Abstract
Dysfunctional glutamate neurotransmission has been implicated in the pathophysiology of schizophrenia. Abnormal expression in schizophrenia of ionotropic glutamate receptors (iGluRs) and the proteins that regulate their trafficking have been found to be region- and subunit-specific in brain, suggesting that abnormal trafficking of iGluRs may contribute to altered glutamatergic neurotransmission. The posttranslational modification N-glycosylation of iGluR subunits can be used as a proxy for their intracellular localization. Receptor complexes assemble in the lumen of the endoplasmic reticulum (ER), where N-glycosylation begins with the addition of N-linked oligomannose glycans, and subsequently is trimmed and replaced by more elaborate glycans while trafficking through the Golgi apparatus.
Previously, we found abnormalities in N-glycosylation of the GluR2 AMPA receptor subunit in schizophrenia. Here we investigated N-glycosylation of NMDA and kainate receptor subunits in dorsolateral prefrontal cortex from subjects with schizophrenia and a comparison group. We used enzymatic deglycosylation with two glycosidases: endoglycosidase H (Endo H), which removes immature high mannose containing sugars, and peptide-N-glycosidase F (PNGase F), which removes all N-linked sugars. The NR1, NR2A, NR2B, GluR6 and KA2 subunits were all sensitive to treatment with Endo H and PNGase F. The GluR6 kainate receptor subunit was significantly more sensitive to Endo H-mediated deglycosylation in schizophrenia, suggesting a larger molecular mass of N-linked high mannose and/or hybrid sugars on GluR6. This finding, taken with our previous work, suggests that a cellular mechanism underlying abnormal glutamate neurotransmission in schizophrenia may involve abnormal trafficking of both AMPA and kainate receptors.
Keywords: schizophrenia, postmortem brain, psychosis, kainate, glutamate receptors, prefrontal cortex
Introduction
Growing evidence implicates glutamate receptor dysfunction in the pathophysiology of schizophrenia, but the underlying mechanism remains unknown. Hypotheses involving glutamate abnormalities in this illness have led to the direct study of glutamate receptors and other proteins associated with glutamate neurotransmission in postmortem brain in schizophrenia. Numerous studies have found altered expression of glutamate receptor subunits in schizophrenia brain, but changes have been modest and inconsistent[1]. Multiple studies, however, have noted robust alterations in the expression of proteins that regulate trafficking and activity of glutamate receptors[1-7]. In addition, we have previously found evidence suggestive of disturbances in the trafficking of glutamate receptors in schizophrenia, including the endosomal trafficking of AMPA receptors[5,8], as well as the trafficking of AMPA[9] and NMDA[10,11] receptors from the endoplasmic reticulum (ER) to the plasma membrane. These data suggest that glutamate dysfunction in schizophrenia may be associated with the processing and trafficking of glutamate receptors rather than abnormal receptor expression.
Trafficking of glutamate receptors, after their exit from the ER, can be studied by measuring the extent of N-glycosylation of receptor subunits[9]. We have previously observed a significantly smaller fraction of GluR2 with high-mannose sugars in schizophrenia, which may indicate accelerated exit from the ER or increased forward trafficking of GluR2-containing AMPA receptors in this disorder[9]. We have also found that EAAT1 and EAAT2, two of the excitatory amino acid transporters (EAATs), are less N-glycosylated in schizophrenia, which also suggests the accelerated exit from the ER and abnormal forward trafficking of these transporters in this disorder[12]. It is possible that abnormal N-glycosylation of glutamate receptors and other molecules associated with glutamate neurotransmission could contribute to the pathophysiology of schizophrenia by altering the forward trafficking of those proteins. Given our previous findings, in this work we studied N-linked glycosylation of subunits of the NMDA and kainate (KA) receptors in postmortem brain in schizophrenia. We hypothesized that abnormalities in N-linked glycosylation of ionotropic glutamate receptor subunits are not restricted to AMPA receptors in schizophrenia and may be found for NMDA and/or KA receptor subunits as well.
