Abstract
To investigate whether obese women can compensate for sucrose added to the diet when it is given blind, rather than gaining weight or exhibiting dysfunctional regulation of intake, in the present study, forty-one healthy obese (BMI 30–35 kg/m2) women (age 20–50 years), not currently dieting, were randomly assigned to consume sucrose (n 20) or aspartame (n 21) drinks over 4 weeks in a parallel single-blind design. Over the 4 weeks, one group consumed 4 × 250 ml sucrose drinks (total 1800 kJ/d) and the other group consumed 4 × 250 ml aspartame drinks. During the baseline week and experimental weeks, body weight and other biometric data were measured and steps per day, food intake using 7 d unweighed food diaries, and mood using ten- or seven-point Likert scales four times a day were recorded. At the end of the experiment, the participants weighed 1·72 (se 0·47) kg less than the value predicted by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) model; the predicted body weight accounted for 94·3 % of the variance in the observed body weight and experimental group accounted for a further 1·1 % of the variance in the observed body weight, showing that women consuming sucrose drinks gained significantly less weight than predicted. The reported daily energy intake did not increase significantly, and sucrose supplements significantly reduced the reported voluntary sugar, starch and fat intake compared with aspartame. There were no effects on appetite or mood. Over 4 weeks, as part of everyday eating, sucrose given blind in soft drinks was partially compensated for by obese women, as in previous experiments with healthy and overweight participants.
Keywords: Sucrose, Fat intake, Energy regulation, Body weight, Soft drinks, Obesity
A recent systematic review and meta-analysis has concluded that ‘Among free living people involving ad libitum diets, intake of free sugars or sugar sweetened beverages is a determinant of body weight’( 1 ). It may be particularly difficult to accurately compensate for energy-dense liquids that lack visual or orosensory cues to their energy content( 2 , 3 ). It has also been hypothesised that compensation when participants know that they are being given sugars differs from that when they are given sugars blind( 4 , 5 ). In the former conditions, psychological expectancy effects are more likely to occur, such as abstinence violation effects( 6 , 7 ), meaning that sugars can have both direct effects on energy intake and indirect effects by being the marker and sign of a less-healthy lifestyle that may include inactivity and surplus energy intake from all sources, notably from fat.
Studies that encourage reductions in the intake of sugars cannot be blind, and participants deliberately choose foods, making it difficult to differentiate the direct effects of sugar consumption from indirect effects. In studies in which isoenergetic high-sugar or low-sugar diets are given, sometimes under blind conditions, no difference in weight status can be found( 1 ). In short, the impact of sugars in the diet depends upon what else people eat( 1 ) and overall energy balance( 2 ). It is not clear whether obese people differ qualitatively, or only quantitatively, in their regulation of eating from people who weigh less( 8 ). There have been few randomised controlled studies carried out in obese participants deliberately increasing sugar intake in the ad libitum diet( 1 ), which was the purpose of the experiment reported herein.
In this line of research, two previous studies gave supplements of 1800 kJ of sucrose as liquid for normal-weight and overweight women under single-blind conditions over 4 weeks and found that women partially compensated for the added energy by reducing ad libitum intake elsewhere in the diet( 4 , 5 ). The participants did not gain weight over the course of the experiments. It was concluded that when given blind to eliminate psychological expectancy effects, women compensate for sucrose added to the diet in soft drinks. The present experiment replicates this procedure using obese participants.
The aim of the present study was to determine the effects of sucrose beverages consumed over 4 weeks when compared with a placebo containing artificial sweeteners in obese women. Previous studies( 4 , 5 ) found no effects of labelling, so in the present study, all the participants were informed that they were being given sucrose-sweetened soft drinks, meaning that half of the participants were misinformed, as they were actually being given artificially sweetened ones.
The present experiment extends the previous experimental design to women who are obese (BMI ≥ 30 kg/m2). The hypothesis was that obese women, similar to overweight and normal-weight women in previous studies, would partially compensate for the energy content of supplementary soft drinks: (1) not gain weight, the primary outcome measure based on body weight; secondary outcomes being (2) reduce energy intake elsewhere in the diet, (3) reduce the total fat content of the diet, as in the previous experiments, and (4) reduce the ad libitum carbohydrate (CHO) content of the diet, measured by the self-reported food intake in the food diaries. Drinks rather than solid foods were used for convenience and because sugary drinks are the form of simple CHO most likely to cause the effects discussed above.
Methods
Design
The present experiment was carried out over a period of 5 weeks: baseline data were collected for 1 week and supplementary drinks were added to the diet for 4 weeks. On the basis of previous research( 5 ), a target cell size of twenty-two was calculated to achieve a 90 % chance of detecting a difference in change in the body weight of 1·5 % of baseline body weight between the groups (assuming sd= 1·5). The subjects were given drinks containing sucrose (achieved n 20) or drinks containing aspartame (with minimal energy content, achieved n 21). All the subjects were informed that they were being given sugar-sweetened drinks.
