Abstract
BACKGROUND & AIMS
Pancreatic mucinous cystic neoplasm (MCN), a cystic tumor of the pancreas that develops most frequently in women, is a potential precursor to pancreatic ductal adenocarcinoma. MCNs develop primarily in the body and tail of the pancreas and are characterized by the presence of a mucinous epithelium and ovarian-like subepithelial stroma. We investigated the involvement of Wnt signaling in KRAS-mediated pancreatic tumorigenesis and development of MCN in mice, and Wnt activation in human MCN samples.
METHODS
LSL-KrasG12D, Ptf1a-cre mice were crossed with elastase-tva mice to allow for introduction of genes encoded by the replication-competent avian sarcoma-leukosis virus long-terminal repeat with splice acceptor viruses to pancreatic acinar cells and acinar cell progenitors, postnatally and sporadically. Repeat with splice acceptor viruses that expressed Wnt1 were delivered to the pancreatic epithelium of these mice; pancreatic lesions were analyzed by histopathology and immunohistochemical analyses. We analyzed levels of factors in Wnt signaling pathways in 19 MCN samples from patients.
RESULTS
Expression of Wnt1 in the pancreatic acinar cells and acinar cell progenitors of female mice led to development of unilocular or multilocular epithelial cysts in the pancreas body and tail, similar to MCN. The cystic lesions resembled the estrogen receptor– and progesterone receptor–positive ovarian-like stroma of MCN, but lacked the typical mucinous epithelium. Activated Wnt signaling, based on nuclear localization of β-catenin, was detected in the stroma but not cyst epithelium. Wnt signaling to β-catenin was found to be activated in MCN samples from patients, within the ovarian-like stroma, consistent with the findings in mice.
CONCLUSIONS
Based on studies of mice and pancreatic MCN samples from patients, the canonical Wnt signaling pathway becomes activated and promotes development of the ovarian-like stroma to contribute to formation of MCNs.
Keywords: Wnt Signaling Pathway, Mucinous Cystic Neoplasm (MCN), Ovarian-Like Stroma, Mouse Model
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related mortality in the United States.1 PDAC commonly arises through the progression of precursor lesions known as pancreatic intraepithelial neoplasias (PanINs).2,3 Genomic analysis of normal pancreatic tissue, PDAC samples, and PanIN lesions has demonstrated the presence of activating KRAS mutations in >90% of PanINs and PDAC.2,4,5
Cystic neoplasms of the pancreas, in particular intraductal papillary mucinous neoplasm and mucinous cystic neoplasm (MCN), represent precursors for PDAC as well.6,7 MCN is characterized by a profound female-sex bias, and the presence of an estrogen receptor (ER)– and progesterone receptor (PR)–positive ovarian-like subepithelial stroma is the pathologic hallmark of this tumor type.6,8 As the name suggests, the epithelium lining the cysts is typically mucinous, but some regions can lack intracellular mucin, and the epithelium resembles that of the normal pancreatic ducts. Recent work has reported the genetic alterations commonly identified in this tumor, including mutations in KRAS.7,9 Yet, other than KRAS-induced pathways, the key molecular signaling pathways that underlie key features of this tumor remain unknown. In particular, the signaling cascades that drive the formation of the stereotypical ovarian-like stroma remain unknown. Given the prominence of the ovarian-like stroma in MCN, it likely plays an important role in the pathology of this tumor. Therefore, identification of the signaling pathways that regulate the MCN-associated stroma is important for understanding this tumor type.
The Wnt signaling pathway regulates multiple developmental processes in vertebrates and invertebrates.10,11 In mammals, there are 19 known Wnt ligands that bind and activate a family of 10 frizzled (FZD) receptors. Engagement of Fzd receptors by Wnt ligands results in the activation of downstream signaling cascades. Typically, this includes the canonicalWnt/β-catenin signaling axis. In addition, some Wnt ligands stimulate signaling through the planar cell polarity (PCP) and Wnt/Ca2+ signaling pathways as well.11 Wnt signaling plays an important role during pancreatic development and dysregulation of Wnt signaling—through either aberrant activation or pathway blockade—disrupts normal pancreatic development.12,13 These findings suggest that activated Wnt signaling might play a role in pancreatic tumorigenesis.
Consistent with a role in pancreatic tumorigenesis, mutational activation of the Wnt/β-catenin signaling axis is observed in rare pancreatic cancer types, such as a solid pseudopapillary neoplasm, pancreatoblastoma, and acinar cell carcinomas.14–18 Heiser and colleagues demonstrated that stabilization of β-catenin resulted in the formation of tumors resembling human solid pseudopapillary neoplasm in mice.19 However, activation of the pathway by mutation is not observed in PDAC. Nonetheless, cytoplasmic and nuclear accumulation of β-catenin, indicative of pathway activation, is commonly observed.20 In addition, recent work suggested that high expression of ataxia-telangiectasia group D complementing gene might underlie the activated β-catenin signaling activity observed in PDAC, at least in some instances.21 However, these studies focused primarily on the activation of β-catenin and not the PCP and Ca2+ signaling cascades, yet earlier work suggested a role for Wnt5a, a Wnt ligand that specifically activates the noncanonical signaling pathways, in pancreatic cancer cell migration and invasion.22,23 In addition, these earlier studies did not explore potential interactions between the tumor epithelium and the reactive stroma in response to Wnt signaling.
Therefore, we explored the consequences of engineered postnatal and sporadic activation of Wnt1-induced signaling in a KRAS-driven mouse model of pancreatic tumorigenesis. We find that concomitant expression of Wnt1 and KRASG12D within the pancreas epithelium stimulates development of MCN-like lesions in female mice. These lesions display specific activation of downstream Wnt signaling cascades within the ovarian-like stroma, but not the cyst epithelium. Finally, we find that human MCN lesions also display specific activation of Wnt-induced signaling within the stroma. These findings suggest that activation of Wnt signaling within the stroma contributes to development of mucinous cystic neoplasms of the pancreas.
Materials and Methods
Genetically Engineered Mice and Animal Care
The elastase-tva, LSL-KrasG12D, and Ptf1a-cre mouse strains have been described previously.24–26 All animals were kept in specific pathogen-free housing with abundant food and water under guidelines approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee. DF1 chicken fibroblasts (2 × 107 cells) transfected with replication-competent avian sarcoma-leukosis virus longterminal repeat with splice acceptor (RCAS)-Wnt1, RCAS-GFP, or RCAS-β-cateninS37A vectors and producing high titer virus (>1 × 106 infectious units/mL), were delivered via intraperitoneal injection into 3-day-old pups.
