Abstract
Extracellular matrix (ECM) synthesis and deposition surrounding the developing vasculature is critical for vessel remodeling and maturation events. Although the basement membrane is an integral structure underlying endothelial cells (ECs), few studies, until recently, have been performed to understand its formation in this context. In this review, we highlight new data demonstrating a co-requirement for ECs and pericytes to properly deposit and assemble vascular basement membranes during morphogenic events. In EC only cultures or under conditions whereby pericyte recruitment is blocked, there is a lack of basement membrane assembly, decreased vessel stability (with increased susceptibility to pro-regressive stimuli) and increased EC tube widths (a marker of dysfunctional EC-pericyte interactions). ECs and pericytes both contribute basement membrane components and, furthermore, both cells induce the expression of particular components as well as integrins that recognize them. The EC-derived factors, platelet derived growth factor-BB (PDGF-BB) and heparin binding-epidermal growth factor (HB-EGF), are both critical for pericyte recruitment to EC tubes and concomitant vascular basement membrane formation in vitro and in vivo. Thus, heterotypic EC-pericyte interactions play a fundamental role in vascular basement membrane matrix deposition, a critical tube maturation event that is altered in key disease states such as diabetes and cancer.
Keywords: Vascular basement membrane assembly, endothelial cells, pericytes, integrins, extracellular matrix, vascular guidance tunnels, laminin, collagen type IV, fibronectin
Introduction
Of critical importance during both embryonic development, and in postnatal life during processes such as wound repair, is formation of new endothelial cell (EC)-lined tubes in three-dimensional (3D) extracellular matrix (ECM) environments (Adams & Alitalo, 2007; Arroyo & Iruela-Arispe, 2010; Davis & Senger, 2005; Davis & Senger, 2008; Davis, et al., 2011; Hynes, 2007; Hynes, 2009; Iruela-Arispe & Davis, 2009; Sacharidou, et al., 2011; Senger & Davis, 2011). These tubes arise through vasculogenesis, where de novo formation of new blood vessels assemble from individual precursor cells, and/or angiogenesis, where sprouting of new vessels occurs from pre-existing parental vessels (Adams & Alitalo, 2007; Carmeliet, 2005; Drake, 2003; Risau & Flamme, 1995). After these EC tube networks are formed, recruitment of supporting cells such as pericytes and vascular smooth muscle cells occurs along the tube abluminal surface (Bergers & Song, 2005; Betsholtz, et al., 2005; Davis, et al., 2011; Stratman, et al., 2009a; Stratman, et al., 2010). This latter step represents a critical event controlling further tube remodeling, maturation and stabilization (Benjamin, et al., 1999; Benjamin, et al., 1998; Bergers, et al., 2003; Davis & Senger, 2008; Davis, et al., 2011; Gaengel, et al., 2009; Hanahan, 1997; Hellstrom, et al., 2001; Hughes, 2008; Jain, 2003; Saunders, et al., 2006; Stratman, et al., 2009a). During these early events in vascular morphogenesis, patterning of the vasculature is very dynamic and prone to remodeling in conjunction with the onset of flow as well as embryo growth (Benjamin, et al., 1998; Hellstrom, et al., 2001; Jain, 2003). With continuing flow as well as increasing shear stress and blood pressure over time during vascular development, greater vessel stability is required (Iruela-Arispe & Davis, 2009; Wagenseil & Mecham, 2009). Such vessel stability is attained through EC-mural cell interactions and concomitant ECM remodeling including deposition and cross-linking of ECM components (i.e. basement membranes, interstitial matrices, and elastin-rich matrices) at distinct places in the vessel wall and depending on the vessel type (i.e. with unique cellular compositions) (Cheng, et al., 1997; Davis & Senger, 2005; Davis, et al., 2011; Li, et al., 2002; Miner & Yurchenco, 2004; Senger & Davis, 2011; Stratman, et al., 2009a; Wagenseil & Mecham, 2009; Yurchenco, et al., 2004; Yurchenco & Patton, 2009). One of the key ECM components that facilitates EC tube stabilization are basement membrane matrices which are in direct contact with the EC layer on its abluminal surface and which provides important signals that control the stability of this layer. Although considerable information exists on the signaling properties of basement membrane components toward different cell types, much remains to be learned concerning how such components affect EC behaviors at distinct stages of vascular morphogenesis and stabilization in embryonic and postnatal life.
Vascular basement membrane matrices {Davis, 2005; Senger, 2011} are largely composed of the structural components laminin (particularly laminins 411, 421, 511 and 521) (Cheng, et al., 1997; Miner, et al., 1998; Miner & Yurchenco, 2004; Scheele, et al., 2007; Thyboll, et al., 2002; Yurchenco, et al., 2004), collagen IV (Paulsson, 1992; Poschl, et al., 2004; Schmidt, et al., 1992), and fibronectin (Astrof, et al., 2007; Astrof & Hynes, 2009; Clark, et al., 1982; Francis, et al., 2002; Hynes, 2007; Risau & Lemmon, 1988). Other proteins provide bridging functions such as nidogens 1 and 2 (Ho, et al., 2008; Paulsson, 1992; Stratman, et al., 2009a; Timpl, et al., 1983; Timpl, et al., 1984) and the heparan sulfate proteoglycan, perlecan (Handler, et al., 1997; Paulsson, 1992; Stratman, et al., 2009a; Timpl, 1994; Yurchenco, et al., 2004) which facilitate the co-assembly of basement membrane components. It has long been known that endothelial cells have the capacity to synthesize most if not all of these proteins so it was generally assumed that basement membrane assembly occurred through ECs alone. However, in a variety of tissues, most notably the skin, it is clear that basement membrane assembly requires more than keratinocytes and was strongly stimulated by the presence of fibroblasts in collagenous matrices underlying the keratinocyte layer (Smola, et al., 1998). Thus, by analogy with these findings, it is likely that vascular basement membrane assembly may require heterotypic cell-cell contacts which was originally suggested by Davis and Senger (Davis & Senger, 2005). A recent set of papers from our laboratory demonstrate that pericyte recruitment to EC-lined tubes in vitro and in vivo is necessary to stimulate vascular basement membrane matrix assembly {Stratman, 2009; Stratman, 2010; Senger, 2011}, a key step in vascular maturation and stabilization. In this review, we will review current concepts related to how ECs and pericytes work together to control EC tubulogenesis and maturation events, and in particular, discuss recent insights into how dynamic EC-pericyte tube coassembly leads to vascular basement membrane matrix deposition and tube stabilization in 3D matrices {Stratman, 2009; Stratman, 2010; Davis, 2011; Senger, 2011}.