Ethics
The experiment was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of Queen Margaret University. Written informed consent was obtained from all the participants. The study was registered at clinicaltrials.gov (NCT01799096). The following significant ethical issues were recognised: first, giving obese people sucrose could possibly have adverse effects on their weight or diet, but the null results of previous studies minimised this risk; second, monitoring the diets of obese people introduced a requirement of care, which was satisfied by providing detailed feedback about dietary intake and potential ways of improving it at the end of the experiment, and this also served as an additional incentive to participate; lastly, the experiment involved deception, so all the participants were informed about the reasons for this at the end of the experiment.
Participants
Participants were recruited by advertising around local universities and businesses. They included university staff, mature students and members of the general public. Volunteers were screened via telephone interviews. Inclusion criteria included being a woman with a BMI between 30 and 35 kg/m2 and aged between 20 and 55 years with at least one period of dietary restriction of 4 weeks or more in the last 24 months, but not in the last 4 weeks. Exclusion criteria included a dislike for popular sweet carbonated drinks, dieting in the last month, history of diabetes, having an eating disorder, being currently treated for depression or scoring >10 on the Beck Depression Inventory II( 9 ), being a smoker, pregnant or lactating, wearing a pacemaker, and currently taking medication for mood or thyroid disorders. A pin-prick method using the Accu-Chek GC was used to measure blood glucose levels. Fasting blood glucose levels ≥ 11 mmol/l were determined as abnormal, and individuals with these levels were not permitted to participate in the study. A screening questionnaire was also used to detect individuals with diabetes symptoms. In fact, nobody with diabetes symptoms was detected.
Of the eighty-five women screened, forty-one were excluded: for having a BMI >35 kg/m2 (n 5) or a BMI < 30 kg/m2 (n 30), currently lactating (n 1), receiving antidepressant treatment (n 3) or having a thyroid disorder (n 2). A further three participants withdrew during the baseline week, giving an achieved sample size of forty-one (age 35 (sd 9·1) years). The participants were assigned alternately to two groups after screening. The participants who withdrew were replaced by the next available participant. This method of assignment was used instead of random assignment because recruitment to previous studies was difficult, given the exclusion criteria and duration of the experiment. It was important that early recruiters and later recruiters (who might differ, for instance, in terms of eagerness to volunteer) be assigned evenly to the two groups. As nobody dropped out of the experiment during the intervention phase, an intention-to-treat analysis was unnecessary. The participants were given a disturbance allowance of £100 (£20 per week), to provide a limited, but not compelling, incentive for participation.
Experimental drinks
Each participant was given four 250 ml bottles per d for 4 weeks (daily total 1800 kJ; 105 g CHO). The sucrose-sweetened Irn Bru (per 100 ml) contained 180 kJ, 10·5 g CHO and 0·35 mg Fe with traces of protein and fat. The diet Irn Bru contained 17 kJ/100 ml and traces of CHO, with no protein, fat or Fe. The amount to be consumed was chosen as 1000 ml/d on the basis of the ethical approval process in our earlier studies (Reid et al., 2007( 4 )), as it was found to be the minimum amount to have an effect on dietary intake and have ecological validity without posing ethical and practical difficulties. In 2012, the consumption of carbonated soft drinks amounted to 102·6 litres per head( 10 ), 281 ml/d, in the UK, although some consumers drink substantially more than this( 11 ).
Experimental measures
Physical measures
Digital scales (Soehnle) were used to measure weight to the nearest 0·1 kg, and a stadiometer (model no. 220; SECA) was used to measure height to the nearest 0·1 cm. Using standardised callipers, skinfold thickness was measured around the abdomen (suprailiac) just above the iliac crest. Waist circumference was measured to the nearest 0·1 inch using a standardised measuring tape. Subsequently, the volunteers rested on a bed for 5 min before body measurements were taken using the Bodystat®1500 bioimpedance machine (Bodystat Limited). The bioimpedance machine was used to measure body fat, water and lean mass, BMI, BMR (in kJ), estimated average requirement and impedance. The BMR (at rest) was estimated using the Brozek and Grande formula. This formula is based on the lean weight of a subject and not on total body weight.
Food, mood and activity diary
Each volunteer was given an electronic pedometer (Digi-Walker; Yamax) and instructions on how to use it. Accordingly, the participants were asked to record the number of steps that they walked each day in their diaries before bedtime. For each week, the mean number of total daily steps was calculated. From this number, activity levels were estimated, where < 5000 steps indicate sedentary, 5000–7499 steps low active, 7500–9999 steps somewhat active, and 10 000+ steps active( 12 ). For entry into the simulation model( 13 ), these categories were coded as activity levels 1·4, 1·6, 1·8 and 2·0, respectively.
The 7 d unweighed prospective diary used in the present experiment was similar to that used previously in similar experiments( 4 , 5 ). The participants were required to record all foods and drinks consumed by them as accurately as possible in hourly time slots. To improve accuracy, all the participants were trained in the use of the diary, including the use of appropriate detailed descriptions of foods, using food atlases showing portion sizes for reference( 14 ) and using labelling and packaging information for data entry. Moreover, the experimenter (M. D.) is a trained nutritionist and each week along with the participants, she reviewed and corrected their diaries to maximise accuracy by checking portion sizes, eliciting further details of the foods actually eaten, checking periods when the participants seemed to have eaten little or nothing and checking that they had remembered to record snacks and drinks consumed. To further enhance compliance, the participants were given a brief individual dietary report at the end of the experiment, based on their data. Mood and activity levels were also recorded in the diary. Mood was measured four times a day using ten- or seven-point bipolar Likert scales designed to comprehensively assess the main dimensions of subjective state (happy–sad; angry–calm; anxious–composed; disgusted–satisfied; tired–energetic; restless–relaxed; hungry–full; thirsty–not thirsty; intoxicated–sober; ill–well).
