Abstract
Evidence supports the premise that maternal psychological distress adversely affects pregnancy outcomes and that inflammatory markers and placentally-produced corticotrophin-releasing hormone (pCRH) are likely mediating factors. The primary aim of the study was to explore the associations between maternal psychological distress, use of selective serotonin re-uptake inhibitors, pCRH, and maternal plasma inflammatory markers during pregnancy. Measures of maternal plasma pCRH, Interleukins-1, 6, & 10, C-Reactive Protein, Macrophage Migration Inhibitory Factor, and Tumor Necrosis Factor-α were completed in 100 pregnant women. Measures of depression, anxiety, and perceived stress were completed, as well as collection of demographic/behavioral data, e.g. use of selective serotonin re-uptake inhibitors (SSRIs). Significant correlations were found at 14–20 weeks gestation between IL-6 & 10, and depression, anxiety, and perceived stress. Also at 14 – 20 weeks gestation, IL10 levels were significantly lower in women with 4th quartile pCRH levels and IL1β, IL6, and IL10 were significantly lower among women who took an SSRI during pregnancy. After controlling for maternal age, BMI, pCRH level, and SSRI use, psychological distress remained to explain variation in maternal inflammatory markers. These results might suggest that future research should focus on whether depression and anxiety are effectively being treated during pregnancy, and how such a scenario might contribute to an immune system pathway to poor pregnancy outcome.
Keywords: Pregnancy, Psychological Distress, Depression, Anxiety, Cytokines, Interleukin-10, Inflammation, CRH, SSRI
1. INTRODUCTION
Significant evidence supports the premise that chronic psychological distress contributes to adverse pregnancy outcome, such as preterm birth [1–4]. Plausible explanations include interactions between stress physiology and the normal physiology of pregnancy and birth, in addition to individual health behaviors and genomic makeup [5]. These interactions frequently involve immune mediators, such as interleukins (IL) 1, 6, 10, and Tumor Necrosis Factor-alpha (TNFα), and hormonal/neurohormonal mediators, such as placental corticotrophin-releasing hormone (pCRH) [6–10]. While there are several reports of significant associations between psychological distress and inflammatory markers in pregnancy [11–13], or between psychological distress and pCRH [14–17], there has been little focus on evaluating the relationships between maternal use of SSRIs during pregnancy and inflammatory markers or pCRH levels. In a previous study [18] we reported that pCRH and SSRI use during pregnancy were independent predictors of preterm birth. Additionally, there are conflicting studies that implicate either depression and anxiety or use of antidepressants (e.g. selective serotonin re-uptake inhibitors—SSRIs) in association with a higher risk of preterm birth [19–21]. At least one study suggests that antidepressants and psychological distress during pregnancy convey an equal risk (20%) of preterm birth [21]. In light of these previous findings, the current study was conducted to address the following aims: 1) evaluate the relationship between inflammatory markers and a) the use of SSRIs during pregnancy, b) pCRH, and c) psychological distress during pregnancy, and 2) determine whether psychological distress, SSRI, or pCRH levels are predictive of inflammatory markers during pregnancy after controlling for maternal BMI and age. Using the data from the same cohort of women from a previous study, and accessing the associated plasma repository in order to conduct inflammatory marker assays, we aimed to further explore these important relationships.
1.1. Psychological Distress Defined
Chronic stress is a multidimensional, composite concept Published Online February 2013 in SciRes. http://www.scirp.org/journal/ojog making measurement difficult [22]. Stress appraisal, personal history and outlook, lifestyle, coping style, and environmental threats (real or imagined) are only some of the contributors to the experience of stress. Psychological distress has a well-documented association with, and is generally presumed to be a common response to chronic stress [23,24]. The three most commonly identified and measured aspects of psychological distress are depression, anxiety, and perceived stress. Within the context of pregnancy, psychological distress may be a marker of an elevated risk for adverse perinatal outcome [2].
1.2. Psychoneuroimmunology (PNI) Framework
Figure 1 provides a PNI framework that explains the theoretical links between psychosocial and behavioral stress, psychological distress, alterations in the neurohormonal and immune systems during pregnancy, and adverse pregnancy outcome. These links form the framework for the current study.
Figure 1.
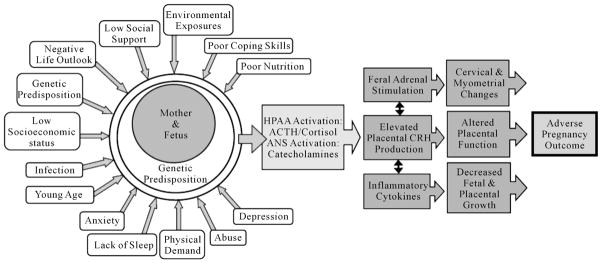
Proposed PNI model linking psycho-social stress, neurohormonal & immune mediators, and adverse pregnancy outcomes. HPAA: Hypothalamic-Pituitary-Adrenal Axis; ACTH: Adrenocorticotrophin Releasing Hormone; ANS: Autonomic Nervous System; C RH: Corticotrophin Releasing Hormone.