Methods
Tissue acquisition and sample preparation
Dorsolateral prefrontal cortex (DLPFC) samples from patients with schizophrenia (N=35: age=74 ± 12 years; postmortem interval=12.5 ± 6.6 hours; tissue pH=6.4 ± 0.3; 66% male, 34% female) and from a comparison group (N=31: age=78 ± 14; postmortem interval=8.1 ± 6.9 hours; tissue pH=6.4 ± 0.2; 39% male, 61% female) were from the Mount Sinai Medical Center brain collection and were used for this study as previously described[9,13]. Not all subjects were available for each separate experiment. Consent was obtained from the next of kin for each subject. Patients were diagnosed with schizophrenia using DSM-III-R criteria, and each subject had psychotic symptoms before the age of 40 and at least ten years of hospitalization, with a diagnosis of schizophrenia made by two experienced clinicians. Patients were recruited prospectively and underwent extensive antemortem clinical assessment. All subjects were without a history of alcoholism, substance abuse, death by suicide, coma for more than 6 h before death, or any neuropathological evidence of neurodegenerative disorders. Subjects used in this study were matched for sex, tissue pH, postmortem interval (PMI), and age as previously described[9,13].
Brains were collected at autopsy and cut in the coronal plane in 10 mm slabs. DLPFC was dissected from tissue slabs, snap frozen, pulverized into powder, and stored at −80°C until used. Samples were reconstituted and homogenized in ice-cold buffer (5 mM Tris-HCl (pH 7.4), 0.32 M sucrose), with a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Mannheim, Germany) using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Waltham, MA), and protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific).
Enzymatic deglycosylation
Samples (50 μg total protein per reaction) were incubated at 37°C for 16 h with endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGase F) (New England Biolabs, Ipswich, MA), according to the manufacturer's protocol. After enzymatic deglycosylation, samples were mixed with 6x protein sample buffer (0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 50 mM dithiothreitol, 6% SDS, 30% glycerol, and bromophenol blue as the tracking dye), heated at 95°C for 10 min and resolved for 1.5 h at 180 V on 4-12% gradient sodium dodecyl sulfate-polyacrylamide (SDS-polyacrylamide) gels (Invitrogen, Carlsbad, CA). Proteins were transferred from SDS-polyacrylamide gels to polyvinylidene fluoride (PVDF) using a BioRad semi-dry transblotter. The membranes were blocked in LiCor blocking buffer (LiCor, Lincoln, NE), and then incubated overnight at 4°C in LiCor blocking buffer with 0.1% Tween 20 and with the following primary antibodies: mouse monoclonal anti-NR1 (118 kDa) BD Biosciences, San Jose, CA, # 556308; 1:1000), mouse monoclonal anti-NR2A (170 kDa) BD Biosciences, San Jose, CA, # 612286; 1:500), mouse monoclonal anti-NR2B (175 kDa) BD Biosciences, San Jose, CA, # 610416; 1:1000), mouse monoclonal anti-NR2C (140 kDa), anti-GluR6 (C18) goat polyclonal (Santa Cruz Biotechnology Santa Cruz, CA, # 7618; 1:250) (105 kDa), anti-KA2 (H-60) rabbit polyclonal (Santa Cruz Biotechnology, # 8915; 1:250) or anti-β-tubulin mouse monoclonal (Millipore, # 05-661; 1:100,000). The membranes were washed in phosphate-buffered saline with 0.1% Tween 20 (PBST) and probed with IR-dye labeled secondary antibodies (LiCor; 1:10,000) for 1 h at room temperature. The membranes were rinsed three times for 15 min with PBST and briefly with deionized water.