Procedure
Further details of the questionnaire are available elsewhere( 4 ). The eligible volunteers visited the laboratory six times over 5 weeks: during the initial screening interview, at the start of each intervention week and at the end of the last intervention week. During the initial screening interview, height and weight were recorded, and the subjects were screened for eating disorders and depression using standard criteria with the Beck Depression Inventory II( 9 ) and the Eating Disorder Inventory( 15 ).
As well as verbal information, the volunteers were given an information sheet about the experiment. The subjects were told that the purpose of the experiment was to examine the effects of certain nutrients in soft drinks on people's psychological well-being. They were informed that the drinks provided would be similar in taste to commercial soft drinks such as Irn Bru and Tizer, but that the drinks had been prepared especially for the experiment. The drinks were actually Irn Bru in plastic bottles with the replacement labels removed and the caps painted a uniform colour. Although artificially sweetened and sucrose soft drinks taste similar, they are discriminable in simultaneous taste comparisons. The participants were given only one type of drink, and they were instructed not to compare theirs with that of other participants in order to maintain the integrity of the experiment. Most participants were unknown to each other. Those eligible participated after the screening session on the basis of informed consent, which included consent to drinking beverages that may or may not contain sucrose.
A taste test was then conducted with a rating form to exclude anyone who disliked drinks of the type to be given. Biometric baseline measurements, including skinfold thickness, waist circumference and bioelectrical impedance measurements, were taken in those still eligible. They were also given a 7 d food diary and a food atlas to record their dietary intake for the baseline week before the experiment. The experimenter spent approximately 30 min training each subject on the accurate completion of the diary (see below).
After recording baseline data, the subjects returned on the following Monday or Tuesday morning each week to keep the weekend constant within each 7 d test period. During these visits, the diaries of the subjects were checked for any ambiguities or missing data, and if necessary, the subjects were given further training on the completion of the diary. Anthropometric measurements were again taken at each visit together with a saliva swab. The participants were told that the experimenter could tell the extent to which they had been consuming the test drinks from the saliva sample, which was in fact discarded. This is an example of a ‘bogus pipeline’, which improves compliance( 16 ). The participants also discussed any perceived effects of consuming the soft drinks and any difficulties that they had experienced on drinking them on schedule. The participants were then given their supply of drinks for the week and were informed to consume the specified amount each day at 11.00, 14.00, 18.00 and 20.00 hours. The subjects rated their mood directly after consuming the drinks in their 7 d food and mood diaries every day. They were instructed to keep the unopened bottles in a refrigerator or in a cool place.
The same procedure was repeated on the following three Monday or Tuesday mornings. Throughout each 7 d period, the subjects were asked to report to the experimenter any problems or symptoms that they felt might be related to the consumption of the soft drinks. At the end of week 4, the subjects returned one last time to the laboratory, when final measurements were taken and diaries were collected.
At the end of the 5-week experiment, written and verbal dietary feedback was provided and a disturbance allowance was given to those who completed the experiment. The subjects were debriefed about the nature and purpose of the study, including explanation of the deceptions involved.
Statistical analyses
Data collected during baseline (week 0) and weeks 1 and 4 of the intervention were compared; data collected during weeks 2 and 3 were not used, as they assessed neither hypothetical early changes in dietary compensation nor final outcome. Possible differences in baseline anthropometric, dietary and psychological variables between the groups were assessed using independent t tests. Using Shapiro–Wilk tests, all the variables entered into the main analyses were found to be normally distributed. Body weight at the end of the intervention was analysed with linear regression modelling to compare the observed body weight with the predicted body weight (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) model) and intervention group. Differences in dietary variables were examined using general linear modelling with the following design: two between (sucrose or aspartame drink groups) by three repeated (baseline, week 1 and week 4) measures with waist circumference as a covariate. In this design, an effect of sucrose would be shown by a significant week × group interaction.
The assessment of mood data was carried out using a general linear modelling repeated-measures design using time of day and week as repeated measures, with type of drink and expectancy as fixed variables. Where sphericity tests failed, this was noted; otherwise, results are reported by assuming sphericity. Where appropriate, post hoc comparisons were made using t tests. All analyses were carried out using SPSS versions 16 and 19 for Windows (SPSS, Inc., IBM).
Results
Table 1 summarises the selected baseline data for the two groups. Similar to that observed in previous studies, the experimental manipulation had no effect on mood, and mood data are not reported here further. The only significant difference between the groups at baseline was with regard to waist circumference (t(39 df) = 2·53, P< 0·05), so waist circumference was included as a covariate in all ANOVA, but its effects were never significant and are not reported. Table 1 also summarises the reported and predicted energy intake (NIDDK model( 13 )). It can be seen that only a mean of 79 % of the predicted energy intake was reported. Moreover, the mean disparity between the predicted and reported intake was larger (2598 kJ) than the value of the intervention (1800 kJ). However, 19·5 % of the sample reported consuming more than 100 % of the value predicted, so there was no uniform under-reporting.