Immunohistochemistry
Mouse tissue samples were fixed and processed as described previously.25 Immunostaining was performed as described25 with primary antibody incubation performed overnight at 4°C. The appropriate horseradish peroxidase –conjugated secondary antibodies (1:100) were incubated at room temperature for 1 hour. For human tissues, ENVISION Plus was used for secondary antibody (Dako Co., Carpinteria, CA). Substrate incubation and color development were performed according to manufacturer’s instructions (Nova Red, Vector Labs, Burlingame, CA; N-Histofine DAB-3S kit; Nichirei Bioscience Inc, Tokyo, Japan). Slides were counterstained with hematoxylin. The primary antibodies used and their concentrations are summarized in the Supplementary Methods.
DNA Extraction and Polymerase Chain Reaction Amplification
Five tissue sections (5 µm) were deparaffinized in Xylene and rinsed in ethanol. Tissues were suspended with 400 µL DNA lysis buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 100 mM NaCl, 1% sodium dodecyl sulfate, and 1 mg/mL proteinase K) and incubated overnight at 50°C. After phenol and chloroform extraction, total DNA was precipitated in ethanol with centrifugation at 12,500g for 15 minutes at 4°C, and resuspended in diethylpyrocarbonate-treated water. Polymerase chain reaction amplification was performed in reaction buffer containing 10 mM Tris- HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 250 µM deoxynucleoside triphosphate, 100 nM primers, 4% (v/v) dimethyl sulfoxide, 0.1 unit Taq DNA polymerase (Applied Biosystems, Carlsbad, CA), and 1 µg extracted total DNA template. An initial denaturation at 95°C for 5 minutes was followed by 40 cycles consisting of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 45 seconds. In the second PCR step, 2 µL of the first amplification product was added to a new reaction mixture and reamplified for 40 cycles as described here.
Results
Postnatal Introduction of Wnt1 Stimulates Development of MCN-Like Lesions in Mice
Jackson and colleagues previously generated an activated Kras allele, KrasG12D, knocked into the endogenous Kras gene locus.27 This allele is preceded by a lox-stop-lox cassette, such that tissue-specific expression of this allele is generated by combination with appropriate cre transgenic lines.24,27,28 This allele has been utilized previously to generate models of pancreatic tumorigenesis.24,29,30 We have previously described a different pancreatic cancer mouse model generated through the tissue-specific expression of the avian leukosis virus subgroup A receptor under the control of the elastase promoter, and the subsequent pancreas-specific delivery of avian leukosis virus–based RCAS viruses encoding oncogenes of interest.25
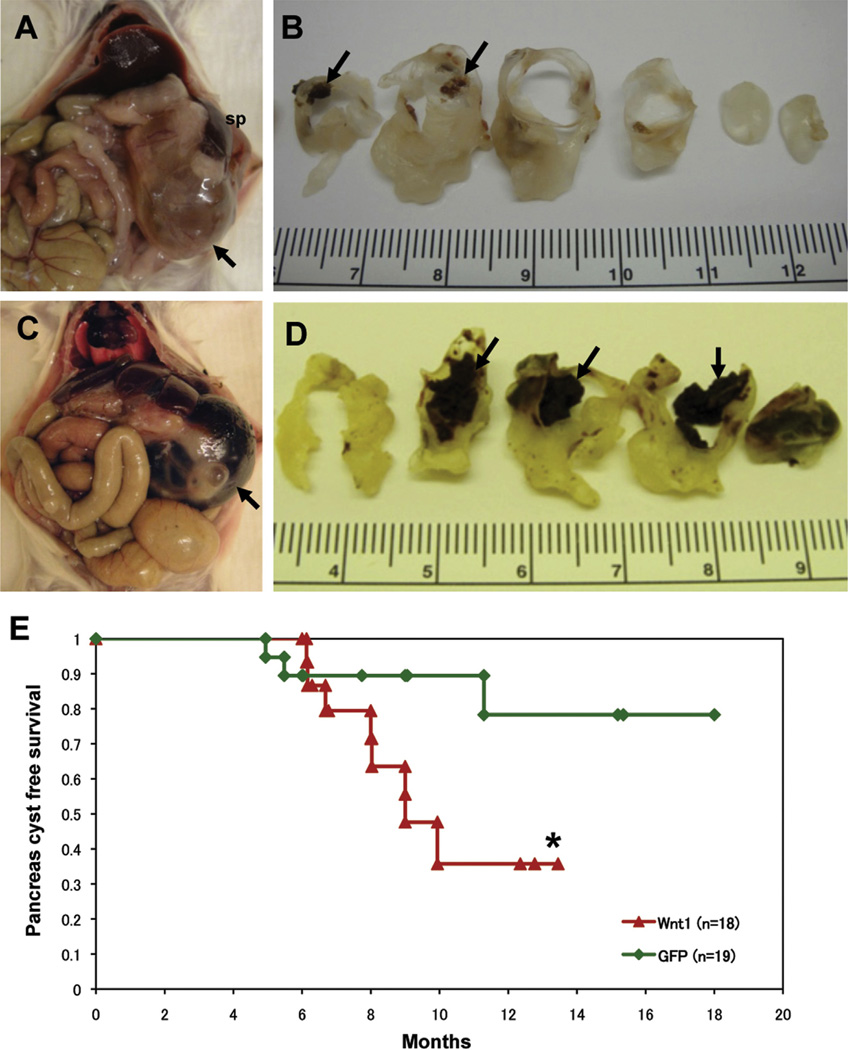
To elucidate the ability of Wnt ligands to cooperate with activated KRAS during pancreatic tumorigenesis, we generated compound LSL-KrasG12D;Ptf1a-cre;elastase-tva mice, and injected them with DF1 chicken fibroblasts producing RCAS viruses encoding Wnt1 or GFP. This resulted in the postnatal and sporadic introduction of genes encoded by the RCAS viruses into pancreatic acinar cells and acinar cell progenitors.25 Compound transgenic mice injected with RCAS-Wnt1 viruses commonly developed large unilocular or multilocular cysts measuring up to 35 mm in diameter in the pancreatic body and tail (Figure 1A–D, Supplementary Figure 1A and B). These cysts were commonly filled with serous or serosanguinous contents (Figure 1A and B), and old hemorrhagic contents were observed in some cases (Figure 1C and D, Supplementary Figure 1B). Direct connection between these cysts and the main pancreatic duct was not demonstrable in any cases. In addition, rupture of the cyst was seen in one case. The clinicopathological phenotypes of the cystic lesions are summarized in Supplementary Table 1. RCAS-Wnt1–injected mice were more likely to succumb to pancreas cyst lesions compared with RCAS-GFP–injected control mice (Figure 1E; P = .052 by log-rank test). Sexual incidence of the cystic lesions was 0 of 4 (0%) male and 8 of 14 (57.1%) female in RCAS-Wnt1 –injected mice, and 0 of 11 (0%) male and 3 of 8 (37.5%) female in RCAS-GFP control mice. Mean age of euthanasia due to cystic lesions in Wnt1 and GFP mice was 8 months, 1 day and 7 months, 11 days, respectively. A cyst occurred in 1 of 5 (20%) elastase-tva–negative, Ptf1a-cre, LSL-KrasG12D mice injected with DF1 cells producing RCAS-Wnt1, indicating spontaneous cyst formation driven by oncogenic KRAS, consistent with the identification of KRAS mutations in cystic lesions of the pancreas.7,9 In contrast, no cystic lesions were observed in 31 elastase-tva–positive, Kras wild-type mice injected with RCAS-Wnt1 producer cells. Interestingly, cystic lesions occurred in 0 of 8 (0%) male and 1 of 7 (14.3%) female mice injected with DF1 cells producing RCAS-β-cateninS37A. Of note, delivery of either RCAS-Wnt1, or RCAS-β-cateninS37A stimulated the development of PDAC in compound LSL-KrasG12D;Ptf1a-cre;elastase-tva mice (M. Sano and B. Lewis, unpublished data).
