Abstract
A recent global gene expression profiling study unexpectedly showed that activated oncogenic NRAS may recruit neural precursor cell expressed, developmentally downregulated 4L (Nedd4L; a human homologue of Nedd4-2) in cultured melanoma cells. However, whether Nedd4L was expressed in melanoma tissues or participated in melanoma carcinogenesis remains to be clarified. Here, we investigated the expression status of Nedd4L in human melanocytes, benign nevi and melanoma tissue specimens and subsequently attempted to determine the role of Nedd4L in melanoma cell growth. Immunohistochemical staining revealed that Nedd4L was not present in any non-tumorous melanocytes or in 18 benign nevi tissues, but it was detected in 34 of 79 cutaneous melanomas and 9 of 32 nodal metastatic melanomas. Downregulation of Nedd4L significantly reduced the growth of cultured G361 melanoma cells in vitro. Moreover, exogenous Nedd4L expression significantly promoted the growth of A2058 melanoma cells in vivo in a xenograft assay. The present findings indicate that Nedd4L expression may be increased to facilitate tumour growth in many melanomas.
Keywords: melanoma, Nedd4L, ubiquitin ligase, ubiquitin pathway
The BRAF kinase inhibitor has greatly improved the short-term prospects of patients with melanoma harbouring the BRAF V600E mutation; however, yet NRAS-mutant melanoma remains without an effective therapy (Chapman et ;al. 2011). Moreover, BRAF(V600E)-positive melanomas drug resistance frequently develops after initial responses (Flaherty et ;al. 2010), possibly via the activation of the RAS-mediated pathway (Nazarian et ;al. 2010). Oncogenic NRAS is found in up to 20% of cutaneous melanomas and is believed to participate in melanoma carcinogenesis by interacting with various cancer-associated molecules (van ‘t Veer et ;al. 1989). Although the suppression of oncogenic NRAS using small interfering RNA (siRNA) impairs the proliferation of human melanoma cells in vitro (Eskandarpour et ;al. 2005), the direct pharmacological inhibition of the RAS proto-oncogenes has thus far been unsuccessful because of their structural and biochemical properties (Ward et ;al. 2012). Therefore, approaches to molecular targeting which can mimic the RAS pathway inhibition are valuable.
Notably, a recent meta-analysis using global gene expression profiling unravelled that the suppression of oncogenic NRAS (NRASQ61R) significantly reduced the expression of a neural precursor cell expressed, developmentally downregulated 4L (Nedd4L) in cultured melanoma cells (Eskandarpour et ;al. 2009). This finding suggests that oncogenic NRAS may recruit Nedd4L in melanoma carcinogenesis.
Nedd4L belongs to the homologous to the E6-AP carboxyl terminus (HECT) family of ubiquitin ligases. Nedd4L is well known to target epithelial Na+ channel (ENaC) for proteasomal degradation (Harvey et ;al. 2001). Furthermore, recent studies show that Nedd4L targets a broad range of molecules as well as ENaC to mediate various biological functions (Persaud et ;al. 2009). Both our own studies and other groups have documented that Nedd4L is markedly overexpressed in several human malignant tumours, such as Sézary syndrome (Booken et ;al. 2008), gallbladder cancer (Takeuchi et ;al. 2011a,b) and prostate cancer (Hellwinkel et ;al. 2011). Therefore, it is believed that Nedd4L expression and regulation play a key role in the pathogenesis of several tumours (Kapoor et ;al. 2012).
Although previous studies showed that Nedd4L was expressed in several cultured melanoma cells (Eskandarpour et ;al. 2009), to the best of our knowledge, whether Nedd4L is overexpressed in melanoma tissues or the expression of Nedd4L is associated with melanoma carcinogenesis has not been clarified previously. The present study aimed to examine the expression of Nedd4L in non-tumourous melanocytes, benign nevi and melanoma tissues and subsequently to explore the role of Nedd4L in melanoma cell growth.