Development of novel EC-pericyte tube co-assembly models in 3D matrices
One of the primary limiting factors toward the study of molecular consequences of EC-pericyte interactions has been the lack of 3D matrix models in which the contributions of each cell to vascular tube morphogenesis and stabilization can be directly assessed. For instance, for many years it has been known that EC-derived PDGF-BB partially contributes to the proper investment of pericytes to the endothelial wall, as a defect is noted in PDGF-BB knockout mice where there are approximately 50% less pericytes around microvessels (Abramsson, et al., 2003; Betsholtz, et al., 2005; Bjarnegard, et al., 2004; Hirschi, et al., 1998; Leveen, et al., 1994; Lindahl, et al., 1997; Lindblom, et al., 2003). However, understanding the direct role of this molecule on pericytes becomes more difficult since this result could be the effect of reduced proliferation, impaired survival or reduced recruitment (perhaps more than one of these). Also, the fact that PDGF-BB signaling in the mouse microvasculature does not fully impair the presence of pericytes around EC tubes, suggests that this molecule might be working in tandem with other growth factors/chemokines to control these events (Iivanainen, et al., 2009; Iivanainen, et al., 2003; Stratman, et al., 2010).
Because of questions like these, we aimed to develop in vitro models of EC-pericyte co-association in 3D collagen type I matrices where ECs undergo lumen formation, branching and tubulogenesis, and recruitment of pericytes occurs to elicit long term tube stabilization and basement membrane deposition (Figs. 1 and 2) (Bayless & Davis, 2002; Bayless, et al., 2000; Bell, et al., 2001; Davis & Camarillo, 1996; Davis, et al., 2007; Davis, et al., 2011; Koh, et al., 2008a; Koh, et al., 2009; Koh, et al., 2008b; Sacharidou, et al., 2010; Saunders, et al., 2006; Stratman, et al., 2009a; Stratman, et al., 2009b). In our first model, we utilized a system that employed the addition of phorbol esters which was necessary to promote EC viability in 3D collagen matrices under serum-free defined conditions (Koh, et al., 2008b). In this study, we demonstrated that pericytes were able to stabilize EC-lined tubes by preventing matrix metalloproteinase (MMP)-1- and MMP-10-dependent tube regression (Saunders, et al., 2005) through the production of the MMP inhibitors, TIMP-2 (predominantly made by ECs) and TIMP-3 (predominantly made by pericytes) (Saunders, et al., 2006). Importantly, we developed a system whereby EC tubes were unstable and susceptible to MMP-dependent tube regression that was prevented by the presence of pericytes due to their influence on the production and MMP inhibitory activity of TIMP-2 and TIMP-3, which together facilitate tube stabilization (Saunders, et al., 2006).
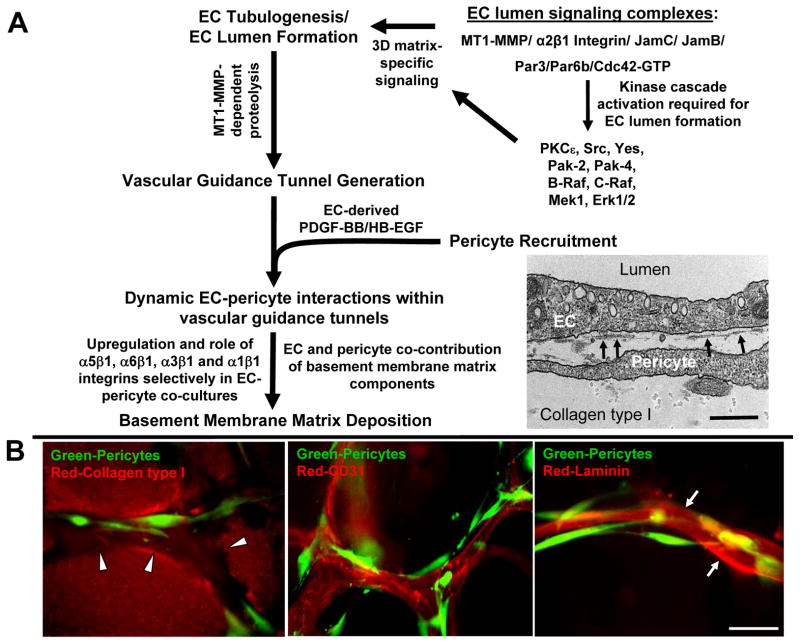
Figure 1. Molecular mechanisms controlling EC tubulogenesis, pericyte recruitment and tube stabilization in 3D matrices.
(A) The schematic depicts the role of MT1-MMP proteolysis, EC lumen signaling complexes, and signal transduction cascades on the promotion of early EC morphogenic events including lumenogenesis and vascular guidance tunnel formation. Pericyte recruitment occurs to these developing tubes through EC-derived PDGF-BB and HB-EGF leading to vascular basement membrane deposition that affects tube maturation and stabilization. EC-pericyte interactions selectively lead to increased basement membrane protein synthesis, deposition and upregulation of integrins that recognize this newly deposited ECM. The image is an electron micrograph showing EC-pericyte tube coassembly and developing vascular basement membranes depositing (arrows) between the two cell types in 3D matrices. Bar equals 0.5 μm. (B) ECs and pericytes were seeded in collagen type I matrix and allowed to assemble over 5 days. Images shown depict varying steps within this assembly process: (left) the generation and presence of vascular guidance tunnels (red: collagen type I matrix, green: GFP-pericytes); (center) the recruitment of pericytes to EC lined tubes (red: CD31, green: GFP-pericytes); (right) the deposition of the vascular basement membrane (red: laminin, green: GFP-pericytes). Bar equals 25 μm.