Figure 1.
Overexpression of Wnt1 ligand induces MCN-like lesions in KrasG12D transgenic mice. Multilocular cysts including serosanguinous contents are located in the pancreatic body and tail in elastase-tva, Ptf1a-cre, LSL-KrasG12D compound transgenic mice infected with RCAS-Wnt1 (A and C; arrows; sp, spleen). (B) Cut surface showing enlarged cyst with slightly thickened cyst wall and precipitation of old coagulation in the lumen (arrows). (D) A unilocular cyst containing hemorrhagic contents is observed in the pancreatic tail of an RCAS-Wnt1–injected elastase-tva, Ptf1a-cre, LSL-KrasG12D compound transgenic mouse (arrows). (E) Kaplan–Meier analysis demonstrates that RCAS-Wnt1–injected mice tended to have poor prognosis under cystic lesion-free survival curve *P = .052 by the log-rank test.
Together, these data indicate that postnatal expression of Wnt1 in the pancreatic epithelium cooperates with activated KRAS to promote development of cystic lesions with a female-sex bias. These findings are consistent with the recent demonstration of KRAS gene mutations in pancreatic cystic neoplasms,7,9 and suggest a role for activated Wnt signaling in the development of these lesions.
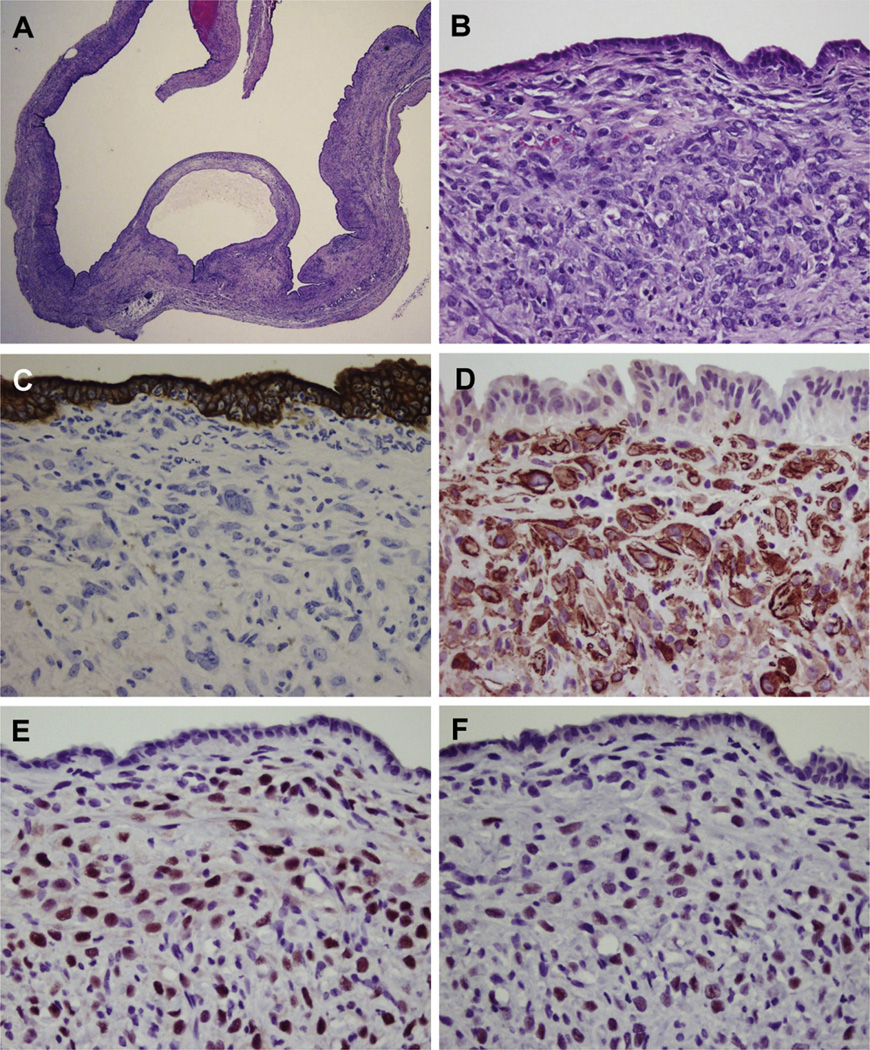
Histopathologically, the Wnt-induced cystic lesions displayed a serrated cystic lumen with a slightly thickened wall composed of ovarian-like stroma that resembles that found in human MCN, a lesion with a predominant female sex bias (Figure 2A and B). In contrast to most human MCNs, the epithelium in the murine lesions produced in this model consisted of low cuboidal cells, as opposed to columnar cells (Figure 2A and B). In addition, when identified, the columnar epithelial cells in the murine lesions lacked the typical mucin production seen in human MCNs. Rare mucinous material was detected on the apical membrane with diastase-digestive periodic acid–Schiff and Alcian blue stains (Supplementary Figure 1C and D). Slight nuclear atypia of the cyst epithelium was observed in association with inflammatory cell infiltration, but there was no high-grade dysplasia or invasive carcinoma, nor was distant metastasis detected in any cases.
Figure 2.
