journal of medical virology. 2000;62(2):127–139. doi: 10.1002/1096-9071(200010)62:2<127::aid-jmv2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 127.Beswick M, Pachnio A, Al-Ali A, Sweet C, Moss PA. An attenuated temperature-sensitive strain of cytomegalovirus (tsm5) establishes immunity without development of CD8 T cell memory inflation. Journal of medical virology. 2013 doi: 10.1002/jmv.23688. [DOI] [PubMed] [Google Scholar]
- 128.Al-Ali A, Timoshenko O, Martin BA, Sweet C. Role of mutations identified in ORFs M27, M36, m139, m141, and m143 in the temperature-sensitive phenotype of murine cytomegalovirus mutant tsm5. Journal of medical virology. 2012;84(6):912–922. doi: 10.1002/jmv.23273. [DOI] [PubMed] [Google Scholar]
- 129.Macdonald MR, Li XY, Stenberg RM, Campbell AE, Virgin HWT. Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by using an attenuated MCMV mutant. Journal of virology. 1998;72(1):442–451. doi: 10.1128/jvi.72.1.442-451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **130.Cicin-Sain L, Bubic I, Schnee M, et al. Targeted deletion of regions rich in immune-evasive genes from the cytomegalovirus genome as a novel vaccine strategy. Journal of virology. 2007;81(24):13825–13834. doi: 10.1128/JVI.01911-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The advent of BAC technology has allowed the rationale design of safer and more immunogenic live, attenuated CMV vaccines. Outstanding overview of the potential of this system.
- 131.Mohr CA, Cicin-Sain L, Wagner M, et al. Engineering of cytomegalovirus genomes for recombinant live herpesvirus vaccines. Int J Med Microbiol. 2008;298(1–2):115–125. doi: 10.1016/j.ijmm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 132.Mohr CA, Arapovic J, Muhlbach H, et al. A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. Journal of virology. 2010;84(15):7730–7742. doi: 10.1128/JVI.02696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Slavuljica I, Busche A, Babic M, et al. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest. 2010;120(12):4532–4545. doi: 10.1172/JCI43961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Medearis DN., Jr. Mouse Cytomegalovirus Infection. 3. Attempts to Produce Intrauterine Infections. Am J Hyg. 1964;80:113–120. [PubMed] [Google Scholar]
- 135.Johnson KP. Mouse cytomegalovirus: placental infection. The Journal of infectious diseases. 1969;120(4):445–450. doi: 10.1093/infdis/120.4.445. [DOI] [PubMed] [Google Scholar]
- *136.Woolf NK, Jaquish DV, Koehrn FJ. Transplacental murine cytomegalovirus infection in the brain of SCID mice. Virology journal. 2007;4:26. doi: 10.1186/1743-422X-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Paper challenges dogma regarding the potential for transplacental transfer of CMV in the murine model
- 137.Tsutsui Y. Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol Int. 1995;45(2):91–102. doi: 10.1111/j.1440-1827.1995.tb03428.x. [DOI] [PubMed] [Google Scholar]
- 138.Li RY, Tsutsui Y. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology. 2000;62(2):79–85. doi: 10.1002/1096-9926(200008)62:2<79::AID-TERA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 139.Shinmura Y, Kosugi I, Kaneta M, Tsutsui Y. Migration of virus-infected neuronal cells in cerebral slice cultures of developing mouse brains after in vitro infection with murine cytomegalovirus. Acta neuropathologica. 1999;98(6):590–596. doi: 10.1007/s004010051123. [DOI] [PubMed] [Google Scholar]
- 140.Tsutsui Y. Effects of cytomegalovirus infection on embryogenesis and brain development. Congenit Anom (Kyoto) 2009;49(2):47–55. doi: 10.1111/j.1741-4520.2009.00222.x. [DOI] [PubMed] [Google Scholar]
- 141.Tsutsui Y, Kosugi I, Kawasaki H. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol. 2005;15(5):327–345. doi: 10.1002/rmv.475. [DOI] [PubMed] [Google Scholar]