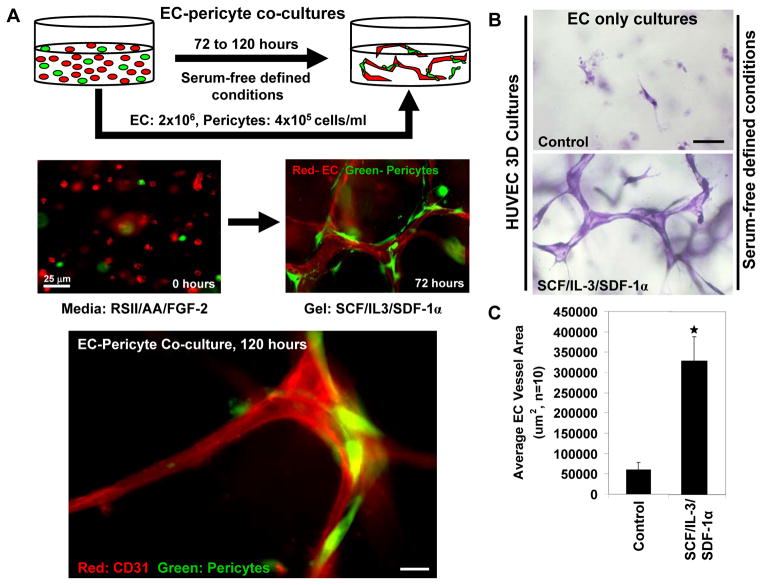
Figure 2. The development of in vitro models of EC tubulogenesis and EC-pericyte tube coassembly under defined serum-free conditions.
(A) Models of EC-pericyte coassembly were developed in collagen type I matrices in which the pericytes recruit to developing EC tubes over a period of three to five days. The two cells are seeded randomly as individual cells and over time the ECs vacuolate, lumenize and interconnect into vascular networks; pericytes then recruit to these vessels to promote maturation and long term stabilization events. Images are shown to display this assembly process with the ECs immunolabeled with anti-CD31 antibodies in red and pericytes stably expressing GFP. Bar equals 10 μm. (B/C) The 3D assay systems utilized are performed using a series of three hematopoietic cytokines, SCF, SDF-1α and IL-3, to promote tubulogenesis. Images and quantification of the morphogenic phenotype observed at day 3 of culture are shown. Bar equals 25 μm.
Our laboratory has recently developed a second system under serum-free defined conditions that does not require phorbol ester addition (Stratman, et al., 2011; Stratman, et al., 2009a). In this novel system, the hematopoietic stem cell cytokines, stem cell factor (SCF), interleukin-3 (IL-3) and stromal derived factor 1-alpha (SDF-1α) were added in combination to support tube formation in the absence or presence of pericytes in 3D collagen matrices (Figs. 1B, 2B, C) (Stratman, et al., 2011; Stratman, et al., 2009a). Pericytes dramatically recruit to developing tubes over a period of days and once they reach the tube surface, we demonstrated that they migrate along this tube surface (Stratman, et al., 2009a; Stratman, et al., 2010). When pericytes are present, vessel tube diameters are narrower due to vascular basement membrane assembly on the EC tube abluminal surface. Also, EC tubes extensively form and stabilize under these conditions for several weeks of culture. Of great interest is that the efficacy of SCF, IL-3, and SDF-1α to support vascular tube morphogenesis was strongly enhanced by priming ECs with vascular endothelial growth factor (VEGF-A) and fibroblast growth factor (FGF-2) (Stratman, et al., 2011). This VEGF and FGF-2 priming step was shown to occur through upregulation of c-Kit, IL-3 receptor alpha, and CXCR4, which represent the receptors for SCF, IL-3 and SDF-1α, respectively (Stratman, et al., 2011). By carrying out a series of manipulations in this model such as the use of chemical inhibitors, blocking antibodies and siRNA technologies, we have been able to rapidly assess the contributions of ECs and pericytes individually during the time course of vessel assembly (Koh, et al., 2008b; Saunders, et al., 2006; Stratman, et al., 2011; Stratman, et al., 2009a; Stratman, et al., 2010). Furthermore, real time video analysis can be performed to determine the effects of different molecules and treatments at any given stage of the tube assembly process (Koh, et al., 2008b). For this work, we utilized two sources of pericytes which were both derived from nervous tissue and were either from bovine retina (Saunders, et al., 2006; Stratman, et al., 2009a) or human brain {Stratman, 2010 }. In each case, the cells appeared completely uniform and expressed known pericyte markers such as NG2 proteoglycan and PDGFRβ. We also labeled our bovine and human pericytes with green-fluorescent protein (GFP) using lentiviral gene transfer to facilitate our ability to image them during morphogenic events {Stratman, 2009 Stratman, 2010 }. A number of studies report that fibroblasts from various sources appear to differentiate into pericyte-like cells {Hirschi, 1998 } and furthermore, they have been reported to support vascular morphogenesis and maturation events {Lilly, 2009 #100; }. Unpublished work from our laboratory suggests that human dermal fibroblasts do not support EC tube formation and maturation nearly as well as pericytes under our serum-free defined conditions. In addition, human vascular smooth muscle cells (aortic and coronary artery) also do not support tube formation and maturation events. Thus, pericytes have very unique features in this ability using our experimental paradigms.
Using these experimental strategies, we aimed to address three primary questions which were to determine; 1) the molecular consequences of EC-pericyte interactions on tube stabilization (Saunders, et al., 2006; Stratman, et al., 2009a; Stratman, et al., 2010); 2) how pericyte recruitment to endothelial tubes is regulated (Stratman, et al., 2010); and 3) what molecular changes occur when pericyte recruitment to developing EC tubes is blocked (Stratman, et al., 2009a; Stratman, et al., 2010). In this review, we address each of these points by way of demonstrating a role for both ECs and pericytes in the formation and maintenance of EC-lined tubes and the underlying vascular basement membrane matrix.
Vascular guidance tunnels provide a matrix template for EC-pericyte tube coassembly
Recently, it has been demonstrated though a series of papers that ECs require cell surface anchored proteases, (specifically MT1-MMP or MMP14) to undergo lumen formation and tubulogenesis in 3D ECM environments (Fig. 1A, B) (Chun, et al., 2004; Davis, et al., 2007; Davis, et al., 2011; Lafleur, et al., 2002; Sacharidou, et al., 2010; Saunders, et al., 2006; Stratman, et al., 2009b). This proteinase allows EC’s to digest the surrounding collagen in a localized manner to create a series of physical empty spaces within the matrix which we term vascular guidance tunnels (Stratman, et al., 2009b). It is clear that EC lumen and tube formation creates vascular guidance tunnels and when lumen formation does not occur, the tunnel spaces are not formed (Sacharidou, et al., 2010; Stratman, et al., 2009b). Suppression of MT1-MMP expression, through the use of siRNAs, leads to blockade of EC lumen and tube formation as well as vascular guidance tunnel formation, while increased expression of MT1-MMP (using viral vectors) leads to increased EC lumen and vascular guidance tunnel formation (Sacharidou, et al., 2010; Saunders, et al., 2006; Stratman, et al., 2009b). Interestingly, ECs appear to create about twice the number of vascular guidance tunnels that they occupy at any given moment during vessel formation and remodeling events (Stratman, et al., 2009b). These spaces remain open in the matrix and allow for EC motility and tube remodeling as well as tube regrowth following tube collapse which was revealed using real-time video analysis (Stratman, et al., 2009b). Although MT1-MMP is required for vascular guidance tunnel formation, once tunnels have formed, ECs and tube networks within tunnel spaces are capable of moving in 3D matrices in an MMP-independent manner (Stratman, et al., 2009b). Thus, vascular guidance tunnels represent a series of ECM conduits in 3D matrices that control tubulogenesis and vessel patterning which are generated throughout the matrix during vascular morphogenic events (Stratman, et al., 2009a; Stratman, et al., 2009b).