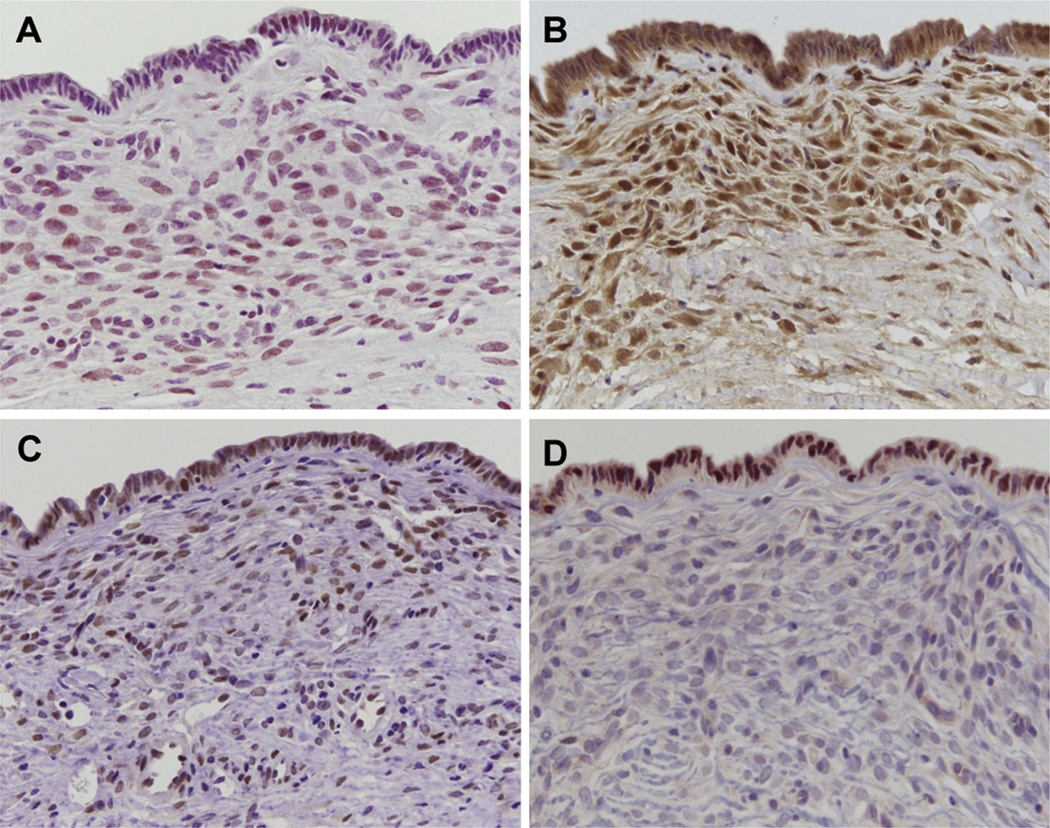
Histopathological phenotype of MCN-like lesions. (A) Multilocular cysts showing serrated cystic lumen and focal thickened wall. (B) High-power view of cyst in panel A reveals dense ovarian-like stroma, and cuboidal epithelium that lacks abundant mucin production. (C) The ovarian-like stoma is immunohistochemically negative for wide-spectrum keratin (C), whereas these cells are positive for α-smooth muscle actin (α-SMA) (D), ER (E), and PR (F).
Immunohistochemical analysis demonstrated that cyst epithelial cells had a low Ki-67 labeling index (9.3%), suggesting that the cyst epithelial cells had, at most, low-grade dysplasia. In contrast, a high Ki-67 labeling index (29.1%) was observed in the ovarian-like stromal cells. In addition, the ovarian-like stroma was negative for the epithelial marker wide-spectrum keratin (Figure 2C), and it was positive for mesenchymal markers vimentin and α-smooth muscle actin (Supplementary Figure 1E and Figure 2D). In addition, ER and PR expression, characteristic features of the ovarian-like stroma in human MCNs, were highly detected in all cases (Figure 2E and F). Interestingly, although all of the cystic lesions identified in RCAS-Wnt1 –injected mice had ER- and PR-positive stromal cells, 2 of the cystic lesions identified in RCAS-GFP and RCAS-βcateninS37A –injected mice were negative for these markers, suggesting they might belong to a separate class of cystic lesion.
Activation of Wnt Signaling in the Stroma of Murine MCN-Like Lesions
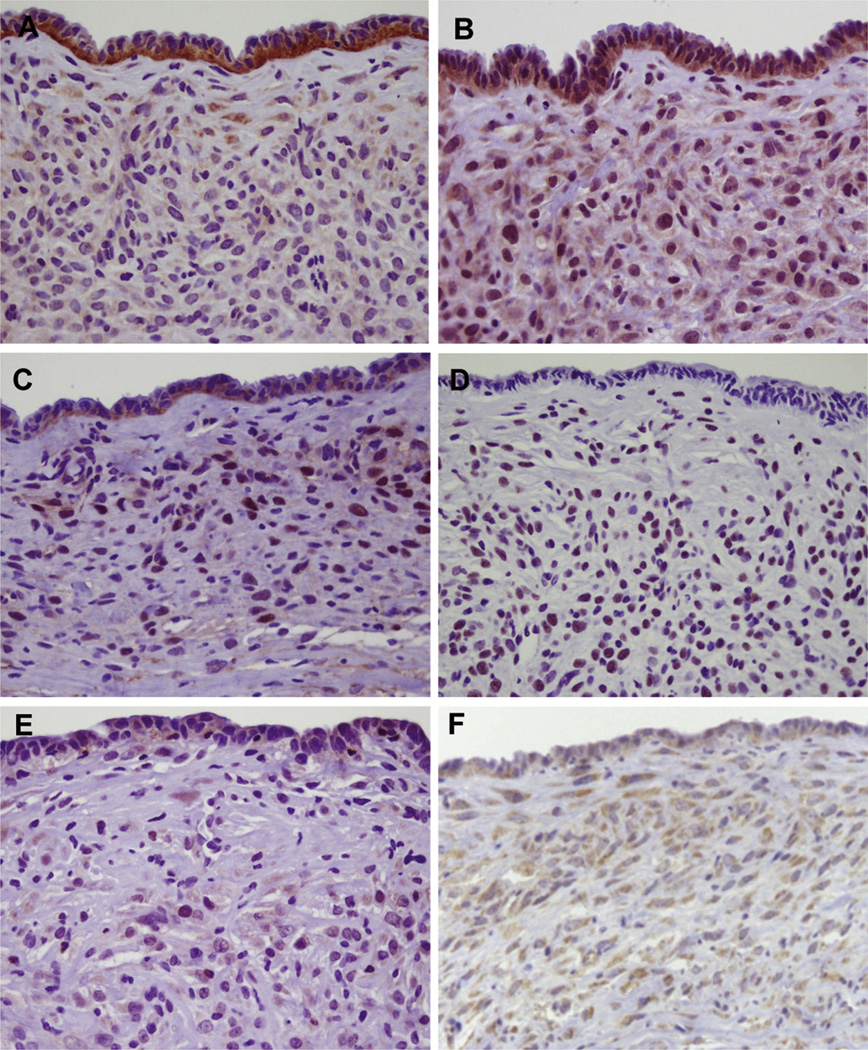
To confirm that RCAS-introduced Wnt1 induced the MCN-like lesions, we stained the cystic lesions with an anti-Wnt1 antibody. As expected, Wnt1 was primarily found in the cyst epithelium, and faint staining was observed in the ovarian-like stroma (Figure 3A). To confirm the integration of RCAS-Wnt1 viruses in the epithelium of the cystic lesions, total DNA was isolated from formalin-fixed and paraffin-embedded tissue sections, and RCAS and tva amplified by polymerase chain reaction (Supplementary Figure 2). Polymerase chain reaction bands corresponding to RCAS and tva were amplified from DNA isolated from tissue sections containing MCN-like lesions in elastase-tva;Ptf1a-cre;LSL-KrasG12D compound mice injected with RCAS-Wnt1. In contrast, RCAS virus was not detected from a MCN-like lesion in an elastase-tva–negative, Ptf1a-cre;LSL-KrasG12D mouse injected with RCAS-Wnt1. In addition, RCAS virus was not detected in 2 noninjected elastase-tva;Ptf1a-cre;LSLKrasG12D compound mice (Supplementary Figure 2).