GluR6 expression level analysis
For total GluR6 expression level studies, the homogenized DLPFC samples were diluted with ultrapure water (Milli-Q A10, Millipore, Bellarica, CA), mixed with 6x protein sample buffer (0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 50 mM dithiothreitol, 6% SDS, 30% glycerol, and bromophenol blue as the tracking dye), heated at 95°C for 10 min, resolved on SDS-polyacrylamide gels and transferred to nitrocellulose as above. The membranes were blocked in LiCor blocking buffer (LiCor, Lincoln, NE), and then incubated overnight at 4°C in LiCor blocking buffer with 0.1% Tween 20 and with anti-GluR6 (C18) goat polyclonal (Santa Cruz Biotechnology, # 7618; 1:250) or anti-β-tubulin mouse monoclonal (Millipore, # 05-661; 1:100 000) antibodies. The membranes were washed in PBST and probed with IR-dye labeled secondary anti-goat or anti-mouse antibodies (LiCor, 1:10 000) for 1 h at room temperature. The membranes were rinsed three times for 15 min with PBST and briefly with deionized water.
Data analysis
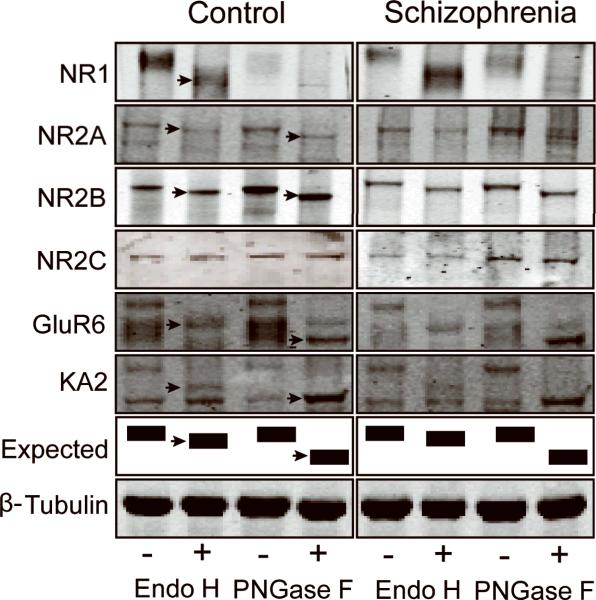
The near-infrared fluorescence value for each protein band were collected using a LiCor Odyssey scanner, and the migration distance for each NMDA or KA receptor subunit was measured using Odyssey V3.0 software. For analysis of N-glycosylation of NMDA and KA receptor subunits, we quantified two dependent variables: (1) Endo H-sensitive molecular mass shifts for NR1, NR2A, NR2B, GluR6, and KA2, and (2) PNGase F-sensitive molecular mass shifts for NR2A, NR2B, GluR6, and KA2. The distance was measured between deglycosylated protein bands of higher mobility after treatment with Endo H or PNGase F and the same protein in the control reaction in the adjacent lane without Endo H or PNGase F, respectively (Fig.). The migration distance for each receptor subunit was converted to molecular mass shift for each subunit, as previously described[9,12]. For total GluR6 expression level studies, the near-infrared fluorescence value for each GluR6 protein band was collected using a LiCor Odyssey scanner, and normalized to the values collected for intralane β-tubulin from each subject[9].
Figure.
Representative immunoblots showing changes in electrophoretic mobility (arrows) of the NMDA receptor subunits NR1, NR2A, and NR2B, and the KA receptor subunits GluR6 and KA2, but not the NMDA receptor subunit NR2C or β-tubulin following enzymatic deglycosylation with either Endo H or PNGase F. The schematic diagram shows predicted band patterns following deglycosylation with either Endo H or PNGase F.
Data were analyzed using the Statistica software package (Statsoft, Tulsa, OK). All dependent measures were determined to be normally distributed. Correlation analyses were performed to determine associations between the dependent variables and age, PMI and tissue pH. No correlations were found between these variables and any dependent measures. Data were analyzed with one-way analysis of variance (ANOVA). For all statistical tests, α= 0.05.