- 142.Tsutsui Y, Kashiwai A, Kawamura N, Kadota C. Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. The American journal of pathology. 1993;143(3):804–813. [PMC free article] [PubMed] [Google Scholar]
- **143.Cekinovic D, Golemac M, Pugel EP, et al. Passive immunization reduces murine cytomegalovirus-induced brain pathology in newborn mice. Journal of virology. 2008;82(24):12172–12180. doi: 10.1128/JVI.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Elegant experimental model for neuropathogenesis in newborn mice caused by MCMV infection; potentially important implications for therapy of infants with congenital HCMV infection
- 144.Schachtele SJ, Mutnal MB, Schleiss MR, Lokensgard JR. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol. 2011;17(3):201–211. doi: 10.1007/s13365-011-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melnick M, Jaskoll T. An in vitro mouse model of congenital cytomegalovirus-induced pathogenesis of the inner ear cochlea. Birth Defects Res A Clin Mol Teratol. 2013;97(2):69–78. doi: 10.1002/bdra.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ostermann E, Macquin C, Bahram S, Georgel P. Use of In vivo Imaging to Monitor the Progression of Experimental Mouse Cytomegalovirus Infection in Neonates. J Vis Exp. 2013;(77) doi: 10.3791/50409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **147.Bia FJ, Griffith BP, Fong CK, Hsiung GD. Cytomegaloviral infections in the guinea pig: experimental models for human disease. Rev Infect Dis. 1983;5(2):177–195. doi: 10.1093/clinids/5.2.177. [DOI] [PubMed] [Google Scholar]; ** Older review but nonetheless a landmark paper chronically the evolution of the GPCMV model for study of congenital CMV infection
- 148.Griffith BP, Mccormick SR, Fong CK, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. Journal of virology. 1985;55(2):402–409. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mess A. The Guinea pig placenta: model of placental growth dynamics. Placenta. 2007;28(8–9):812–815. doi: 10.1016/j.placenta.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 150.Mess A, Zaki N, Kadyrov M, Korr H, Kaufmann P. Caviomorph placentation as a model for trophoblast invasion. Placenta. 2007;28(11–12):1234–1238. doi: 10.1016/j.placenta.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 151.Markham FS, Hudson NP. Susceptibility of the Guinea Pig Fetus to the Submaxillary Gland Virus of Guinea Pigs. The American journal of pathology. 1936;12(2):175–182. 171. [PMC free article] [PubMed] [Google Scholar]
- 152.Hsiung GD, Choi YC, Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. The Journal of infectious diseases. 1978;138(2):191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
- 153.Kumar ML, Nankervis GA. Experimental congenital infection with cytomegalovirus: a guinea pig model. J Infect Dis. 1978;138(5):650–654. doi: 10.1093/infdis/138.5.650. [DOI] [PubMed] [Google Scholar]
- 154.Johnson KP, Connor WS. Guinea pig cytomegalovirus: transplacental transmission. Brief report. Arch Virol. 1979;59(3):263–267. doi: 10.1007/BF01317422. [DOI] [PubMed] [Google Scholar]
- 155.Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 2006;47(1):65–72. doi: 10.1093/ilar.47.1.65. [DOI] [PubMed] [Google Scholar]
- *156.Griffith BP, Lucia HL, Hsiung GD. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr Res. 1982;16(6):455–459. doi: 10.1203/00006450-198206000-00010. [DOI] [PubMed] [Google Scholar]; * Study that demonstrates relevance of congenital GPCMV model to human health; neuropathogenesis with brain involvement
- 157.Woolf NK, Koehrn FJ, Harris JP, Richman DD. Congenital cytomegalovirus labyrinthitis and sensorineural hearing loss in guinea pigs. J Infect Dis. 1989;160(6):929–937. doi: 10.1093/infdis/160.6.929. [DOI] [PubMed] [Google Scholar]