Materials and methods
Tissue specimens and immunohistochemical staining
Immunohistochemical detection of the Nedd4L antigen was performed using tissue microarrays (US Biomax, Rockville, MD, USA). All the tissues were collected according to the ethical standards and protocols approved by the Institutional Review Board and Health Insurance Portability and Accountability Act. Written informed consent was obtained from all the donors. All the specimens were obtained surgically, fixed in 10% buffered formalin, and embedded in paraffin. Tissues were immunostained with antibodies using the ImmPRESS™ polymerized reporter enzyme staining system (Vector laboratories, Inc., Burlingame, CA, USA), as previously reported (Takeuchi et ;al. 2000). The antibody specific to Nedd4L was purchased from ProteinTech Group (Chicago, IL, USA).
Cell culture and siRNA silencing
A2058, G361 and Mewo melanoma cells were obtained from the Japan Health Science Research Resources Bank (Osaka, Japan). Colo679 cells were obtained from the RIKEN Biosource Center (Tsukuba, Japan). Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco BRL–Life Technologies, Grand Island, NY, USA) containing 10% heat-inactivated foetal bovine serum (FBS).
A detailed procedure for siRNA silencing of target genes using Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA) has been described previously (Takeuchi et ;al. 2012). We used the siRNA duplex 5′-AACCACAACACAAAGUCACAG-3′ (Snyder et ;al. 2004) and Silencer Select predesigned and validated siRNA (Ambion, Austin, TX, USA) for silencing the Nedd4L gene and the GFP siRNA duplex, target sequence 5′-CGGCAAGCUGACCCUGAAGUUCAU-3′ as a non-silencing control. Cells were used for subsequent studies 48 ;h after transfection.
Reverse transcriptase–polymerase chain reaction, plasmids and transfection
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed as previously described (Takeuchi et ;al. 2012). Briefly, total cellular RNA was prepared from cell lysates using RNA-zol B (Biotex Laboratory, Houston, TX, USA). cDNA synthesis from total RNA and subsequent PCR were performed using an RNA LA (long and accurate) PCR kit (Takara Bio Inc.). The procedure was performed according to the manufacturer's protocol. The primer sets used in this study were sense 5′-CAGTGGAGATTTGTGAACAGGG-3′ and antisense 5′-CTAGAATCCACCCCTTCAAATCCTTG-3′ for Nedd4L, and sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR-amplified products were electrophoretically separated on a 2% agarose gel.
For stable expression of Nedd4L in A2058 cells, a previously described Nedd4L-expression vector (Takeuchi et ;al. 2011a,b) was modified to express the product of the neomycin-resistant gene and transfected into cells using N-[1-(2,3-dioleoyloxy) propyl]-N,N,N-trimethylammonium methylsulphate (DOTAP) transfection reagents (Boehringer Mannheim, Indianapolis, IN, USA) according to the manufacturer's protocol. Briefly, A2058 cells were seeded at approximately 50% confluency in 10-cm culture dishes. On the next day, the culture medium was replaced with 5 ;ml of Opti-MEM1 medium (Invitrogen). We added to the medium 5 ;mg of plasmid with a Nedd4L expression vector linearized using BsaB1or a control empty vector incubated with DOTAP-reagent. Colonies resistant to G418 (Gibco BRL; final concentration, 600 ;μg/ml) were selected after 4 ;weeks and subcultured as described previously (Takeuchi et ;al. 2012). Independent clones that expressed Nedd4L were screened using immunoblotting.
Immunoblotting
Immunoblotting was performed according to the modified method of Towbin et ;al. (Towbin et ;al. 1979), as previously reported (Takeuchi et ;al. 2012). Briefly, proteins were electrophoresed in sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gels and electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). After blocking in bovine serum albumin (BSA), the membranes were incubated with antibodies. To evaluate the protein loading, anti-β-actin antibody (Sigma-Aldrich) was used as control.
Immunocytostaining
Immunocytostaining was performed by indirect immunofluorescent staining. Briefly, cells grown on tissue culture chamber slides (Lab TekII Chamber Slide; Nalge Nunc International, Rochester, NY, USA) were fixed in methanol and permeabilized with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 for 5 ;min at room temperature and then were placed in a blocking buffer (1% BSA in PBS) for 20 ;min. Cells were stained by indirect immunofluorescence with rabbit anti-Nedd4L or control rabbit antibody and subsequently with Alexa Fluo 488-conjugated anti-rabbit IgG, F(ab′)2 Fragment (Cell Signaling Technology Inc, Beverly, MA, USA).