Table 1.
Selected data at baseline for the experimental and control groups (Mean values and standard deviations)
| Sugar | Aspartame | |||
|---|---|---|---|---|
| Variables | Mean | sd | Mean | sd |
| n | 20 | 21 | ||
| Age (years) | 35·1 | 9·9 | 34·6 | 8·5 |
| BMI (kg/m2) | 32·9 | 1·8 | 32·7 | 2·2 |
| Bodyweight (kg) | 88·4 | 1·5 | 89·2 | 1·5 |
| Waist circumference from skinfold thickness (cm) | 87·9 | 2·8 | 84·9 | 1·8 |
| Waist circumference from tape measurements (cm)* | 94·3 | 1·6 | 87·8 | 2·0 |
| How often did you exercise during the last week? | 4·6 | 3·2 | 3·3 | 2·3 |
| Mean steps per d | 7929·5 | 3649·4 | 8997·3 | 2955·4 |
| Reported energy intake (kJ/d) | 8875·0 | 2639·7 | 8920·8 | 1925·3 |
| Predicted energy intake (kJ/d, Hall et al.'s( 13 ) model) | 11351·1 | 1870·7 | 11635·9 | 1707·9 |
| Reported energy intake as a percentage of the predicted value | 78·7 | 22·2 | 78·9 | 23·5 |
P< 0·05.
Body weight
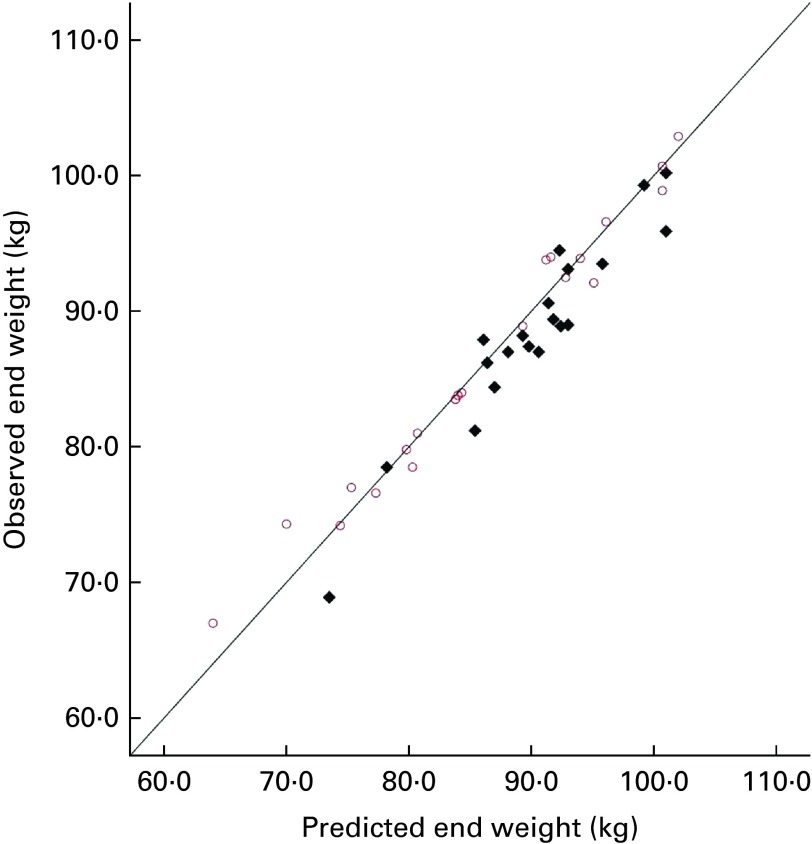
For each participant, the predicted body weight was calculated using the NIDDK model( 13 ), assuming that the sucrose group consumed an additional 1800 kJ/d, with no change in activity levels, and that the aspartame group changed neither their diet nor their activity (i.e. predicted body weight was the same as that at baseline). Fig. 1 shows the plots of the predicted and observed body weight at the end of week 4. In the aspartame group, there was a highly significant correlation between the predicted and observed body weight (r 0·988, P< 0·001). In the sucrose group also, there was a highly significant correlation (r 0·956, P< 0·001), but this was slightly lower than that observed in the aspartame group. It can be seen in Fig. 1 that approximately two-thirds of the sucrose group (14/20) weighed less than the value predicted, but this applied to fewer individuals (n 11/21) of the aspartame group. A linear regression analysis was conducted to predict the observed body weight from the predicted body weight (entered in step 1) and experimental group (entered in step 2). The predicted body weight accounted for 94·3 % of the variance in the observed body weight (F(1,39 df) = 659·80, P< 0·0001), while experimental group accounted for a further 1·1 % of the variance in the observed body weight (F(1,38) = 9·15, P< 0·001). The arithmetic difference between the predicted and observed body weight was calculated, and the sucrose and aspartame groups were compared using a between-group t test. The groups differed significantly (sucrose 1·71 (sd 2·09) kg; aspartame − 0·31 (sd 1·71) kg; t(39 df) = 3·40, P< 0·005), confirming that the observed weight of the aspartame group was very close to the predicted (baseline) value. The differences between the baseline weight and the observed end weight were compared for the aspartame group using a paired t test, confirming that there was no significant change in weight (t(20 df) = 0·842, P= 0·41), so they had not reduced voluntary food intake because they expected to receive sugars.