Importantly, later vessel tube maturation events are dependent on vascular guidance tunnels. Using our novel EC-pericyte co-culture systems, we have demonstrated that pericytes are recruited to EC-lined tubes along their abluminal surface within vascular guidance tunnels (Stratman, et al., 2009a). Thus, pericytes are in direct juxtaposition with the ECs lining the tube wall, with no initial matrix between the two cells, but both cells are present within vascular guidance tunnel spaces (Fig. 1B) (Stratman, et al., 2009a). Thus, ECs and pericytes assemble so that they directly contact each other within these spaces, allowing for both direct and indirect regulation of each cell by its interacting counterpart. Real-time video analysis of these events reveals the highly motile nature of both cell types during tubulogenesis and maturation events (Stratman, et al., 2011; Stratman, et al., 2009a; Stratman, et al., 2010). Pericyte motility observed along the EC tube abluminal surface occurs within the pre-defined vascular guidance tunnel spaces. As mentioned above for ECs, once pericytes enter the tunnels, they are also able to migrate within these spaces in an MMP independent manner (Stratman, et al., 2009a). Also, as pericytes assemble along the vessel wall, they are induced to produce TIMP-3, an endogenous MMP inhibitor, which serves to suppress further EC tube morphogenic and sprouting events by inhibition of MT1-MMP (Saunders, et al., 2006). In addition, pericyte-derived TIMP-3 protects EC tubes from MMP-1 and MMP-10-dependent tube regression responses (Saunders, et al., 2006). Interestingly, this work also identified a role for EC-derived TIMP-2 in this process (Saunders, et al., 2006). Thus, EC-pericyte interactions lead to involvement of both TIMP-2 and TIMP-3 in controlling vascular tube stabilization (Saunders, et al., 2006). Of interest are other studies revealing the ability of TIMP-2 to suppress angiogenesis in a manner that was not dependent on its ability to inhibit MMPs. In contrast, TIMP-2 was shown to bind the α3β1 integrin leading to Shp-1 activation and dephosphorylation of pro-angiogenic receptor tyrosine kinases such as VEGFR2. As mentioned below, α3β1 integrin becomes important during EC-pericyte tube coassembly due to its ability to bind vascular laminin isoforms in the basement membrane and it is very interesting that TIMP-2 also interacts with this integrin during these events. Overall, these studies focused on new mechanisms controlling how pericyte recruitment leads to tube stabilization, a process that was previously not well understood.
Pericyte recruitment induces vascular basement membrane assembly around developing EC-lined tubes in 3D matrices
We have further identified a new function for pericytes and EC-pericyte interactions to control the critical process of vascular basement membrane matrix assembly (Stratman, et al., 2009a; Stratman, et al., 2010). These studies have revealed that there is a co-requirement for both ECs and pericytes to assemble and maintain the vascular basement membrane matrix during vascular tube morphogenesis, maturation and stabilization in 3D extracellular matrices (Fig. 3) (Stratman, et al., 2009a; Stratman, et al., 2010). This work focused on the following key basement membrane structural and cross-linking proteins: laminins, fibronectin, collagen IV, nidogens 1/2 and perlecan. By using a detergent free immunostaining protocol, to detect only deposited proteins in the ECM outside of cells, it was shown that in EC only or pericyte only cultures there is little to no deposition of any of these basement membrane proteins (Stratman, et al., 2009a; Stratman, et al., 2010). We further demonstrated that both cell types are important co-contributors to the deposition of the vascular basement membrane matrix. In contrast, when ECs and pericytes are cocultured together there is a dramatic increase in the extracellular deposition of these same molecules on the abluminal surface of EC tubes (Fig. 3) (Stratman, et al., 2009a; Stratman, et al., 2010). This same phenomenon holds true in vivo as well, as the appearance of the quail vascular basement membrane directly correlates with the arrival of pericytes around developing microvascular tubes (Stratman, et al., 2009a; Stratman, et al., 2010). In all cases, deposition of basement membrane leads to restrictions in vessel diameter and patterning, while disruption of basement membrane assembly, proper EC-pericyte interactions or cell-matrix communication (by blocking integrins) leads to unregulated and mispatterned vessel growth including increased vessel widths (Stratman, et al., 2009a; Stratman, et al., 2010).
Figure 3. EC-pericyte interactions promote tube remodeling, maturation and stabilization through deposition of the vascular basement membrane.
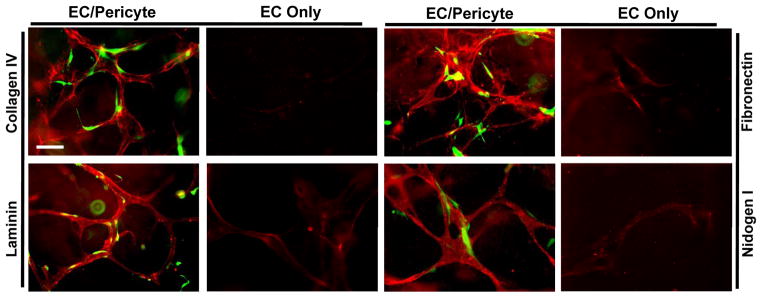
EC only versus EC-pericyte cocultures were established for a period of 5 days in 3D collagen matrices. Immunostaining analysis was performed, using detergent free staining protocols to recognize only extracellular proteins, of the EC only versus EC-pericyte cocultures. Vascular basement membrane matrix assembly reveals extracellular deposition of the indicated basement membrane proteins only in the coculture setting. Pericytes are GFP labeled while the varying basement membrane proteins indicated are immunolabeled in red. Bar equals 25 μm.