Figure 3.
Activation of Wnt signaling pathways in murine MCN-like lesions. (A) Wnt1 is predominantly detected in the cystic epithelium, while faint immunoreactivity is seen in the ovarian-like stroma. (B) The putative Wnt1 receptor FZD3 is expressed in both the cystic epithelium and stroma. (C) Nuclear translocation of β-catenin is specifically observed in the stromal cells, but not the cyst epithelium. The presence of p-JNK (D), p-Rock2 (E), and the Wnt target Axin2 (F) are also observed in the stromal cells.
We next determined the location of the putative Wnt1 receptors FZD3 and FZD6 by immunohistochemistry. FZD3 expression was predominantly identified in ovarian-like stroma cells, while cyst epithelial cells were focally positive for FZD3 (Figure 3B). Expression of FZD6 was focally observed in ovarian-like stromal cells with abundant cytoplasm that resembled the luteinized cells found in human MCN (Supplementary Figure 3A). However, in contrast to their human counterparts, these cells were negative for inhibin, a marker for luteinized cells (data not shown).31 Interestingly, the distribution pattern of FZD6 tended to overlap with PR staining in the stromal cells. In addition, we examined the staining patterns of the Wnt receptors FZD1 and FZD4 that are up-regulated by luteinizing hormone in murine ovaries32 and that have the potential to bind Wnt1.33,34 FZD1 was weakly and focally detected in the luteinized ovarian-like stromal cells (Supplementary Figure 3B). Meanwhile, faint staining for FZD4 was seen in focal luteinized cells, but not the in cyst epithelium (Supplementary Figure 3C, and data not shown).
To identify the cells in which Wnt1-induced signaling pathways are active, we immunohistochemically investigated the status of major Wnt signaling pathways—the Wnt/β-catenin (β-catenin staining), Wnt/PCP (p-JNK and p-Rock2 staining), and Wnt/Ca2+ signaling pathways (NFATc1 staining). Interestingly, nuclear accumulation of β-catenin, p-JNK, and p-Rock2 (Figure 3C–E), but not NFATc1 (data not shown), was observed in the ovarian-like stroma. In contrast, in the cyst epithelium, β-catenin was localized to the plasma membrane, focal nuclear p-JNK was observed, and no immunoreactivity to p-Rock2 and NFATc1 was seen (Figure 3C–E, and data not shown). Consistent with activation of the canonical Wnt signaling pathway, staining for the target gene Axin2 was readily observed in the MCN stroma (Figure 3F).
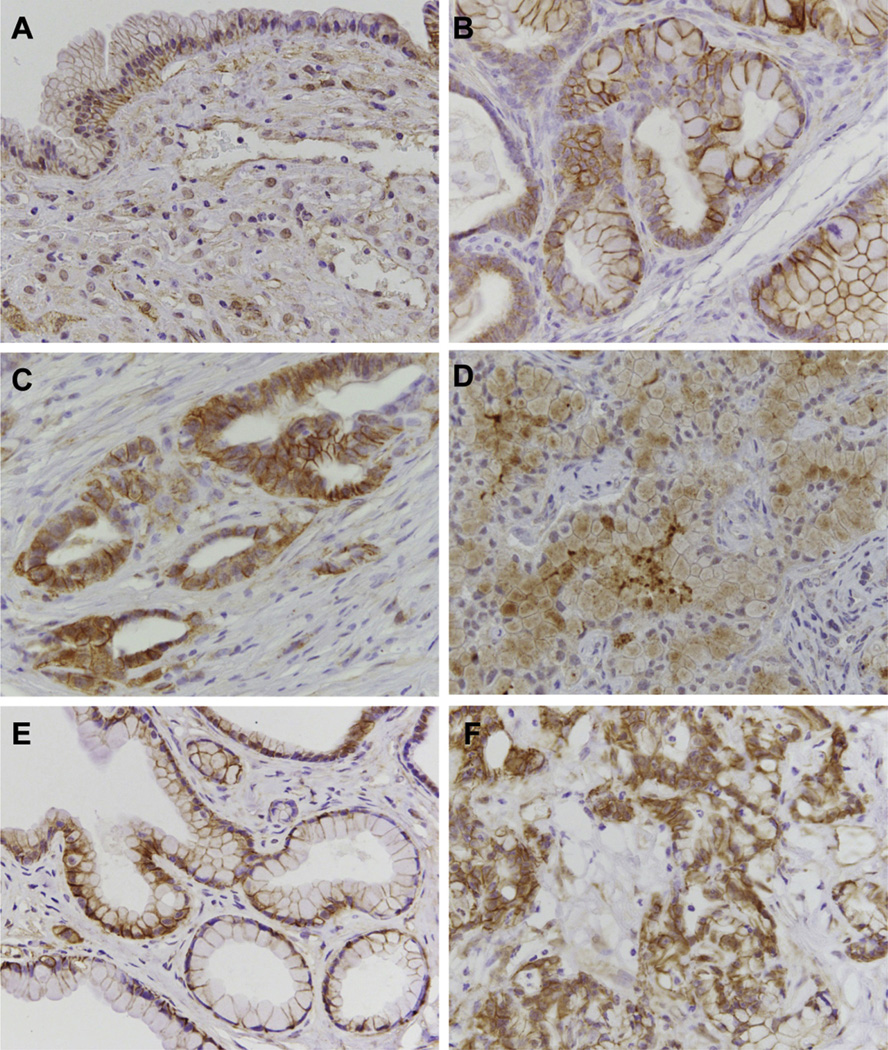
Izeradjene and colleagues previously described an MCN mouse model (KDD) induced via concomitant activation of KRAS and deletion of Smad4.35 To determine whether stromal activation of Wnt signaling in MCN occurs irrespective of initiating genetic lesion, we evaluated β-catenin localization in 2 MCN specimens from this model, and observed nuclear staining in the stroma in this model (Figure 4A). In the epithelium, β-catenin was primarily localized to the cell membrane, although occasional nuclear staining could be observed.
Figure 4.