Fig. 1.
Predicted body weight (National Institute of Diabetes and Digestive
and Kidney Diseases (NIDDK) model) and observed weight at the end of
the experiment. Drinks given over 28 d:  , sucrose;
, sucrose;  , aspartame. (A
colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, aspartame. (A
colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
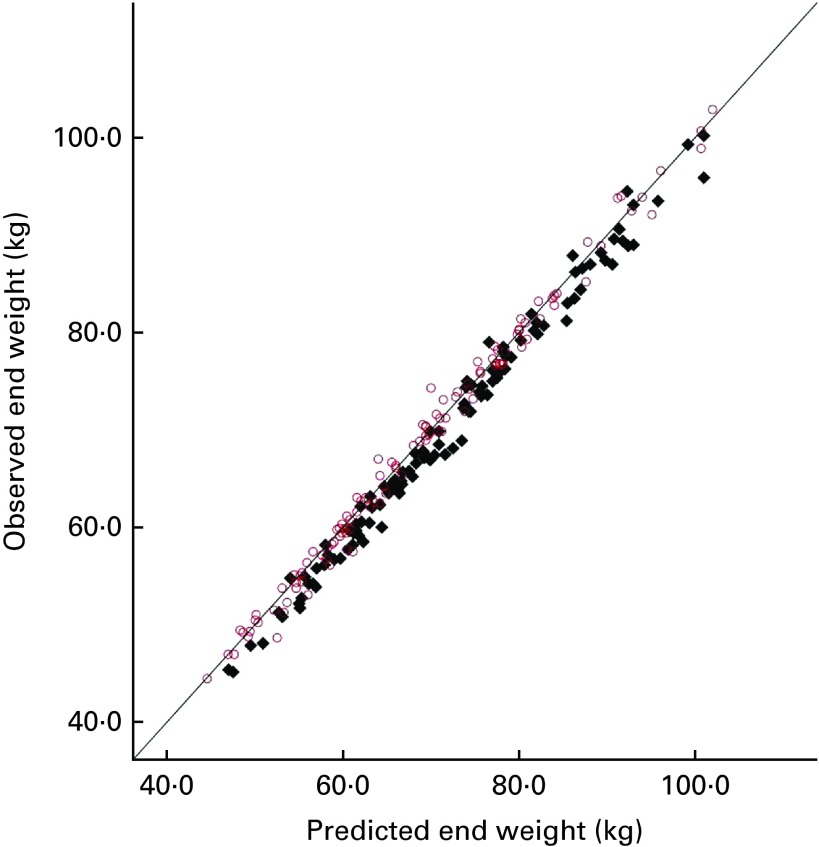
Additionally, the data on the predicted and observed weight at the end of the intervention were compared with the data obtained from the two earlier experiments carried out in normal-weight and overweight women( 4 , 5 ), as shown in Fig. 2. It can be seen that across all the three experiments, the observed weight tended to be lower than the predicted weight for women given sucrose, indicating that partial compensation had occurred.
Fig. 2.
Predicted body weight (National Institute of Diabetes and Digestive
and Kidney Diseases (NIDDK) model) and observed weight at the end of
the three experiments with healthy-weight, overweight and obese
participants. Drinks given over 28 d:  , sucrose;
, sucrose;  , aspartame. (A
colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
, aspartame. (A
colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
A linear regression analysis was conducted to predict the observed body weight from the predicted body weight (entered in step 1), experimental group (entered in step 2) and study (entered in step 3). For this analysis, study was dummy-coded into two binary variables of healthy weight/not healthy weight and obese/not obese. The predicted body weight indicated 98·6 % of the variance in the observed body weight (F(1,225) = 16 416·87, P< 0·0001). Experimental group predicted only a further 0·4 % of the variance, but this was significant (F(1,224) = 81·40, P< 0·0001). Study had a further significant effect (F(1,222) = 3·77, P< 0·05), but predicted less than 0·1 % of the variance. Fig. 2 shows that there is no clear trend for compensation to vary as a function of initial weight. Looking at the means for women who were given sucrose in the three experiments, healthy-weight women compensated by 1·87 (sd 0·13) kg that they did not gain and overweight women compensated by 1·32 (sd 0·27) kg, while obese women compensated by 1·72 (sd 0·47) kg. So, overweight women compensated least well, but the standard error for obese women was approximately twice that for the other participants, showing more variability. To address the question of whether weight change after sucrose consumption differed between the groups, a one-way ANOVA on weight change (observed end weight − baseline weight) was conducted only for the participants who were given sucrose, with study (healthy, overweight and obese) as the independent variable. There was no effect of study (F(2,109) = 1·46, P= 0·236).