Results
We measured the extent of N-glycosylation of the NMDA receptor subunits NR1, NR2A, NR2B, and NR2C, and the KA receptor subunits GluR6 and KA2, in DLPFC of schizophrenia patients and a comparison group. Samples were incubated with glycosidases that differ in their specificity toward N-linked glycans: Endo H removes immature high mannose-containing and hybrid sugars, whereas PNGase F removes all N-linked sugars leading to total N-linked deglycosylation. The removal of sugars is inferred by a decrease of the molecular mass.
All studied subunits except NR2C were sensitive to enzymatic deglycosylation (Fig.), reflected by measurable changes in electrophoretic mobility of each subunit after enzymatic treatment. We assayed the NR1 subunit after Endo H treatment only, because NR1 immunoreactivity was undetectable in samples incubated in the PNGase F specific buffer. Of all subunits studied, only GluR6 was significantly more sensitive to Endo H-mediated deglycosylation in schizophrenia than in the comparison subjects (12.4 ± 1.3 vs. 11.5 ± 1.0 kDa, p<0.05). We compared expression levels of GluR6 protein normalized to β-tubulin and did not find any significant difference between schizophrenia (0.7 ± 0.4) and comparison subjects (0.9 ± 0.4). We did not observe any differences in molecular mass shifts for the other subunits between schizophrenia and comparison subjects after either Endo H or PNGase F treatment.
Discussion
Previously, we have found that proteins associated with glutamate neurotransmission are abnormally N-glycosylated in schizophrenia, including the GluR2 subunit of the AMPA receptor[9,12]. In this work, we have extended the characterization of N-glycosylation in schizophrenia to subunits of the other families of ionotropic glutamate receptors, NMDA and KA receptors. We found that the GluR6 KA receptor subunit is abnormally N-glycosylated in schizophrenia. We have also determined the N-glycosylation status of NR1, NR2A, NR2B, NR2C, and KA2; all analyzed subunits except NR2C were sensitive to Endo H or PNGase F treatment, but no significant changes were found between schizophrenia and comparison subjects for those subunits.
GluR6 was significantly more sensitive to Endo H-mediated deglycosylation in schizophrenia, suggesting that either a larger fraction of this subunit contains N-linked high mannose and/or hybrid sugars or that sugars attached to GluR6 are a larger molecular weight in schizophrenia than in comparison subjects. This is intriguing as all of our previous findings of N-glycosylation abnormalities of proteins associated with glutamatergic neurotransmission in schizophrenia have been of decreased N-glycans on these proteins[9,12]. Endo H recognizes and removes only glycans that are specific to proteins residing in the proximal secretory pathway (ER to medial-Golgi)[14,15], suggesting that in schizophrenia a larger fraction of the GluR6 subunit may be retained in the ER or Golgi apparatus, limiting the amount of GluR6 that can be assembled into active KA receptors. Given our previous finding of decreased ER content of the NR2B NMDA receptor subunit[11], it is surprising the we did not find any changes in N-glycosylation of this or other NMDA receptor subunits. These data taken together, however, are consistent with abnormal trafficking of ionotropic receptors in schizophrenia, but the mechanism of such dysregulation is likely multifactorial.
Abnormalities of N-glycosylation and forward trafficking of GluR6 may have functional consequences in schizophrenia. GluR6 is the major subunit of KA receptors, and it regulates KA receptor function. Most KA receptors contain the GluR6 subunit, and the cell-surface expression of KA receptors containing this subunit or other subunits/their splice variants is regulated by GluR6[16,17]. Unlike NMDA or AMPA receptors, KA receptors are found on both sides of the synapse, and play a major role in neurotransmitter release, neuronal excitability and plasticity[18-20]. They can act as ionotropic glutamate receptors, but also regulate metabotropic neuronal signaling pathways[21]. GluR6 containing KA receptors have been demonstrated to be important in generation of brain oscillatory activity[22]. The expression of a nonfunctional GluR6 subunit has been proposed to disrupt development and lead to cognitive dysfunction[23,24].