Wnt signaling occurs specifically in the MCN stroma. (A) Nuclear translocation of β-catenin is specifically observed in the stromal cells, but not the cyst epithelium in the KDD mouse model. β-catenin staining of PanIN lesions (B) and PDAC (C) induced in compound LSL-KrasG12D;Ptf1a-cre; elastase-tva mice injected with RCAS-Wnt1. Cytoplasmic β-catenin staining can be observed in some epithelial cells in PDAC. (D) Staining for Axin2 in PDAC cells (arrows), but not stroma. β-catenin staining in PanIN lesions (E) and PDAC (F) identified in the KDD mouse model.
Interestingly, ER- and PR-positive lesions identified in mice injected with RCAS-GFP also contained stromal cells positive for nuclear β-catenin, and the ER- and PR-negative cystic lesions induced in mice injected with RCAS-GFP or RCAS- β-cateninS37A were negative for nuclear β-catenin in the stroma, suggesting that activation of the Wnt signaling pathway can occur specifically within the stroma of MCN-like lesions, but not other cystic lesions.
We next determined whether nuclear β-catenin staining occurs in other pancreatic lesions in mice. We stained PanIN lesions and PDAC arising in the compound KRAS/Wnt1 model. Consistent with published findings,36 we observed membrane-associated β-catenin staining in PanIN lesions (Figure 4B), and membrane and cytoplasmic β-catenin staining in PDAC (Figure 4B and C). In contrast to the MCN lesions induced in this model, the PanIN and PDAC stroma were negative for β-catenin (Figure 4B and C). In agreement, the Wnt target Axin2 could be observed within the epithelium of PDAC lesions (Figure 4D), but not the stroma. Similar findings were observed in PanIN and PDAC lesions induced in the KDD model (Figure 4E and F). Taken together, these data suggest that activation of Wnt signaling in the stroma occurs specifically in MCN.
Nuclear translocation of the p-Smad2/3/4 complex has been shown to be promoted by p-JNK signaling.37,38 We detected nuclear translocation of p-Smad2/3 in almost all ovarian-like stromal cells, and focal nuclear p-Smad2/3 was seen in the cyst epithelium (Figure 5A). The presence of focal nuclear p-Smad staining in the epithelial cells suggests that the nuclear translocation observed might be mediated in part by canonical transforming growth factor–βsignaling. Indeed, epithelial cells in Wnt1-induced MCN lesions displayed SMAD4 staining in the nucleus and cytoplasm (Figure 5B), suggesting intact transforming growth factor–β-signaling, and further suggesting that loss of SMAD4 is not required for MCN development. Izeradjene et al previously showed that Hes1, a Notch-responsive gene, is induced in the epithelium of MCN induced in the KDD model.35 We found Hes1-positive cells in the epithelium of Wnt1-induced MCN (Figure 5C), confirming a potential role for epithelial Notch signaling in this lesion type. The origin of the MCN stroma remains unclear, and recent work from Rhim et al suggested that some cells in the PDAC stroma are derived from dedifferentiated epithelial cells identified by lineage tracing and Pdx1 staining.39 Therefore, we stained MCN lesions for Pdx1. We found that Pdx1 staining occurs specifically within the epithelium, but not the stroma, suggesting that the MCN stroma is not derived from dedifferentiated epithelial cells (Figure 5D).
Figure 5.
Analysis of signaling pathways in MCN. Analysis of transforming growth factor–βsignaling in MCN lesions by immunostaining for the nuclear translocation of phosphorylated Smad2/3 (A) and immunostaining for Smad4 (B). Assessment of active Notch signaling via immunostaining for Hes1 (C). Immunostaining for the pancreas progenitor marker Pdx1 (D).
The immunohistochemical features of the mouse MCN-like lesions are summarized in Supplementary Table 2.
Activation of Wnt Signaling Pathways in Human MCN
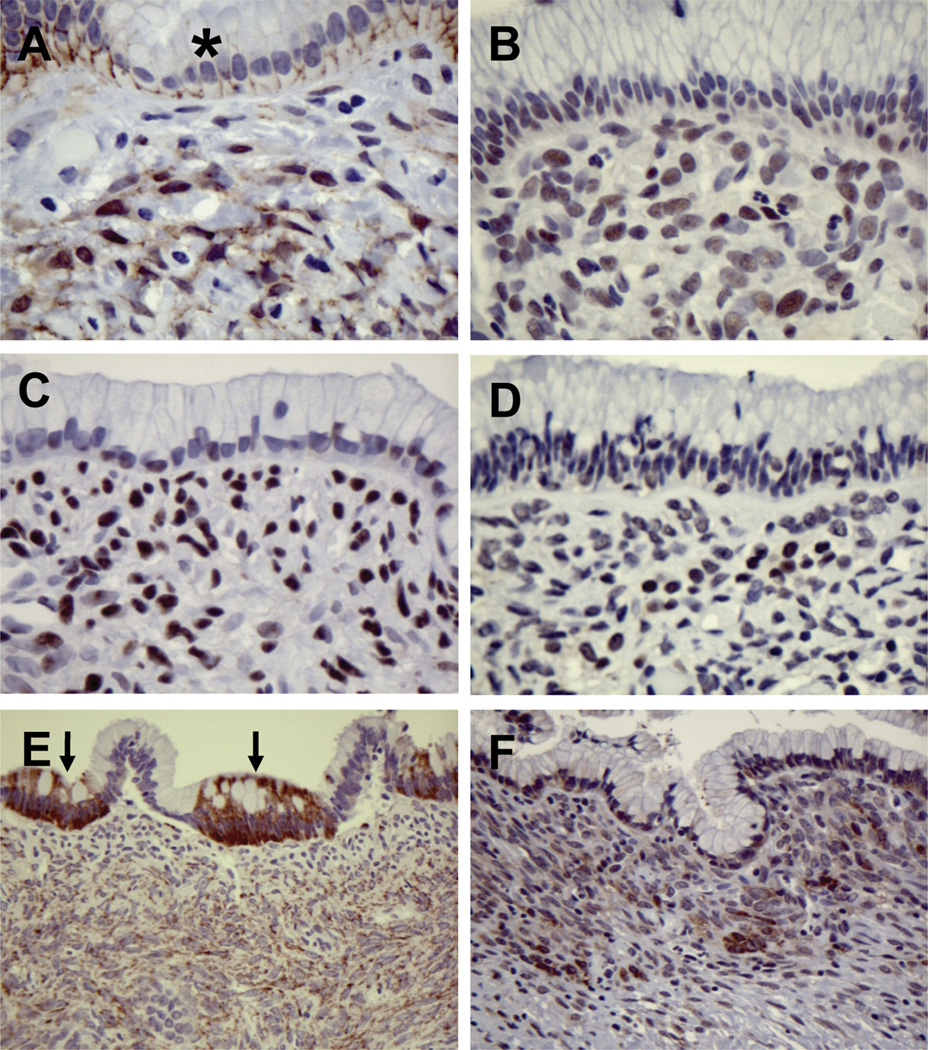
Our mouse model data indicated that Wnt pathway activation might be a signature feature of stromal cells in MCN. We therefore ascertained whether activated Wnt signaling is observed in human MCN. β-catenin localization was evaluated in 19 MCN cases. Sixteen of 19 specimens displayed nuclear and/or cytoplasmic staining for β-catenin in the stroma, and in the majority of cases this staining was focal (Figure 6A, Supplementary Figure 4A). In the cyst epithelium, β-catenin was primarily localized to the cell membrane. The extent of β-catenin staining in the stroma did not correlate with degree of dysplasia within the epithelium. We further investigated the status of the Wnt/β-catenin and PCP signaling pathways in 8 cases (summarized in Supplementary Table 3). We found that the stromal cells were additionally positive for p-JNK, p-Smad2/3, and p-Rock2 (Figure 6B–D), suggesting that both the Wnt/β-catenin and PCP signaling pathways are activated in the ovarian-like stroma of human MCN cases, consistent with our findings in the murine MCN-like lesions. In human MCN, expression of Wnt1 was detected in focal cyst epithelial cells in the majority of cases (Figure 6E), although in some cases no detection of Wnt1 expression was observed in the epithelium (Supplementary Figure 4B). However, the ovarian-like stroma was diffusely positive for Wnt1 in all of the human MCN cases examined (Figure 6E and Supplementary Figure 4B). In addition, consistent with our findings in the mouse, FZD3 was expressed in the ovarian-like stroma and focally in the cyst epithelium (Figure 6F and Supplementary Figure 4C). Of the other FZD receptors evaluated, FZD6 was found focally within the stroma, FZD1 was found only in the cyst epithelium (Supplementary Figure 4D), and FZD4 staining was not observed. Together with our mouse model data, these results suggest that epithelial and/or stromal produced Wnt ligands promote the activation of FZD3 and the subsequent stimulation of Wnt signaling cascades in the ovarian-like stroma in human MCN.