On comparing the difference between the predicted and observed body weight, as discussed above, it was found that the observed body weight of the sucrose group differed significantly from the predicted value (sucrose 1·72 (sd 1·37); aspartame 0·16 (sd 1·27); t(225 df) = 8·93, P< 0·0001). As in the present experiment, combining all three experiments the aspartame group's weight had not changed since baseline by paired t test (t(114 df) = − 1·373, P= 0·17).
Dietary intake data
Table 2 summaries the mean reported daily macronutrient intake over the course of the experiment. Data are reported as g/d and include the supplementary drinks. Analyses were conducted by including the supplements to demonstrate that the participants had consumed the drinks and by excluding the supplements to ascertain the extent of compensation for the supplements elsewhere in the diet.
Table 2.
Reported macronutrient composition of the diet over the course of the experiment (data include the supplementary drinks) (Mean values and standard deviations)
| Sugar | Aspartame | |||
|---|---|---|---|---|
| Mean | sd | Mean | sd | |
| Baseline | ||||
| Reported energy intake (kJ/d) | 8875·0 | 2639·7 | 8920·8 | 1925·3 |
| Carbohydrate (g/d) | 261·7 | 70·4 | 259·5 | 82·8 |
| Sugars (g/d) | 106·8 | 47·8 | 100·8 | 41·1 |
| Fat (g/d) | 83·6 | 34·9 | 80·3 | 20·2 |
| Protein (g/d) | 74·0 | 22·2 | 77·2 | 16·1 |
| Week 1 | ||||
| Reported energy intake (kJ/d) | 9908·4 | 2095·0 | 8610·0 | 9243·4 |
| Carbohydrate (g/d) | 335·0 | 67·0 | 249·5 | 72·0 |
| Sugars (g/d) | 199·5 | 47·3 | 101·9 | 41·4 |
| Fat (g/d) | 75·0 | 23·4 | 76·6 | 21·4 |
| Protein (g/d) | 72·7 | 16·3 | 74·7 | 20·5 |
| Week 4 | ||||
| Reported energy intake (kJ/d) | 9091·3 | 1454·1 | 7996·0 | 1930·3 |
| Carbohydrate (g/d) | 307·8 | 52·3 | 224·1 | 61·0 |
| Sugars (g/d) | 177·5 | 29·2 | 88·4 | 44·0 |
| Fat (g/d) | 69·8 | 16·0 | 73·4 | 24·7 |
| Protein (g/d) | 64·9 | 14·3 | 67·8 | 17·8 |
Dietary intake including the supplement
The mean reported daily dietary energy intake did not change over the weeks (F(2,76) = 1·15, P= 0·32) and there was no group × week interaction (F(2,76) = 2·49, P= 0·09), indicating that the supplementary drinks did not significantly affect the reported energy intake.
For grams of total sugars ingested, there was no main effect of week (F(2,76) = 1·5, P= 0·23), but there was a large week × group interaction (F(2,76) = 23·6, P< 0·0001), indicating that, as one would expect, the supplementary sucrose drinks increased sugar intake. In subsequent analyses, the effects of the drink supplements on voluntary intake excluding the supplements were examined more thoroughly.
Voluntary carbohydrate intake excluding the supplement
Because the supplements contained sucrose, voluntary total sugar intake and voluntary starch intake were analysed separately. For the percentage of energy obtained from total sugars, there were no main effects of group (F(1,38) = 2·94, P= 0·09) or week (F(2,76) = 0·36, P= 0·70), but there was a significant week × group interaction (F(2,76) = 4·87, P< 0·05). For the percentage of energy obtained from starch, sphericity could not be assumed (W(2 df) = 0·60, P< 0·001). Similar to that observed for total sugars, there were no main effects of group (F(1,38) = 0·84, P= 0·37) or week (F(2,76) = 2·94, P= 0·07), but, again, there was a significant week × group interaction (F(2,76) = 6·03, P< 0·01).
Because the effects were similar for sugars and starches, post hoc analyses were used to examine the voluntary intake of CHO (sugars plus starches): participants given the sucrose supplement significantly decreased their voluntary intake of CHO by 23 % (mean reduction 31 g CHO, 29 % of the supplement, range 129 g reduction–208 g increase) at week 1 (t(19 df) = 5·77, P< 0·001) and by 27 % (mean reduction 59 g, 56 % of the supplement, range 208 g reduction–88 g increase) at week 4 (t(19 df) = 14·30, P< 0·001) compared with that at baseline. In the aspartame group, there was a 4 % decrease (non-significant) in CHO intake at week 1 and an 18 % decrease at week 4 (t(20 df) = 19·98, P< 0·001) compared with that at baseline.
Protein intake
For the percentage of energy obtained from protein, there was a main effect of group (F(1,38) = 5·69, P< 0·05), but no effect of week (F(2,76) = 0·49, P= 0·61) and no interaction (F(2,76) = 2·43, P= 0·10). Using independent groups t tests, it was found that the groups did not differ in the percentage of energy obtained from protein at baseline (t(39 df) = 0·65, P= 0·52), but that they differed at week 1 (t(39 df) = 2·61, P< 0·05) and week 4 (t(39 df) = 3·38, P< 0·005). Using paired t tests, it was found that in the sucrose group, the percentage of energy obtained from protein at week 1 (t(19 df) = 2·37, P< 0·05) and week 4 (t(19 df) = 3·76, P< 0·001) was reduced compared with that at baseline. This was not the case in the aspartame group.