The primary cell surface receptors for recognizing matrix proteins are integrins. Integrins function by dimerization of α and β receptor subunits, allowing for the transfer of signaling cascades both in an outside-in manner and an inside-out manner from the cell surface (Davis & Senger, 2005; Hynes, 2007; Somanath, et al., 2009). In ECs the primary α chain integrin subunits include α1–6 (with the exception of α4 which is variably expressed by ECs) pairing with the β1 subunit and αV pairing with either β1, β3 or β5 (Davis & Senger, 2005; Hynes, 2007; Stratman, et al., 2009a; Stupack & Cheresh, 2004). Each α/β integrin subunit pairing has specificity for differential matrix components, with α1 and α2 primarily being collagen receptors, α5 and αV being fibronectin receptors, and α3 and α6 (and in part α1) being laminin receptors (Hynes, 2007). ECs seeded within type I collagen gels, such as in our 3D assays, are dependent upon α2β1 integrin for tubulogenesis (Davis & Camarillo, 1996; Sacharidou, et al., 2010; Stratman, et al., 2009a). However, as the basement membrane begins to assemble around the tubes (when pericytes are present and recruit to tubes), ECs lose their reliance on α2 (a type I collagen receptor) and instead become dependent on other α chain integrins, primarily α5, α3, α6, and α1 (which recognize basement membrane components), to maintain tube stability, restrict EC vessel width and interact with the newly assembled basement membrane matrix (Fig. 1) (Stratman, et al., 2009a). Importantly, this new integrin dependence only occurs when ECs and pericytes are co-cultured together and does not occur with EC only cultures (Stratman, et al., 2009a). Thus, we demonstrated that pericytes are required to work with ECs to properly assemble the vascular basement membrane and that while this basement membrane is being constructed and remodeled, both cell types recognize and respond to this matrix (as demonstrated by requirements for new integrin chains recognizing this new ECM and loss of requirements for integrins recognizing collagen type I) during the time course of vascular morphogenesis and maturation (Figs. 1 and 3) (Stratman, et al., 2009a).
By way of understanding the co-requirement of ECs and pericytes to assemble the basement membrane, an additional series of studies were performed to determine the regulation patterns of each of the key basement membrane matrix molecules, collagen IV chains, laminin isoform chains, fibronectin, nidogens 1/2 and perlecan, at the mRNA level in either ECs or pericytes alone versus each cell individually within the coculture. By carrying out these studies, three major conclusions became apparent which were; 1) in the absence of the co-culture setting, the mRNA transcript level of most of the basement membrane genes decreased over the 5 day time course as well as the expression levels of their correlating integrin chains in EC only and pericyte only cultures; 2) both ECs and pericytes were able to contribute components of the basement membrane (Stratman, et al., 2009a); and 3) that EC-pericyte interactions dramatically increased expression of laminin alpha5 and beta2 chains, necessary for the formation of laminins 511 and 521 that can self-assemble into basement membranes (Miner & Yurchenco, 2004; Yurchenco, et al., 2004), as well as other structural components such as fibronectin and the collagen IV alpha1 chain along with bridging molecules such as nidogen 1 and perlecan (Stratman, et al., 2009a). These changes in gene expression describe the molecular consequences observed downstream of heterotypic cell-to-cell interactions, such as those seen between interacting ECs and pericytes during vascular tube co-assembly, and begin to elucidate the role that pericytes play in supporting long-term tube stabilization.
We also observed that pericyte-derived TIMP-3 played a key role in stabilizing the newly deposited vascular basement membrane. siRNA suppression of TIMP-3 led in particular to markedly decreased collagen type IV assembly and/or stability (Stratman, et al., 2009a). Interestingly, the EC tubes were significantly wider when pericyte TIMP-3 expression was suppressed (Stratman, et al., 2009a). Thus, EC-pericyte interactions not only control synthesis and deposition of basement membrane components, but contribute to stability of this matrix by inhibiting proteolysis.
PDGF-BB and HB-EGF regulate pericyte recruitment to EC lined tubes to induce vascular basement membrane deposition and tube stability
Due to the critical nature of EC-pericyte heterotypic interactions which are necessary for basement membrane deposition and tube stabilization, we sought to understand the signals controlling directional pericyte recruitment to EC-lined tubes under defined serum-free conditions in 3D matrices. As mentioned previously, a considerable amount of work has been done to understand the role that PDGF-BB plays in mural cell investment of the vasculature (Gaengel, et al., 2009; Stratman, et al., 2010). As shown through the use of knockout mice, loss of EC-derived PDGF-BB leads to reduced pericyte coverage in microvascular beds (Abramsson, et al., 2003; Bjarnegard, et al., 2004; Lindblom, et al., 2003). In more recent work, EGF family members appear to play a similar role in affecting pericyte investment of the vasculature (Iivanainen, et al., 2009; Iivanainen, et al., 2003; Stratman, et al., 2010; Weskamp, et al., 2010). The mechanisms by which these growth factors act to control mural cell investment of vessels needs to be investigated in greater detail.