- 158.Katano H, Sato Y, Tsutsui Y, et al. Pathogenesis of cytomegalovirus-associated labyrinthitis in a guinea pig model. Microbes Infect. 2007;9(2):183–191. doi: 10.1016/j.micinf.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 159.Park AH, Mann D, Error ME, et al. Comparative analysis of detection methods for congenital cytomegalovirus infection in a Guinea pig model. JAMA Otolaryngol Head Neck Surg. 2013;139(1):82–86. doi: 10.1001/jamaoto.2013.1090. [DOI] [PubMed] [Google Scholar]
- 160.Park AH, Gifford T, Schleiss MR, et al. Development of cytomegalovirus-mediated sensorineural hearing loss in a Guinea pig model. Arch Otolaryngol Head Neck Surg. 2010;136(1):48–53. doi: 10.1001/archoto.2009.210. [DOI] [PubMed] [Google Scholar]
- 161.Bia FJ, Griffith BP, Tarsio M, Hsiung GD. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis. 1980;142(5):732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- 162.Nozawa N, Yamamoto Y, Fukui Y, et al. Identification of a 1.6 kb genome locus of guinea pig cytomegalovirus required for efficient viral growth in animals but not in cell culture. Virology. 2008;379(1):45–54. doi: 10.1016/j.virol.2008.06.018. [DOI] [PubMed] [Google Scholar]
- *163.Yamada S, Nozawa N, Katano H, et al. Characterization of the guinea pig cytomegalovirus genome locus that encodes homologs of human cytomegalovirus major immediate-early genes, UL128, and UL130. Virology. 2009;391(1):99–106. doi: 10.1016/j.virol.2009.05.034. [DOI] [PubMed] [Google Scholar]; * Novel finding by Inoue laboratory of a previously undiscovered locus in GPCMV genome associated with in vivo pathogenesis and encoding homologs of HCMV UL128-131 proteins
- 164.Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. Journal of virology. 2012;86(13):7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *165.Auerbach M, Yan D, Fouts A, et al. Characterization of the guinea pig CMV gH/gL/GP129/GP131/GP133 complex in infection and spread. Virology. 2013;441(1):75–84. doi: 10.1016/j.virol.2013.03.008. [DOI] [PubMed] [Google Scholar]; *Important paper from Feierbach group identifying GPCMV pentameric complex; implications for preclinical modeling of vaccine studies based on the fact that HCMV homologs of these proteins are known to be critical for epithelial and endothelial cell entry/infection
- 166.Bia FJ, Lucia HL, Fong CK, Tarsio M, Hsiung GD. Effects of vaccination on cytomegalovirus-associated interstitial pneumonia in strain 2 guinea pigs. J Infect Dis. 1982;145(5):742–747. doi: 10.1093/infdis/145.2.742. [DOI] [PubMed] [Google Scholar]
- 167.Bia FJ, Miller SA, Lucia HL, Griffith BP, Tarsio M, Hsiung GD. Vaccination against transplacental cytomegalovirus transmission: vaccine reactivation and efficacy in guinea pigs. J Infect Dis. 1984;149(3):355–362. doi: 10.1093/infdis/149.3.355. [DOI] [PubMed] [Google Scholar]
- 168.Crumpler MM, Choi KY, Mcvoy MA, Schleiss MR. A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine. 2009;27(31):4209–4218. doi: 10.1016/j.vaccine.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Britt WJ, Harrison C. Identification of an abundant disulfide-linked complex of glycoproteins in the envelope of guinea pig cytomegalovirus. Virology. 1994;201(2):294–302. doi: 10.1006/viro.1994.1294. [DOI] [PubMed] [Google Scholar]
- 170.Harrison CJ, Britt WJ, Chapman NM, Mullican J, Tracy S. Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J Infect Dis. 1995;172(5):1212–1220. doi: 10.1093/infdis/172.5.1212. [DOI] [PubMed] [Google Scholar]
- 171.Bourne N, Schleiss MR, Bravo FJ, Bernstein DI. Preconception immunization with a cytomegalovirus (CMV) glycoprotein vaccine improves pregnancy outcome in a guinea pig model of congenital CMV infection. J Infect Dis. 2001;183(1):59–64. doi: 10.1086/317654. [DOI] [PubMed] [Google Scholar]