Fat intake
The percentage of energy obtained from fat did not vary by week (F(2,76) = 1·81, P= 0·17), but there was a small effect of group (F(1,38) = 5·97, P< 0·05), because the percentage of energy obtained from fat was reduced in the sucrose group. There was no week × group interaction (F(2,76) = 1·97, P= 0·15).
Hunger and thirst
Hunger and thirst were rated four times daily as part of the mood rating procedure. Because of substantial individual differences in scale use, ratings were converted to z-scores, using the grand mean of all ratings for each subject, and then the means of four ratings across 7 d were computed across the baseline week and week 4. Paired t tests were carried out separately for each drink, comparing baseline hunger and thirst with hunger and thirst after 4 weeks of the experiment. There were no differences in the rated hunger or thirst.
Discussion
The present experiment carried out in obese women replicates earlier findings on normal-weight and overweight women; however, the use of the NIDDK model( 13 ) allowed more precise estimation of the magnitude of compensation: obese women who were given 1800 kJ sucrose per d in soft drinks for 4 weeks gained a mean of 1·72 kg less than the value predicted by the model. Weight did not change any more than that observed in previous studies with normal-weight and overweight participants. The reported energy intake, including the supplement, did not change. The reported voluntary intake of CHO of the participants was reduced to as much as 27 % of the energy value of the supplement by week 4 of the intervention. The reported fat and protein intake was also reduced. There were no effects on mood, hunger or thirst. There was no significant weight change in the group given aspartame, suggesting that any deliberate attempts to refrain from consuming the diet in case the drinks contained sugar were ineffective.
Study limitations
The present study has at least four limitations. First, dietary intake recorded in the unweighed diaries was poorly related to the energy expenditure estimated using the NIDDK model( 13 ), suggesting about 21 % under-reporting. Consequently, it is important to avoid overinterpreting the self-reported dietary intake data. Second, there were some differences between the results of the present experiment and those of earlier experiments( 4 , 5 ), with the reported compensation being smaller and individual differences being larger. The participants may have altered their behaviour in response to the experimental intervention. Self-monitoring is important for the weight control of obese people( 17 ), and taking part in a study monitoring dietary behaviour may have had a generic influence on behaviour. Thus, the present experiment does not show that obese people compensate identically to healthy-weight people.
Third, the participants may simply have avoided drinking some or all the supplementary drinks, despite regular monitoring and the bogus pipeline. However, the fact that overall CHO intake increased only in the sucrose group suggests adequate compliance. Lastly, sweetness may have reduced energy intake in both conditions. However, while there is some evidence that sweetness increases short-term satiety( 18 ), the limited evidence suggests that, if anything, in the long term the consumption of sweet drinks leads to weight gain( 19 ), which was not the case in the present study for either the sucrose or the aspartame group.
Generalisability
The findings of the present study apply only to those experiments where sucrose is added to the diet blind, with the diet being monitored. If some participants were restraining voluntary consumption because their diets were being monitored, then liberal use of sucrose in the unmonitored diet might not be compensated for so well. The soft drinks used in the present experiment were sucrose sweetened as is common in the UK, rather than being sweetened with high-fructose maize syrup, as is common in the USA. Specific concerns about fructose and obesity remain, although they may have been overstated( 20 ).
Implications
Generally, obese women were able to compensate for the addition of sucrose to the diet by mainly reducing CHO intake. Although these effects were relatively small and the experiment is limited in other ways, the present study suggests that the cognitive control of eating in obesity is important. Expectancy effects may play a role in failures to compensate for sucrose, hence in any consequent weight gain.
Partially controlling psychological expectancy effects, by giving sucrose blind, reduces the chances of people being affected by cognitive factors, which may be important for determining the effects of sugars in everyday life. The accepted cognitive factors include the emotional valence of food( 21 ), which may be associated with eating binges; interactions between expectancy, orosensory experience, and satiety( 2 , 3 ); the abstinence violation effect( 6 ) that having eaten a restricted food, one may as well eat more; outcome expectancy effects( 22 ), so that people's behaviour after eating sugars is in accordance with the expectation that it leads to sugar craving and more consumption; and experimental (or social) demand characteristics( 23 ), so that participants tend to behave as the experiment (or social setting) suggests that they should behave.
If under controlled conditions people partially compensate for sucrose added to the diet, then there is a need for more research on what people think and what they feel about sugars, and it is premature to consider sucrose to be exceptionally problematic for weight management. Indeed, widely publicised warnings about sugars( 24 , 25 ) may make cognitive factors more likely to support dysregulation of energy intake, perpetuating the everyday weight management problems that the warnings are supposed to address.
Conclusions
The present study demonstrates that obese women partially compensate for sucrose added blind to the diet over 4 weeks and do not gain weight. Their response is not fundamentally different from the response of normal-weight and overweight women observed in earlier studies, despite concerns that obese people regulate diet differently, or more poorly, compared with normal-weight people. If cognitive factors are important determinants of dietary compensation, then there is a need for an improved understanding of how cognition interacts with physiological mechanisms of appetite regulation.