Figure 6.
Activation of Wnt/β-catenin signaling pathways in human MCN. (A) Nuclear accumulation of β-catenin is observed in the ovarian-like stoma of human MCN, but not in the cyst epithelium (asterisk). Nuclear translocation of p-JNK (B), p-Smad2 (C), and p-Rock2 (D) are seen in the ovarian-like stroma. (E) Strong expression of Wnt1 is seen focally in the cyst epithelium (arrows), and the ovarian-like stromal cells are diffusely positive for Wnt1. (F) Expression of the Wnt1 receptor FZD3 is observed in focal epithelial cells and in the ovarian-like stroma.
Discussion
Primary pancreatic cystic lesions have been classified on the basis of clinicopathological features, namely non-neoplastic pseudocysts and neoplastic cysts, such as serous cystic neoplasm, MCN and intraductal papillary neoplasm, as well as solid-pseudopapillary neoplasm and lymphoepithelial cyst.40–43 Recent studies have begun to characterize in greater detail the genomic alterations that occur in pancreatic cystic neoplasms. In particular, attention has been focused on intraductal papillary mucinous neoplasm and MCN, as they can serve as precursor lesions for development of invasive PDAC.6 Despite these efforts, the molecular mechanisms underlying the development of these lesions remain poorly understood. Indeed, MCNs were shown to have a relatively low number of gene mutations.9 In addition, these genomic studies have focused exclusively on the changes occurring within the cyst epithelium, and not the abundant ovarian-like stroma that is the cardinal diagnostic feature of MCN, the origin of which remains uncertain.
In this article, we found that postnatal introduction of Wnt1 into pancreatic epithelial cells cooperates with activated KRAS to stimulate the development of cystic lesions with the stereotypical ovarian-like stroma found in human MCN. The occurrence of these lesions in our model reflected the female-sex and middle-age bias associated with this lesion in humans. In addition, the lesions were predominantly localized to the body and tail of the pancreas, mimicking the human neoplasm. Although the cystic lesions lacked the columnar mucin-producing epithelium typically found in MCN, they harbored the ER- and PR-positive ovarian-like stroma found in human MCNs. Importantly, immunohistochemical characterization of the mouse lesions demonstrated that activation of the canonical Wnt/β-catenin signaling cascade occurs specifically within the stroma, but not the epithelium, despite the demonstrated production of Wnt1 by the epithelial cells. Our analysis of human MCN specimens confirmed that the specific activation of Wnt-induced signaling cascades in the stroma is a hallmark feature of MCN. Our experiments reveal for the first time a cardinal feature of this cystic neoplasm.
Significantly, we observed nuclear β-catenin staining in the stroma of MCN lesions arising in the KDD mouse model, suggesting that the initiating genetic alterations do not influence this phenotype. In addition, cystic lesions arising in animals injected with RCAS-GFP or RCAS-β-cateninS37A, and which did not have an ovarian-like stroma, also failed to show nuclear accumulation of β-catenin within the stromal cells. Analysis of PanIN lesions and PDAC in the Wnt1 and KDD models demonstrated the absence of β-catenin staining in the stroma, although cytoplasmic localization of β-catenin, and its target gene Axin2, could be observed in the epithelial cells in PDAC. Together, these data confirm the uniqueness of the MCN stroma, and suggest that activation of Wnt signaling within the stroma is specific to MCN.
However, it is likely that activation of Wnt signaling pathways is insufficient to induce the features associated with the ovarian-like stroma. More likely, multiple signaling pathways converge to promote the recruitment and stimulation of the ovarian-like stroma. We find that the MCN stroma is more proliferative than the cyst epithelium. This finding suggests that ER- and PR-stimulated proliferation might play an important contributory role in MCN, a hypothesis consistent with the observed female-sex bias of this neoplasm. The convergence of activated Wnt signaling (and other signaling pathways) with hormone receptor signaling might provide the required stimulus that results in MCN development. Additional studies will be needed to model this integrated signaling, and to elucidate the identity of other contributing pathways in the MCN stroma.
The inability of epithelial expression of β-cateninS37A to induce MCN in our model is instructive and suggests that although stimulated Wnt signaling in the stroma is an important driver in MCN development, activated canonical Wnt signaling in the epithelium is not. Nuclear β-catenin staining was very rarely observed in the epithelium of human and murine MCN. This observation also provides a potential explanation for the differences between the tumor types induced in our model and that of Heiser et al, who observed that expression of activated β-catenin in the pancreas induced the formation of solid pseudopapillary neoplasm-like tumors, and that concomitant activation of β-catenin and KRAS resulted in the development of pancreatic ductal lesions with features resembling intraductal tubular neoplasms.19
Interestingly, recent sequencing studies identified potentially inactivating mutations in the E3 ubiquitin ligase RNF43 in MCN,9 and other recent studies have demonstrated that RNF43 acts as a negative regulator of Wnt signaling by stimulating the endocytosis of Wnt receptors.44,45 Thus, nuclear accumulation of β-catenin within the cyst epithelium would be expected to be a common finding in MCN. However, we failed to commonly identify nuclear β-catenin within the cyst epithelium, suggesting that other proteins and pathways are likely the primary targets of RNF43 in MCN, if it indeed acts as a tumor suppressor in this neoplasm.