Cell proliferation assay
Cell proliferation was evaluated, as previously described, by counting the number of viable cells (Takeuchi et ;al. 2000). Briefly, 5 ;× ;104 cells were cultured on standard 35-mm tissue culture dishes (BD Falcon, San Jose, CA, USA). Viable cells were counted after 24 and 48 ;h. The assay was performed in triplicate and repeated twice. Statistical analysis was performed using the Student's t-test for unpaired observations. Findings of P ;< ;0.05 were considered significant.
Xenograft of A2058 cells in BALB/c nude mice
Nedd4L-expressing or control A2058 melanoma cells (5 ;× ;105) were subcutaneously injected into the dorsal flank of 8-week-old BALB/c nude mice (Charles River Laboratories, Sizuoka, Japan). The detailed procedure of the xenograft assay is described previously (Takeuchi et ;al. 2011a,b). The length and width of the tumours were measured using a caliper with a precision of 1.0 ;mm. The tumour volume was calculated using the following formula: volume ;=(d1 ;× ;d2 ;× ;d3) ;× ;0.5236, where ‘d(no.)’ represents the three orthogonal diameter measurements. The experimental protocol was approved by the Animal Care Committee of Kochi Medical School, Kochi, Japan. The statistical analysis was performed using the Student's t-test for unpaired observations. Values with P ;< ;0.05 were considered significant.
Results
Nedd4L is expressed in many cutaneous invasive and nodal metastatic melanoma cells but not in non-tumourous melanocytes or benign nevus cells
A representative immunostaining result is shown in Figure ;1. The 18 benign nevus cells or non-tumourous epidermal melanocytes from several intradermal nevus tissue specimens showed minimal or no immunoreactivity against the specific anti-Nedd4L antibody. In contrast, Nedd4L immunoreactivity was observed in 34 of 79 cutaneous melanomas. The most common melanomas are generally thought to arise from the de novo proliferation of basal epidermal melanocytes. During the course of the malignant transformation, various molecular alterations occur in the melanocytes. Taken together, the present findings indicate that Nedd4L expression may be increased in melanoma cells during the malignant transformation.
Figure 1.
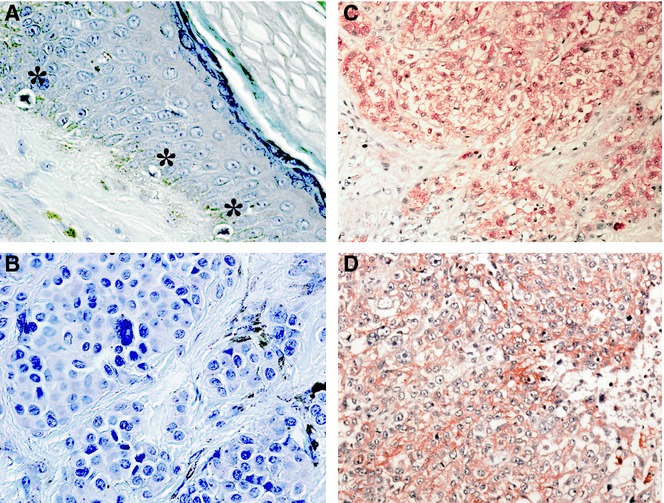
Overexpression of Nedd4L in melanoma tissues. The immunohistochemical staining showed that the immunoreactivity of Nedd4L was minimal or none in non-tumourous melanocytes or in benign nevus cells. In contrast, immunoreactivity of Nedd4L was observed in the cytoplasm of 34 of 79 cutaneous melanomas and 9 of 32 nodal metastatic melanomas. The figure shows representative stainings. (A) Non-tumourous melanocytes indicated by asterisk (*) were not stained. (B) No significant Nedd4L immunoreactivity was observed in benign nevus. (C and D) Representative Nedd4L staining in cutaneous melanoma (C) and nodal metastatic melanoma (D).
Downregulation of Nedd4L significantly impaired the melanoma cell growth
Immunoblotting showed that all the melanoma cell lines examined, except A2058, expressed Nedd4L at various degrees. We found an intense Nedd4L band in G361 cells and a significant Nedd4L band in Colo679 and Mewo cells but a weak band in A2058 cells (Figure ;2A).
Figure 2.