Acknowledgements
The authors thank an anonymous reviewer for helpful suggestions, including directing them to use the NIDDK model.
The present study was funded by the Biotechnology and Biological Sciences Research Council (BBSRC, grant no. D12497) and Sugar Nutrition UK (formerly the Sugar Bureau). Neither BBSRC nor Sugar Nutrition UK had any role in the design, analysis and interpretation of the study or in the writing of the paper.
The authors' contributions are as follows: M. R. oversaw the conduct of the experiment, addressed any issues arising with regard to clinical concerns and assisted with the writing of the paper; R. H. oversaw the initial statistical analyses, conducted additional analyses, including those using the NIDDK model, and assisted with the writing of the paper; M. D. conducted the experiment, coded the nutritional data and conducted the preliminary statistical analyses; C. B. carried out further statistical analyses and assisted with the writing of the paper.
None of the authors has any conflicts of interest.
Abbreviations: CHO, carbohydrate; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases
References
- 1.Morenga LT, Mallard S & Mann J (2013) Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. Br Med J 346, e7492. [DOI] [PubMed] [Google Scholar]
- 2.Houchins JA, Burgess JR, Campbell WW, et al. (2012) Beverage vs. solid fruits and vegetables: effects on energy intake and body weight. Obesity 20, 1844–1850 [DOI] [PubMed] [Google Scholar]
- 3.Cassady BA, Considine RV & Mattes RD (2012) Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr 95, 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid M, Hammersley R, Hill AJ, et al. (2007) Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr 97, 193–203 [DOI] [PubMed] [Google Scholar]
- 5.Reid M, Hammersley R & Duffy M (2010) Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite 55, 130–136 [DOI] [PubMed] [Google Scholar]
- 6.Johnson F, Pratt M & Wardle J (2012) Dietary restraint and self-regulation in eating behavior. Int J Obes 36, 665–674 [DOI] [PubMed] [Google Scholar]
- 7.Carels R, Cacciapaglia H, Rydin S, et al. (2006) Can social desirability interfere with success in a behavioral weight loss program? Psychol Health 21, 65–78 [Google Scholar]
- 8.Prentice AM, Black AE, Murgatroyd PR, et al. (1989) Metabolism or appetite questions of energy balance with particular reference to obesity. J Hum Nutr Diet 2, 95–104 [Google Scholar]
- 9.Beck A (1996) Beck Depression Inventory II. San Antiono, TX: Harcourt Assessment Inc [Google Scholar]
- 10.British Soft Drinks Association (2013) Refreshing the Nation: The 2013 UK Soft Drinks Report. London: British Soft Drinks Association [Google Scholar]
- 11.Duffey KJ, Huybrechts I, Mouratidou T, et al. (2012) Beverage consumption among European adolescents in the HELENA study. Eur J Clin Nutr 66, 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tudor-Locke C & Bassett D (2004) How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 34, 1–8 [DOI] [PubMed] [Google Scholar]
- 13.Hall KD, Sacks G, Chandramohan D, et al. (2011) Obesity 3. Quantification of the effect of energy imbalance on bodyweight. Lancet 378, 826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson M, Atkinson M & Meyer JA (1997) Photographic Atlas of Food Portion Sizes. London: Ministry of Agriculture, Fisheries and Food [Google Scholar]
- 15.Brown I, Thompson J, Tod A, et al. (2006) Primary care support for tackling obesity: a qualitative study of the perceptions of obese patients. Br J Gen Pract 56, 666–672 [PMC free article] [PubMed] [Google Scholar]
- 16.Roese NJ & Jamieson DW (1993) 20 years of bogus pipeline research – a critical-review and meta-analysis. Psychol Bull, 114, 363–375 [Google Scholar]
- 17.Carels RA, Darby LA, Rydin S, et al. (2005) The relationship between self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Ann Behav Med 30, 182–190 [DOI] [PubMed] [Google Scholar]
- 18.Carroll NC & Young AW (2005) Priming of emotion recognition. Q J Exp Psychol Sect A Hum Exp Psychol 58, 1173–1197 [DOI] [PubMed] [Google Scholar]
- 19.Dennis EA, Flack KD & Davy BM (2009) Beverage consumption and adult weight management: a review. Eat Behav 10, 237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan LC, Potter SM & Burdock GA (2010) Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr 50, 889–918 [DOI] [PubMed] [Google Scholar]
- 21.Brown MA, Goldstein-Shirley J, Robinson J, et al. (2001) The effects of a multi-modal intervention trial of light, exercise, and vitamins on women's mood. Women Health 34, 93–112 [DOI] [PubMed] [Google Scholar]
- 22.Reid M, Bunting J & Hammersley R (2005) Relationships between the food expectancy questionnaire (FEQ) and the food frequency questionnaire (FFQ). Appetite 45, 127–136 [DOI] [PubMed] [Google Scholar]
- 23.Davies JB (1997) The Myth of Addiction. London: Psychology Press [Google Scholar]
- 24.Lustig RH, Schmidt LA & Brindis CD (2012) The toxic truth about sugar. Nature 482, 27–29 [DOI] [PubMed] [Google Scholar]
- 25.Dufty WF (1975) Sugar Blues. Radnor, PA: Chilton [Google Scholar]