Significantly, the origin of the ovarian-like stroma remains unknown. Pancreatic stellate cells are believed to be the source of stromal cells in PDAC,46 and these cells are a possible source of the ovarian-like stroma in MCN. If indeed the MCN stroma is derived from stellate cells, understanding the signaling pathways that stimulate this cell population to adopt the unique features of the MCN stroma vs those of the PDAC-associated stroma will be critical to understanding the origins of this neoplasm.
Other cell populations can also serve as the origin of this stroma. Previous work has demonstrated that mesenchymal stem cells are commonly recruited into developing tumors and contribute to tumor pathology.47 In addition, recent work by Rhim and colleagues demonstrated the presence of epithelium-derived stromal cells in PDAC.39 The absence of Pdx1-positive cells in the MCN stroma suggests that this is not a likely source, although data from our laboratory demonstrate that dedifferentiated pancreatic cancer cells frequently lose Pdx1 expression (M. Sano, W. De Jesus-Monge, and B. Lewis, unpublished observations). Additional studies utilizing a lineage-tracing approach will be required to definitively rule out this possibility.
In summary, our findings reported here identify stromal activation of Wnt signaling as a hallmark feature of MCN. Although additional studies are needed to completely understand how this signaling promotes MCN development, these findings suggest that active stromal Wnt signaling might serve as an additional histological marker for this lesion, as well as a potential therapeutic target for MCN.
Supplementary Material
Acknowledgments
The authors thank Dr Leslie Shaw for reagents; Dr Sunil Hingorani for KDD tissue specimens; Victor Adelanwa, Jiu-Feng Cai, and Sharon A Magnusson for animal care and technical assistance; and Drs Leslie Shaw, Karl Simin, Junhao Mao, and members of the Lewis laboratory for useful comments and discussion.
Funding
Supported by National Institutes of Health research grants CA113896 and CA113896-S1 to BCL and by the Verville Foundation. BCL is a member of the University of Massachusetts DERC (DK32520). The funders had no role in the design or conduct of the study.
Abbreviations used in this paper
- ER
estrogen receptor
- FZD
frizzled
- MCN
mucinous cystic neoplasm
- PanIN
pancreatic intraepithelial neoplasia
- PCP
planar cell polarity
- PDAC
pancreatic ductal adenocarcinoma
- PR
progesterone receptor
- RCAS
replication-competent avian sarcoma-leukosis virus long-terminal repeat with splice acceptor.
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at http://www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.09.044.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 4.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 5.Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730 e9–733 e9. doi: 10.1053/j.gastro.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthaei H, Schulick RD, Hruban RH, et al. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8:141–150. doi: 10.1038/nrgastro.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002543. 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klimstra DS. Cystic, mucin-producing neoplasms of the pancreas: the distinguishing features of mucinous cystic neoplasms and intraductal papillary mucinous neoplasms. Semin Diagn Pathol. 2005;22:318–329. doi: 10.1053/j.semdp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 12.Murtaugh LC, Law AC, Dor Y, et al. Beta-catenin is essential for pancreatic acinar but not islet development. Development. 2005;132:4663–4674. doi: 10.1242/dev.02063. [DOI] [PubMed] [Google Scholar]
- 13.Heiser PW, Lau J, Taketo MM, et al. Stabilization of beta-catenin impacts pancreas growth. Development. 2006;133:2023–2032. doi: 10.1242/dev.02366. [DOI] [PubMed] [Google Scholar]
- 14.Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–962. doi: 10.1016/s0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koesters R, von Knebel Doeberitz M. The Wnt signaling pathway in solid childhood tumors. Cancer Lett. 2003;198:123–138. doi: 10.1016/s0304-3835(03)00367-7. [DOI] [PubMed] [Google Scholar]
- 17.Nishimori I, Kohsaki T, Tochika N, et al. Non-cystic solid-pseudopapillary tumor of the pancreas showing nuclear accumulation and activating gene mutation of beta-catenin. Pathol Int. 2006;56:707–711. doi: 10.1111/j.1440-1827.2006.02034.x. [DOI] [PubMed] [Google Scholar]
- 18.Min Kim S, Sun CD, Park KC, et al. Accumulation of beta-catenin protein, mutations in exon-3 of the beta-catenin gene and a loss of heterozygosity of 5q22 in solid pseudopapillary tumor of the pancreas. J Surg Oncol. 2006;94:418–425. doi: 10.1002/jso.20509. [DOI] [PubMed] [Google Scholar]
- 19.Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasca di Magliano M, Biankin AV, Heiser PW, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Heidt DG, Lee CJ, et al. Oncogenic function of ATDC in pancreatic cancer through Wnt pathway activation and beta-catenin stabilization. Cancer Cell. 2009;15:207–219. doi: 10.1016/j.ccr.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 23.Ripka S, Konig A, Buchholz M, et al. WNT5A-target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007;28:1178–1187. doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 24.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 25.Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003;17:3127–3138. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 27.Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuveson DA, Shaw AT, Willis NA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 29.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh MM, Tang LH, Wang S, et al. Inhibin expression in ovarian-type stroma in mucinous cystic neoplasms of the pancreas. Appl Immunohistochem Mol Morphol. 2004;12:148–152. doi: 10.1097/00129039-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh M, Johnson MA, Greenberg NM, et al. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology. 2002;143:898–908. doi: 10.1210/endo.143.3.8684. [DOI] [PubMed] [Google Scholar]
- 33.Bhanot P, Brink M, Samos CH, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 34.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Morris JP, IV, Yan W, et al. Canonical Wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013;73:4909–4922. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He S, Liu X, Yang Y, et al. Mechanisms of transforming growth factor beta(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br J Dermatol. 2010;162:538–546. doi: 10.1111/j.1365-2133.2009.09511.x. [DOI] [PubMed] [Google Scholar]
- 38.Holm TM, Habashi JP, Doyle JJ, et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham SC, Hruban RH, Schulick RD. Differentiating intraductal papillary mucinous neoplasms from other pancreatic cystic lesions. World J Gastrointest Surg. 2010;2:331–336. doi: 10.4240/wjgs.v2.i10.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hruban RH, Fukushima N. Cystic lesions of the pancreas. Diagn Histopathol (Oxf) 2008;14:260–265. doi: 10.1016/j.mpdhp.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimstra DS, Pitman MB, Hruban RH. An algorithmic approach to the diagnosis of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:454–464. doi: 10.5858/133.3.454. [DOI] [PubMed] [Google Scholar]
- 43.Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, et al. Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol. 2010;20:e84–e92. doi: 10.1016/j.suronc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 45.Hao HX, Xie Y, Zhang Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 46.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 47.Stagg J. Mesenchymal stem cells in cancer. Stem Cell Rev. 2008;4:119–124. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.