- 172.Schleiss MR. Cloning and characterization of the guinea pig cytomegalovirus glycoprotein B gene. Virology. 1994;202(1):173–185. doi: 10.1006/viro.1994.1333. [DOI] [PubMed] [Google Scholar]
- 173.Schleiss MR, Mcgregor A, Jensen NJ, Erdem G, Aktan L. Molecular characterization of the guinea pig cytomegalovirus UL83 (pp65) protein homolog. Virus Genes. 1999;19(3):205–221. doi: 10.1023/a:1008136714136. [DOI] [PubMed] [Google Scholar]
- 174.Schleiss MR, Bourne N, Jensen NJ, Bravo F, Bernstein DI. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Viral Immunol. 2000;13(2):155–167. doi: 10.1089/vim.2000.13.155. [DOI] [PubMed] [Google Scholar]
- 175.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188(12):1868–1874. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 176.Schleiss MR, Stroup G, Pogorzelski K, Mcgregor A. Protection against congenital cytomegalovirus (CMV) disease, conferred by a replication-disabled, bacterial artificial chromosome (BAC)-based DNA vaccine. Vaccine. 2006;24(37–39):6175–6186. doi: 10.1016/j.vaccine.2006.06.077. [DOI] [PubMed] [Google Scholar]
- 177.Schleiss MR, Jensen NJ. Cloning and expression of the guinea pig cytomegalovirus glycoprotein B (gB) in a recombinant baculovirus: utility for vaccine studies for the prevention of experimental infection. J Virol Methods. 2003;108(1):59–65. doi: 10.1016/s0166-0934(02)00258-6. [DOI] [PubMed] [Google Scholar]
- 178.Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis. 2004;189(8):1374–1381. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]
- 179.Schleiss MR, Choi KY, Anderson J, et al. Glycoprotein B (gB) vaccines adjuvanted with AS01 or AS02 protect female guinea pigs against cytomegalovirus (CMV) viremia and offspring mortality in a CMV-challenge model. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schleiss MR, Lacayo JC, Belkaid Y, et al. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2007;195(6):789–798. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- *181.Hashimoto K, Yamada S, Katano H, et al. Effects of immunization of pregnant guinea pigs with guinea pig cytomegalovirus glycoprotein B on viral spread in the placenta. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.078. [DOI] [PubMed] [Google Scholar]; * Interesting paper elucidating mechanism of how gB vaccine protects at level of placenta/virus interface in the GPCMV model
- 182.Oxford KL, Eberhardt MK, Yang KW, et al. Protein coding content of the UL)b' region of wild-type rhesus cytomegalovirus. Virology. 2008;373(1):181–188. doi: 10.1016/j.virol.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oxford KL, Strelow L, Yue Y, et al. Open reading frames carried on UL/b' are implicated in shedding and horizontal transmission of rhesus cytomegalovirus in rhesus monkeys. Journal of virology. 2011;85(10):5105–5114. doi: 10.1128/JVI.02631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rivailler P, Kaur A, Johnson RP, Wang F. Genomic sequence of rhesus cytomegalovirus 180.92: insights into the coding potential of rhesus cytomegalovirus. Journal of virology. 2006;80(8):4179–4182. doi: 10.1128/JVI.80.8.4179-4182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Malouli D, Nakayasu ES, Viswanathan K, et al. Reevaluation of the coding potential and proteomic analysis of the BAC-derived rhesus cytomegalovirus strain 68–1. Journal of virology. 2012;86(17):8959–8973. doi: 10.1128/JVI.01132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hansen SG, Strelow LI, Franchi DC, Anders DG, Wong SW. Complete sequence and genomic analysis of rhesus cytomegalovirus. Journal of virology. 2003;77(12):6620–6636. doi: 10.1128/JVI.77.12.6620-6636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yue Y, Barry PA. Rhesus cytomegalovirus a nonhuman primate model for the study of human cytomegalovirus. Adv Virus Res. 2008;72:207–226. doi: 10.1016/S0065-3527(08)00405-3. [DOI] [PubMed] [Google Scholar]