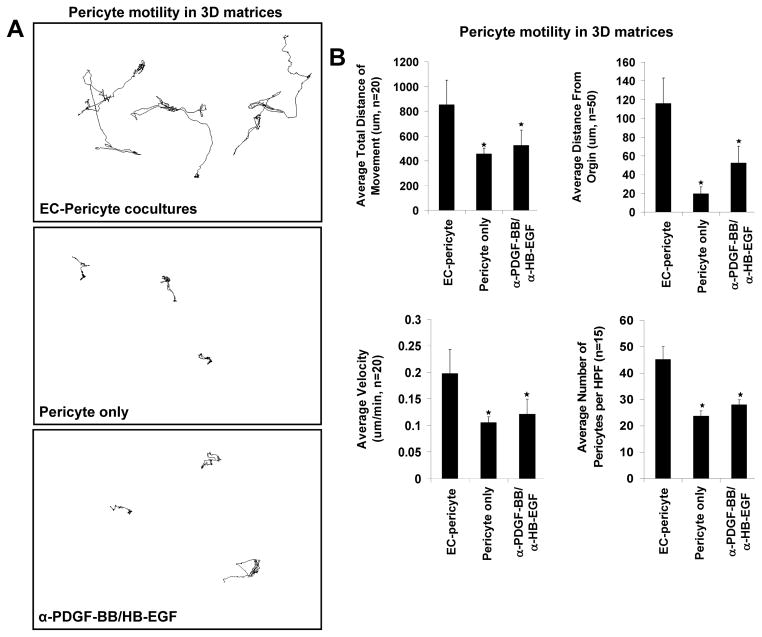
Because of this previous information, we aimed to understand the functional role of PDGF isoforms and EGF family molecules, both individually and in combination, in the molecular control of pericyte motility, proliferation and recruitment during vascular development, tube maturation and vessel stabilization using our serum-free defined system (Stratman, et al., 2011; Stratman, et al., 2009a). Utilizing a pericyte nuclear tracking assay during these events (to assess pericyte motility of nuclear GFP-labeled pericytes in real-time in 3D matrices), we determined that pericytes on their own failed to migrate or proliferate in 3D matrices (Stratman, et al., 2010). In stark contrast, in the presence of ECs and coincident with EC tubulogenesis, marked pericyte motility and proliferation was shown to occur (Fig. 4A) (Stratman, et al., 2010). We also determined that both EC-derived PDGF-BB and HB-EGF are major growth factors produced by ECs during these events, so we performed experiments to address their specific roles (Stratman, et al., 2010). A combinatorial approach utilizing neutralizing reagents specific for either PDGF-BB or HB-EGF (i.e. blocking antibodies and soluble receptor traps) and siRNA suppression of the key receptors for these ligands in pericytes, namely PDGFRβ, EGFR and ErbB4, were employed to demonstrate that these key ligands are derived from ECs and act on pericytes to direct their recruitment to developing tubes (Fig. 4B) (Stratman, et al., 2010). In cases where pericytes are unable to respond to these ligands (i.e. treatment with neutralizing antibodies/receptor traps to both ligands simultaneously or siRNA suppression of the receptors) their motility within 3D matrices was dramatically compromised as shown by decreases in average cellular velocity, average total distance of movement and average distance from the origin (Fig. 4B). In conjunction with this reduced motility there is also a marked decrease in pericyte proliferation and recruitment of pericytes to EC tubes (Fig. 4B) (Stratman, et al., 2010). From these data obtained in vitro, it appears that PDGF-BB and HB-EGF work in tandem to control these processes, as treatments to inhibit either ligand individually do not elicit the maximal effects noted with combination treatments (Stratman, et al., 2010).
Figure 4. EC-derived PDGF-BB and HB-EGF are required to induce pericyte motility and proliferation during EC-pericyte tube coassembly in 3D matrices.
(A) Nuclear GFP-pericytes were seeded in 3D collagen matrices in the presence or absence of ECs and their motility tracked over a period of 3 days. Images of nuclear tracking of pericytes reveal the requirement of ECs to promote pericyte motility. Further, inhibition of PDGF-BB and HB-EGF in combination leads to marked suppression of pericyte motility in 3D matrices. (B) Quantification of nuclear tracking of pericytes in the presence of EC, in the absence of EC or in EC-pericyte cocultures in which PDGF-BB and HB-EGF are inhibited demonstrating that PDGF-BB and HB-EGF in combination are required to stimulate EC motility and proliferation in 3D collagen matrices.
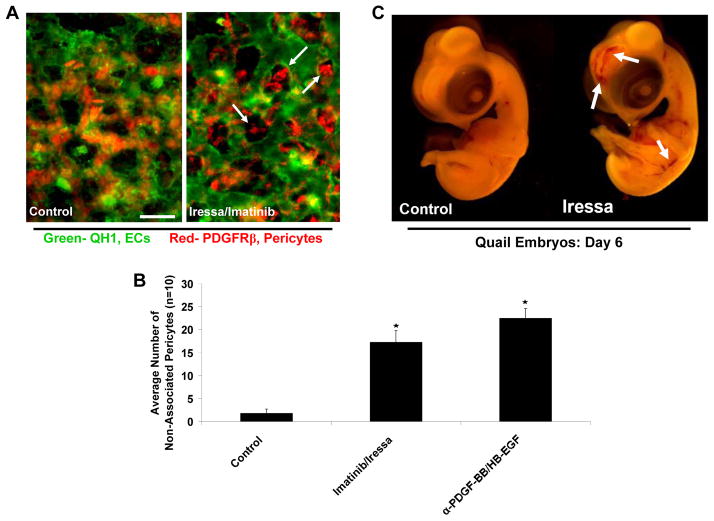
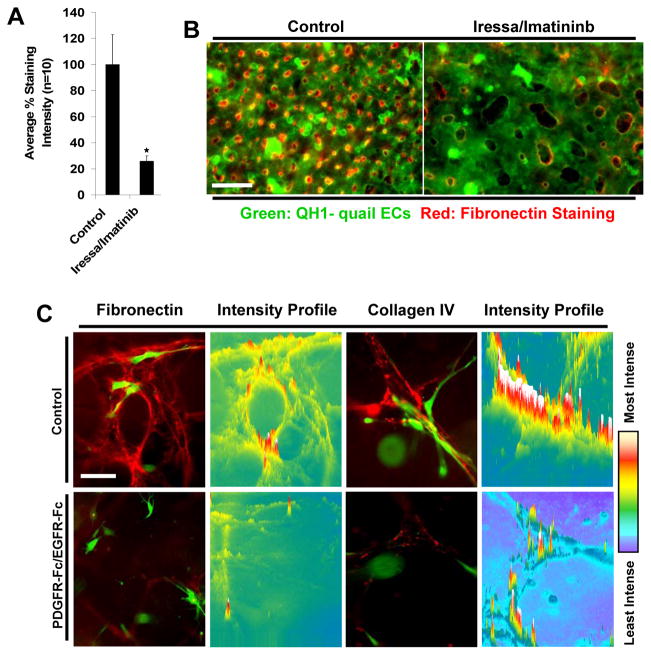
To further support these data in vivo, models of quail vascular development were utilized to investigate the combined influence of PDGF-BB and HB-EGF signaling during these processes. Upon treatment of quail embryos with chemical inhibitors to EGFR (i.e. Iressa) and PDGFRβ (i.e. Imatinib) or using neutralizing antibodies specific to PDGF-BB and HB-EGF during developmental time points of pericyte recruitment, we demonstrated that disruption of both of these ligands in combination leads to a dramatic decrease in the number of pericytes associated with EC tubes versus controls or individual treatment conditions (Fig. 5A, B) (Stratman, et al., 2010). Associated with these recruitment defects is a concomitant increase in EC tube width, decrease in the number of EC branch points and the presence of leaky, hemorrhagic vessels throughout the embryo, which is highly suggestive of improperly remodeled and unstable blood vessels (Fig. 5A, C) (Stratman, et al., 2010). Importantly, the increase in EC tube width and loss of pericyte recruitment to EC vessels noted in vivo is completely consistent with the data obtained in in vitro models.