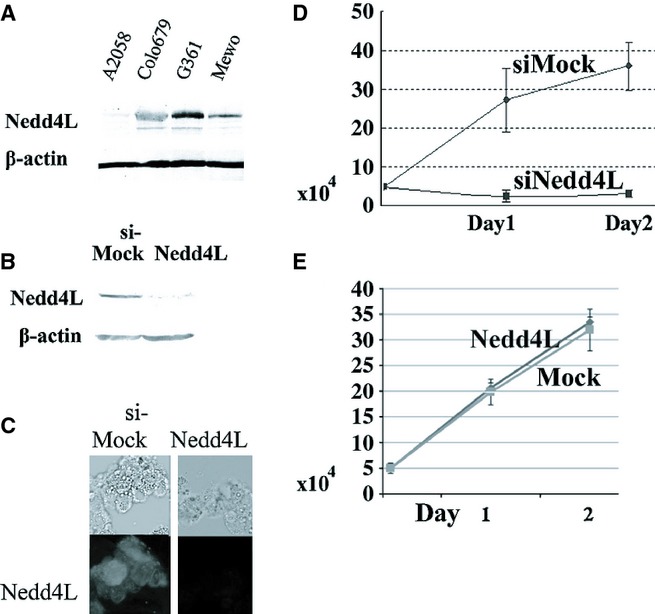
Downregulation of Nedd4L impaired melanoma cell growth in vitro. (A) Nedd4L expression was observed in human melanoma cell lines, Colo679, G361 and Mewo; however, the expression of Nedd4L was weak in the human A2058 cell line. To confirm an equal protein loading, the membrane was incubated with anti-β-actin antibody (Sigma, St. Louis, MO, USA). (B and C) Nedd4L protein expression was reduced after siRNA-mediated silencing of the Nedd4L gene but not after control siRNA treatment in G361 cells (B: Immunoblotting: C: Immunocytostaining). (D) Nedd4L silencing significantly inhibited the growth of G361 cells. We cultured 5 ;× ;104 cells 48 ;h after siRNA treatment on standard 35-mm tissue culture dishes in triplicate and further cultured them for 24 and 48 ;h. Cell proliferation was evaluated by counting the number of viable cells. The assay was performed in triplicate and repeated twice. Statistical analysis was performed using Student's t-test for unpaired observations. Findings of P ;< ;0.05 were considered significant. Data are represented as mean ;± ;standard error of mean (SEM) from three independent experiments, each run in triplicate. (E) Exogenous Nedd4L expression did not alter the growth of A2018 cells. Clones of A2058 cells exogenously expressing Nedd4L (indicated as Nedd4L), and control cells not expressing Nedd4L (indicated as Mock) were established by transfection with an expression vector, which contained the entire coding region of Nedd4L cDNA, or a vector alone. Notably, this representative cell clone was used for xenograft in Figure ;3. Expression status of Nedd4L in transfectants is shown in Figure ;3.
A successful downregulation of the Nedd4L protein was achieved in G361 cells by siRNA-mediated gene silencing (Figure ;2B and C). Interestingly, the downregulation of Nedd4L significantly reduced the growth of G361 cells (Figure ;2D). This finding indicates that Nedd4L plays an important role in the melanoma cell growth in vitro. By contrast, enforced Nedd4L expression did not alter the growth of A2018 cells in vitro. A representative result using three independent clones is shown. See the result of xenograft assay using the same clone in Figure ;3.
Figure 3.