In conclusion, we found altered N-glycosylation of the GluR6 KA receptor subunit, which may result in attenuation of KA receptor function thus affecting glutamate neurotransmission and in turn contributing to the glutamatergic and cognitive dysfunction in schizophrenia. This finding, together with our previous findings, suggests that abnormal trafficking and/or recycling of glutamate receptors and transporters may contribute to the mechanism underlying dysfunctional glutamate neurotransmission in this disorder. It remains to be determined how potentially dysfunctional KA receptors contribute to pathogenesis or pathophysiology of schizophrenia, but given their modulatory roles they may be involved in disturbances in excitatory neurotransmission associated with this disorder.
Table.
Molecular mass shift (kDa) for NR1, NR2A, NR2B, GluR6, and KA2 in schizophrenia (SCZ) and a comparison group (C).
| Endo H | PNGase F | |||
|---|---|---|---|---|
| C |
SCZ | C | SCZ | |
| NR1 | 19.2 ± 3.04 | 18.8 ± 3.57 | nd | nd |
| NR2A | 3.2 ± 1.37 | 3.7 ± 1.41 | 7.5 ± 2.41 | 8.1 ± 1.77 |
| NR2B | 4.7 ± 1.68 | 4.5 ± 1.67 | 9.4 ± 1.13 | 9.8 ± 1.27 |
| GluR6 | 11.5 ± 1.02 | 12.4 ± 1.31* | 22.4 ± 1.33 | 21.81 ± 1.5 |
| KA2 | 11.4 ± 2.88 | 11.4 ± 1.82 | 18.21 ± 2.16 | 17.96 ± 2.69 |
p<0.05
Acknowledgments
This work was supported by MH53327 (JMW), MH064673 (VH) and MH066392 (VH).
References
- 1.Rubio MD, Drummond JB, Meador-Woodruff JH. Biomolecules and Therapeutics. 2012 doi: 10.4062/biomolther.2012.20.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beneyto M, Meador-Woodruff JH. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- 3.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 4.Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, et al. Journal of Neurochemistry. 2002;83:797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- 5.Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Neuropsychopharmacology. 2010;35:2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond JB, Simmons M, Haroutunian V, Meador-Woodruff JH. Neuroreport. 2012;23:1031–1034. doi: 10.1097/WNR.0b013e32835ad229. [DOI] [PubMed] [Google Scholar]
- 7.Drummond JB, Tucholski J, Haroutunian V, Meador-Woodruff JH. Schizophr Res. 2013 doi: 10.1016/j.schres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond JC, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Schizophr Res. 2011;130:260–265. doi: 10.1016/j.schres.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Schizophr Res. 2013;146:177–183. doi: 10.1016/j.schres.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Mol Psychiatry. 2006;11:737–747. 705. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Synapse. 2010;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- 12.Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Schizophr Res. 2010;117:92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Neuroreport. 2009;20:1019–1022. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greger IH, Khatri L, Ziff EB. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 15.Nasu-Nishimura Y, Jaffe H, Isaac JTR, Roche KW. Journal of Biological Chemistry. 2010;285:2847–2856. doi: 10.1074/jbc.M109.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, et al. Neuron. 2005;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Nasu-Nishimura Y, Hurtado D, Braud S, Tang TT-T, Isaac JTR, Roche KW. J. Neurosci. 2006;26:7014–7021. doi: 10.1523/JNEUROSCI.0573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contractor A, Mulle C, Swanson GT. Trends in Neurosciences. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerma J. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro P, Mulle C. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 21.Melyan Z, Wheal HV, Lancaster B. Neuron. 2002;34:107–114. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- 22.Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. The Journal of Neuroscience. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motazacker MM, Rost BR, Hucho T, Garshasbi M, Kahrizi K, Ullmann R, et al. The American Journal of Human Genetics. 2007;81:792–798. doi: 10.1086/521275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanore F, Labrousse VF, Szabo Z, Normand E, Blanchet C, Mulle C. The Journal of Neuroscience. 2012;32:17882–17893. doi: 10.1523/JNEUROSCI.2049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]