- 188.Barry PA, Lockridge KM, Salamat S, et al. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2006;47(1):49–64. doi: 10.1093/ilar.47.1.49. [DOI] [PubMed] [Google Scholar]
- 189.Powers C, Fruh K. Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol. 2008;197(2):109–115. doi: 10.1007/s00430-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **190.Tarantal AF, Salamat MS, Britt WJ, Luciw PA, Hendrickx AG, Barry PA. Neuropathogenesis induced by rhesus cytomegalovirus in fetal rhesus monkeys (Macaca mulatta) The Journal of infectious diseases. 1998;177(2):446–450. doi: 10.1086/514206. [DOI] [PubMed] [Google Scholar]; ** Highly relevant to human health; convincing case for why rhesus CMV is the most relevant animal model for congenital CMV infection in light of similarities to neuropathogenesis in infants induced in RhCMV model
- 191.Dela Pena MG, Strelow L, Barry PA, Abel K. Use of specific-pathogen-free (SPF) rhesus macaques to better model oral pediatric cytomegalovirus infection. J Med Primatol. 2012;41(3):225–229. doi: 10.1111/j.1600-0684.2012.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abel K, Strelow L, Yue Y, Eberhardt MK, Schmidt KA, Barry PA. A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus. Vaccine. 2008;26(47):6013–6025. doi: 10.1016/j.vaccine.2008.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yue Y, Kaur A, Eberhardt MK, et al. Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65–2, and viral interleukin-10 in rhesus macaques. Journal of virology. 2007;81(3):1095–1109. doi: 10.1128/JVI.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abel K, Martinez J, Yue Y, et al. Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques. Journal of virology. 2011;85(6):2878–2890. doi: 10.1128/JVI.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yue Y, Wang Z, Abel K, et al. Evaluation of recombinant modified vaccinia Ankara virus-based rhesus cytomegalovirus vaccines in rhesus macaques. Medical microbiology and immunology. 2008;197(2):117–123. doi: 10.1007/s00430-008-0074-5. [DOI] [PubMed] [Google Scholar]
- *196.Wussow F, Yue Y, Martinez J, et al. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. Journal of virology. 2013;87(3):1322–1332. doi: 10.1128/JVI.01669-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First paper demonstrating potential superiority of a pentameric complex based vaccine for control of CMV infection
- 197.Marsh AK, Willer DO, Ambagala AP, et al. Genomic sequencing and characterization of cynomolgus macaque cytomegalovirus. Journal of virology. 2011;85(24):12995–13009. doi: 10.1128/JVI.05840-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **198.Hansen SG, Sacha JB, Hughes CM, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340(6135):1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** An unexpected function of the UL128-131 locus is to divert CD8+ T cell targeting away from “unconventional” epitopes; this discovery creates the potential for generating more diverse T cell response to novel generations of CMV vaccines
- 199.Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. Journal of virology. 2002;76(3):1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *200.Eberhardt MK, Deshpande A, Chang WL, Barthold SW, Walter MR, Barry PA. Vaccination against a Virus-Encoded Cytokine Significantly Restricts Viral Challenge. Journal of virology. 2013;87(21):11323–11331. doi: 10.1128/JVI.01925-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Intriguing paper with novel approach of vaccination targeting a CMV-encoded immunoregulatory cytokine as a strategy for limiting CMV disease