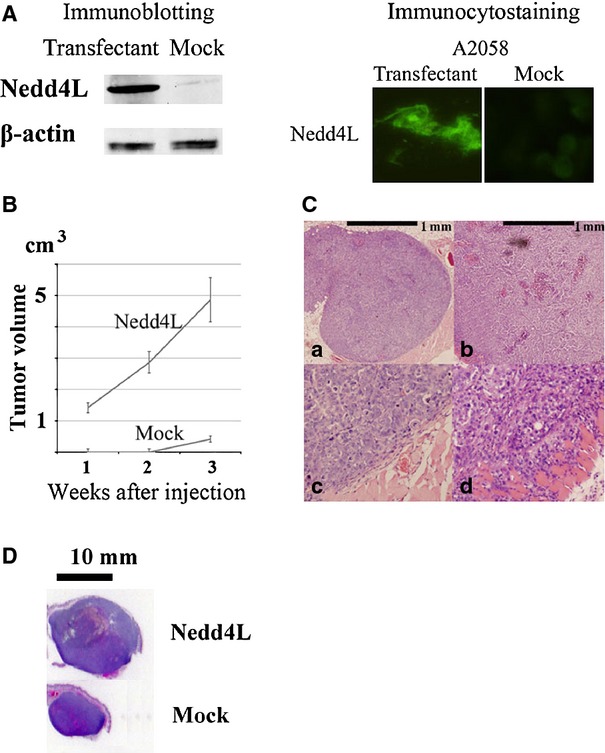
Nedd4L expression accelerated melanoma cell growth in vivo. (A) Representative data of immunoblotting and immunocytostaining of A2058 melanoma cells showing the exogenous expression of Nedd4L. Original A2058 cells expressed less amount of Nedd4L protein (Figure ;2A). Clones of A2058 cells exogenously expressing Nedd4L and control cells not expressing Nedd4L were established by transfection with an expression vector, which contained the entire coding region of Nedd4L cDNA, or a vector alone. (A) representative result from three independent immunoblotting experiments using different transfectant clones is shown. (B) We subcutaneously injected 5 ;× ;105 cells into nude mice. Nedd4L-expressing A2058 cells formed a visible tumour in all the five transplanted BALB/c nude mice after 1 ;week of inoculation (indicated as Nedd4L). By contrast, control A2058 cells formed a visible tumour in none of the five transplanted BALB/c nude mice after 1 ;week or 2 ;weeks of inoculation (indicated as Mock). The tumour volume of each mouse was calculated every week using the formula: volume ;= ;(d1 ;× ;d2 ;× ;d3) ;× ;0.5236, where dn represents the three orthogonal diameter measurements. Representative data are demonstrated as mean ;± ;standard error of the mean (SEM). Similar results were obtained from another independent experiment using different transfectants. (C) Representative histological features of xenograft specimens. In total, 5 ;× ;105 cells were subcutaneously injected into nude mice. Haematoxylin-eosin-stained tissue specimens of transplanted tumours harbouring non-Nedd4L-expressing A2058 cells (a and c) and Nedd4L-expressing A2058 cells (b and d) at 3 ;weeks are shown. Note the rounded tumour border of the non-Nedd4L-expressing A2058 cells with a thin fibrous capsule (c). By contrast, Nedd4L-expressing A2058 tumour exhibited deep invasion with destruction of the muscle layer (d). (D) Whole tumour silhouette of the representative transplanted Nedd4L-expressing A2058 cells (indicated as Nedd4L) and control non-Nedd4L-expressing cells (indicated as Mock) after 3 ;weeks of inoculation to compare the tumour size at glance.
Exogenous Nedd4L expression increased the melanoma cell growth in vivo
Subsequently, we determined whether exogenous Nedd4L expression could support the melanoma cell growth in a xenograft assay. For this study, we transfected A2058 melanoma cells, which expressed little Nedd4L, with a Nedd4L expression vector and obtained three Nedd4L-expressing transfectant clones. The present xenograft assay demonstrated that the enforced Nedd4L expression facilitated A2058 melanoma cell growth in BALB/c nude mice. Representative data are shown in Figure ;3. We think that the overexpression of Nedd4L might support melanoma cell growth in vivo.
Discussion
Melanoma is a cancer of the skin that has high mortality rates and an increasing incidence (Markovic et ;al. 2007; Erdei & Torres 2010; Godar 2011). Despite the recent advancements in melanoma treatment including the application of potent inhibitors of the BRAF proto-oncogene, the long-term prognosis of advanced melanoma remains dismal (Flaherty et ;al. 2010). Thus, studies to develop new therapeutic approaches are important.
Here, we found that Nedd4L shows immunoreactivity in many melanoma cells. By contrast, non-tumourous melanocytes did not show detectable levels of Nedd4L immunoreactivity (Figure ;1). Our findings indicate that the expression of Nedd4L might increase during the neoplastic transformation of melanocytes. Subsequently, we found that the siRNA-mediated downregulation of Nedd4L significantly decreased tumour cell growth in vitro (Figure ;2). This finding indicated that Nedd4L expression may have a direct effect on melanoma cell growth. Introduction of exogenous Nedd4L expression did not alter the A2058 melanoma cell growth in vitro (Figure ;2E), but it supported A2058 melanoma cell growth in a xenograft assay (Figure ;3). We think that robust growth of A2058 cells might shed the effect of Nedd4-expression in vitro. Alternatively, Nedd4L might also affect the tumour microenvironments to promote the melanoma cell growth in vivo. These findings indicate that Nedd4L-related pathway could potentially be a novel molecular target for melanoma therapy.