Figure 5. PDGF-BB and HB-EGF direct pericyte recruitment to endothelial cell tubes during quail vascular morphogenesis.
(A) Images of ECs (QH1-green) overlaid with the associated pericyte field (PDGFRβ-red) are shown demonstrating the lack of pericytes associated with EC tubes in conditions where quail embryos were treated with chemical inhibitors to PDGFRβ (Imatinib) and EGFR (Iressa) (both added at 100 nM). Bar equals 15 μm. (B) Quantification of the number of non-associated pericytes in control versus PDGF-BB/HB-EGF double inhibited treatment conditions (both antibodies added at 50 μg/ml). At day 6 of development, treatments that inhibit PDGF-BB and HB-EGF activity on pericytes lead to an increase in the number of non-associated pericytes versus controls. (C) Images of control versus treatment embryos reveals the increased incidence of cranial vascular hemorrhage phenotypes in the embryos in which pericyte recruitment is impaired.
Of particular interest is whether blockade of pericyte recruitment to EC tubes in vivo (i.e. using PDGF-BB/HB-EGF neutralizing antibodies or chemical inhibitors to their receptors) leads to defects in vascular basement membrane matrix assembly in the developing vasculature. Data generated both in vitro and in vivo show that in the absence of direct EC-pericyte interactions that there is dramatic inhibition of basement membrane protein deposition (Fig. 6) (Stratman, et al., 2010). This data suggests that physical interactions between the two cell types strongly enhances the formation of the basement membrane, and that just the presence of pericytes within the surrounding tissue is insufficient to control this process (Stratman, et al., 2011; Stratman, et al., 2009a; Stratman, et al., 2010). These physical interactions are likely required for the assembly of ECM proteins such as fibronectin, which is known to require mechanical tension exerted on these molecules to expose matricryptic sites that facilitate fibronectin-fibronectin interactions essential for the matrix assembly reaction (Vogel, 2006; Zhong, et al., 1998). Furthermore, our data demonstrates that in the absence of fibronectin assembly there is a concomitant loss in collagen type IV assembly and an increase in EC vessel width (a consistent marker of EC-pericyte dysfunction) (Stratman, et al., 2009a). Such increases in EC vessel width have been noted in both in vitro culture settings and in vivo in the quail vasculature and fibronectin knockout mice (Astrof, et al., 2007; George, et al., 1997; Stratman, et al., 2009a; Stratman, et al., 2010).
Figure 6. EC-pericyte interactions markedly stimulate basement membrane deposition and assembly in 3D matrices in vitro and in vivo.
(A) Quantification of fibronectin immunostaining intensity levels reveals that in conditions with disrupted EC-pericyte interactions (i.e. Imatinib/Iressa) there is a dramatic decrease in the amount of fibronectin deposition around developing quail EC-lined tubes at embryonic day 6. (B) Images of this phenotype are shown in overlay images of the vasculature (QH1-green) versus fibronectin deposition (red) in control versus treatment conditions in which pericyte recruitment is markedly inhibited. Bar equals 25 μm. (C) Images displaying collagen IV or fibronectin staining (red) versus GFP-pericytes reveals a lack of deposition in the treatment condition when pericyte recruitment is blocked. Intensity mapping of this phenotype is also included demonstrating the increased localization of deposited collagen IV around EC tubes in the control versus treated condition.
The role of dysfunctional EC-pericyte interactions in the pathogenesis of disease
Our new findings showing that EC-pericyte interactions are necessary for basement membrane assembly suggests that abnormalities in this process, which lead to decreased vessel integrity, might be an important pathogenic feature of diseases such as diabetes and cancer (where abnormal EC-pericyte interactions have been observed) (Baluk, et al., 2005; Baluk, et al., 2003; Bergers, et al., 2003; Carmeliet, 2005; Hammes, et al., 2002; Hammes, et al., 2004; Hellstrom, et al., 2001; Morikawa, et al., 2002). In the case of diabetes, there is an abnormal increase in non-perfused EC vessels and a dramatic reduction of pericytes within the native vasculature as well as a lack of recruitment to newly forming vessels during angiogenic responses (Hammes, 2005; Hammes, et al., 2002; Hammes, et al., 2004; Hellstrom, et al., 2001). In these cases, our recent data demonstrating that EC-pericyte interactions are required to induce basement membrane protein deposition would suggest that abnormal vascular basement membranes (i.e. disrupted or reduced basement membrane) are present along these vessels, leading to potentially increased vascular permeability and decreased EC tube stability (Stratman, et al., 2009a; Stratman, et al., 2010). These phenotypes might result in reduced nutrient delivery and perfusion to the affected areas and potential long term tissue damage (with reduced tissue repair ability) which are common defects associated with diabetes. These same concepts also apply to the newly forming vasculature within the tumor microenvironment which controls the development and progression of malignant tumors. Overall, these vessels are known to have fewer numbers of associated pericytes, with abnormal heterotypic cell-to-cell interactions along these vessels (Baluk, et al., 2005; Bergers, et al., 2003; Morikawa, et al., 2002; Sennino, et al., 2007). As these vessels are known to be leaky with markedly increased vascular permeability, the data remains consistent with the possibility that vascular basement membranes surrounding tumor vessels may be highly abnormal. It is clear that a greater understanding of the functional role and molecular details underlying how vascular basement membranes are formed and maintained (under normal versus disease conditions) and how this relates to EC tube maturation and stabilization events is an important question for future investigation.