Besides ion channels, Nedd4L targets diverse substrates that were comprehensively identified by a recent metaproteome analysis (Persaud et ;al. 2009). The study to unravel the detailed molecular pathway with which Nedd4L might support the melanoma carcinogenesis is undergoing.
In summary, the present study indicated that the ubiquitin ligase Nedd4L may act as an oncogenic factor in melanoma. Subsequent studies are required to develop a therapeutic approach against Nedd4L-mediating pathway.
Acknowledgments
This study was supported by grants from the Ministry of Education of Japan (KAKEN 24590384). We thank Ms. Rumi Matsumura for her skilled assistance (Division of Molecular Biology, Kochi Medical School).
Conflict of interest
The authors have no conflict of interests.
References
- Booken N, Gratchev A, Utikal J, et al. Sézary syndrome is a unique cutaneous T-cell lymphoma as identified by an expanded gene signature including diagnostic marker molecules CDO1 and DNM3. Leukemia. 2008;22:393–399. doi: 10.1038/sj.leu.2405044. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer Ther. 2010;10:1811–1823. doi: 10.1586/era.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandarpour M, Kiaii S, Zhu C, Castro J, Sakko AJ, Hansson J. Suppression of oncogenic NRAS by RNA interference induces apoptosis of human melanoma cells. Int. J. Cancer. 2005;115:65–73. doi: 10.1002/ijc.20873. [DOI] [PubMed] [Google Scholar]
- Eskandarpour M, Huang F, Reeves KA, Clark E, Hansson J. Oncogenic NRAS has multiple effects on the malignant phenotype of human melanoma cells cultured in vitro. Int. J. Cancer. 2009;124:16–26. doi: 10.1002/ijc.23876. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar DE. Worldwide increasing incidences of cutaneous malignant melanoma. J. Skin Cancer. 2011;2011:858425. doi: 10.1155/2011/858425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Dinudom A, Cook DI, Kumar S. The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J. Biol. Chem. 2001;276:8597–8601. doi: 10.1074/jbc.C000906200. [DOI] [PubMed] [Google Scholar]
- Hellwinkel OJ, Asong LE, Rogmann JP, et al. Transcriptional alternations of members of the ubiquitin-proteasome network in prostate carcinoma. Prostate Cancer Prostatic Dis. 2011;14:38–45. doi: 10.1038/pcan.2010.48. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Takeuchi T, Goto N, Kito Y, Furihata M. Role of altered expression of Nedd4L in the pathogenesis of systemic malignancies. Int. J. Exp. Pathol. 2012;93:463. doi: 10.1111/j.1365-2613.2012.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin. Proc. 2007;82:364–380. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud A, Alberts P, Amsen EM, et al. Comparison of substrate specificity of the ubiquitin ligases Nedd4 and Nedd4-2 using proteome arrays. Mol. Syst. Biol. 2009;5:333. doi: 10.1038/msb.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J. Biol. Chem. 2004;279:5042–5046. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Misaki A, Liang SB, et al. Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J. Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Nagayama T, Furihata M. Nedd4L modulates the transcription of metalloproteinase-1 and-13 genes to increase the invasion activity of gallbladder cancer. Int. J. Exp. Pathol. 2011a;92:79–86. doi: 10.1111/j.1365-2613.2010.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Nagayama T. Expression of a secretory protein C1qTNF6, a C1qTNF family member, in hepatocellular carcinoma. Anal. Cell Pathol. (amst) 2011b;34:113–121. doi: 10.3233/ACP-2011-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Nagayama T. A WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis. 2012;33:548–554. doi: 10.1093/carcin/bgs001. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Veer LJ, Burgering BM, Versteeg R, et al. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol. Cell. Biol. 1989;9:3114–3116. doi: 10.1128/mcb.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AF, Braun BS, Shannon KM. Targeting oncogenic Ras signaling in hematologic malignancies. Blood. 2012;120:3397–3406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]