Conclusions
In this review, we have described new findings that demonstrate that vascular basement membrane assembly is controlled by EC-pericyte interactions in 3D extracellular matrices. This process occurs following EC tubulogenesis which leads to vascular guidance tunnel formation (a matrix-free physical space in 3D matrices generated through MT1-MMP-dependent proteolysis) and also the production of PDGF-BB and HB-EGF which are critical EC-derived factors that direct pericytes to the EC tube abluminal surface and vascular guidance tunnel spaces in vitro and in vivo. Pericyte motility along this tube abluminal surface stimulates ECs and pericytes to deposit basement membrane matrix proteins to facilitate tube maturation and stabilization events. Pericyte recruitment to EC tubes was shown to be necessary for vascular basement membrane assembly in vitro and in vivo. EC-pericyte interactions selectively induce basement membrane components as well as integrins such as α5β1, α6β1, α3β1 and α1β1, which recognize this newly deposited matrix. Thus, heterotypic EC and pericyte interactions induce specific molecular events that control vascular morphogenesis and stabilization by affecting basement membrane matrix synthesis, deposition of this remodeled ECM, recognition of the ECM through differential integrin expression, and protection of this basement membrane matrix by the presentation of TIMP-2 and TIMP-3, which block proteolysis and further tube morphogenesis as well as tube regression mechanisms through inhibition of MMPs.
As discussed above, considerable recent progress has occurred elucidating how EC-pericyte interactions lead to tube maturation and stabilization, but clearly more work is necessary to further understand these processes. One area that needs to be emphasized in future work is how growth factor- and cytokine-mediated signaling interfaces with vascular basement membrane-mediated signaling. Co-signaling between growth factor and ECM receptors is a key aspect of these events, but the specific molecular interactions necessary for such signals needs to be investigated in more detail. It is interesting that specific growth factors show selective affinities for different basement membrane components so this is likely to be a critical area that needs to be further explored. Also, how such co-signaling affects other key EC events necessary for tube maturation such as EC-EC junction formation and stability as well as EC flow responsiveness is fundamental to our understanding of these processes. Finally, the advances discussed in this review have strong implications for our understanding of the underlying pathogenesis of disease states, where abnormalities in EC-pericyte interactions are known to occur (e.g. diabetes and cancer). Furthermore, these insights could lead to novel therapeutic possibilities.
Acknowledgments
This work was supported by NIH grants HL59373, HL79460, HL87308, and HL105606 to GED.
References
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112(8):1142–51. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86(2):226–35. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Crowley D, Hynes RO. Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev Biol. 2007;311(1):11–24. doi: 10.1016/j.ydbio.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12(2):165–75. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15(1):102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163(5):1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115(Pt 6):1123–36. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Salazar R, Davis GE. RGD-dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the alpha(v)beta(3) and alpha(5)beta(1) integrins. Am J Pathol. 2000;156(5):1673–83. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114(Pt 15):2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103(2):159–65. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. Exs. 2005;94:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131(8):1847–57. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem. 1997;272(50):31525–32. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167(4):757–67. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, DellaPelle P, Manseau E, Lanigan JM, Dvorak HF, Colvin RB. Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol. 1982;79(5):269–76. doi: 10.1111/1523-1747.ep12500076. [DOI] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224(1):39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today. 2007;81(4):270–85. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97(11):1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15(3):197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011;288:101–65. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69(1):73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22(6):927–33. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–8. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90(8):3073–81. [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm Metab Res. 2005;37(Suppl 1):39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–12. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53(4):1104–10. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277(5322):48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210(2):130–45. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153(3):543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SAD, Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141(3):805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N. Nidogens-Extracellular matrix linker molecules. Microsc Res Tech. 2008;71(5):387–95. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15(3):204–9. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen E, Lauttia S, Zhang N, Tvorogov D, Kulmala J, Grenman R, Salven P, Elenius K. The EGFR inhibitor gefitinib suppresses recruitment of pericytes and bone marrow-derived perivascular cells into tumor vessels. Microvasc Res. 2009;78(3):278–85. doi: 10.1016/j.mvr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. Faseb J. 2003;17(12):1609–21. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16(2):222–31. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9(6):685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008a;121(Pt 7):989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- Koh W, Sachidanandam K, Stratman AN, Sacharidou A, Mayo AM, Murphy EA, Cheresh DA, Davis GE. Formation of endothelial lumens requires a coordinated PKC{epsilon}-, Src-, Pak- and Raf-kinase-dependent signaling cascade downstream of Cdc42 activation. J Cell Sci. 2009;122(Pt 11):1812–22. doi: 10.1242/jcs.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008b;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs) J Cell Sci. 2002;115(Pt 17):3427–38. doi: 10.1242/jcs.115.17.3427. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157(7):1279–90. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17(15):1835–40. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143(6):1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160(3):985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27(1–2):93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–28. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125(2):441–50. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010;115(25):5259–69. doi: 10.1182/blood-2009-11-252692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cell Tissues Organs. 2011 doi: 10.1159/000331410. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118(Pt 10):2325–40. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175(1):179–91. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele S, Nystrom A, Durbeej M, Talts JF, Ekblom M, Ekblom P. Laminin isoforms in development and disease. J Mol Med (Berl) 2007;85(8):825–36. doi: 10.1007/s00109-007-0182-5. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Pollner R, Poschl E, Kuhn K. Expression of human collagen type IV genes is regulated by transcriptional and post-transcriptional mechanisms. FEBS Lett. 1992;312(2–3):174–8. doi: 10.1016/0014-5793(92)80929-b. [DOI] [PubMed] [Google Scholar]
- Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a005090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67(15):7358–67. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smola H, Stark HJ, Thiekotter G, Mirancea N, Krieg T, Fusenig NE. Dynamics of basement membrane formation by keratinocyte-fibroblast interactions in organotypic skin culture. Exp Cell Res. 1998;239(2):399–410. doi: 10.1006/excr.1997.3910. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Ciocea A, Byzova TV. Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. 2009;53(2):53–64. doi: 10.1007/s12013-008-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Davis MJ, Davis GE. VEGF and FGF prime vascular tube morphogenesis and sprouting directed by hematopoietic stem cell cytokines. Blood. 2011;117(14):3709–19. doi: 10.1182/blood-2010-11-316752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009a;114(24):5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009b;114(2):237–47. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116(22):4720–30. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–38. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22(4):1194–202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Proteoglycans of basement membranes. Exs. 1994;70:123–44. doi: 10.1007/978-3-0348-7545-5_8. [DOI] [PubMed] [Google Scholar]
- Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983;137(3):455–65. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Timpl R, Fujiwara S, Dziadek M, Aumailley M, Weber S, Engel J. Laminin, proteoglycan, nidogen and collagen IV: structural models and molecular interactions. Ciba Found Symp. 1984;108:25–43. doi: 10.1002/9780470720899.ch3. [DOI] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–88. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106(5):932–40. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22(7):521–38. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15(12):1277–94. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141(2):539–51. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]