2. METHODS
A previously conducted prospective cohort study of chronic maternal stress and preterm birth resulted in a maternal blood sample repository for use in this study. For in-depth study detail readers are referred to the original study reports [18]. Approval was obtained from the institutional review board at the University of Utah Health Sciences Center, which included explicit participant consent for the use of collected blood samples in future studies. The current study made use of the previously collected samples for the measurement of inflammatory cytokines. Briefly, blood samples were collected from pregnant women at 14 – 20 weeks (T1) and 26 – 30 weeks (T2) gestation into chilled 6-ml lavender top vacuum tubes containing ethylenediaminetetraacetic acid (EDTA) and aprotonin (500 KIU/ml blood), centrifuged at 4°C and 3000 rpm for 15 min, plasma aliquots placed into 0.5 ml cryotubes, and stored at −80°C (+10°) until time of assay (<2 years). IL1β, 6, 10, TNFα, were measured at T1 and T2. CRP and Macrophage Migration Inhibitory Factor (MMIF) were measured at T2. Previously collected data also included measures of maternal pCRH (measured at T1), as well as completion of a self-administered participant questionnaire (T1) and were available for inclusion into the current study’s analyses.
2.1. Participants
Participants (n = 100) were enrolled prior to 20 weeks gestation from community prenatal clinics, were 18 years of age or older, and were excluded for any of the following conditions (to reduce potential influence from confounding variables): Hypertension, diabetes, cardiovascular disease, immune or autoimmune disorders, threatened abortion, vaginal bleeding, or systemic corticosteroid therapy. Gestational age was determined by clinical dating and ultrasound examination prior to 20 weeks for all women. Women were provided with a $10 gift certificate to a local grocery/department store upon completion of the study questionnaire.
2.2. Measures of Psychological Distress
A self-administered participant questionnaire completed at T1 included the Centers for Epidemiological Studies Depression scale, the Perceived Stress Scale, the Pregnancy-Related Anxiety scale, and demographic/behavioral data (including tobacco, drug, and alcohol exposure, and use of psychotherapeutic medications during pregnancy). Self-reported data were corroborated via medical record review, specifically those related to the use of medications (i.e. selective serotonin reuptake inhibitors). For further detail on the validity and reliability of these instruments, please refer to the original study reports (citations). The questionnaires were completed at home, taking approximately 45 minutes to complete, and returned via a pre-paid postage envelope.
2.3. Placental CRH Measurement
Placentally-derived CRH concentrations were measured by specific and highly sensitive radioimmunoassay (RIA) kit (Phoenix Pharmaceuticals, Burlingame, CA). Briefly, the CRH RIA is 100% specific for human CRH with 0% cross-reactivity to ACTH, luteinizing hormone, vasopressin, and urocortin. The RIA has a sensitivity of 0.00095 ng/ml to 0.02 ng/ml and an intra and inter assay precision (%) of 2 – 6/8.5% – 10%, respectively. Assays were conducted in the Biobehavioral Laboratory, School of Nursing and Allied Health, at the University of Texas Medical Branch—Galveston.
2.4. Inflammatory Markers
Cytokine (IL1β, IL6, IL10, TNFα, MMIF) and CRP concentrations were determined using multiplex bead-based xMAP (Merck Millipore, Darmstadt, Germany) technology and the Luminex Liquichip 200 System (Qiagen, Valencia, CA), also in the Biobehavioral Laboratory at the University of Texas Medical Branch—Galveston. Cytokine-specific antibody-coated beads (Millipore Corporation, Billerica, MA) were used for these experiments. The assay was performed in duplicate according to the manufacturer’s instructions. Cytokine concentrations were calculated by using a standard curve derived from a recombinant cytokine standard. The multiplex bead assay is 100% specific for all analytes, with 0% cross-reactivity. The assay has a sensitivity of 3.2 – 10,000 pg/mL and an intra and inter assay precision (%) of 4 – 13 and 5 – 17, respectively.
2.5. Statistical Approach
Bivariate analyses, parametric and non-parametric, were conducted to test the associations between inflammatory markers, pCRH, SSRI use, and psychological distress measures. Reverse coding for questionnaire items (i.e. in the CES-D and PSS) was completed prior to analyses, where necessary. Placental CRH displayed a bi-modal distribution, essentially rendering the variable into a dichotomous “high” and “low” (4th quartile vs below the 4th quartile) thus treated most usefully as a dichotomous variable for all analyses.
Subsequently, multiple linear regression modeling was used to investigate the relationship of pCRH, SSRI usage, and psychological distress with individual cytokine levels. To deal with multicollinearity among the three psychological measures (CESD, PSS, PSA) we conducted a one-factor principal component analysis to form a single measure of psychological distress before loading into the regression models.
3. RESULTS
3.1. Demographic Characteristics
The questionnaire return rate was 85%. Tables 1 and 2 provide the sample demographic data (n = 100). Participants were predominantly non-Hispanic white, married, and multiparous, with an average age of 25.8 (range 18 – 40 years). Over half of the participants had an annual family income less than $40,000, and 62% had not completed more than a high school education.
Table 1.
Sample demographics, ethnicity & race.
| Study Demographics | % of Sample (n = 100) |
|---|---|
| Ethnicity | |
| Hispanic | 24 |
| Non-Hispanic | 76 |
| Race | |
| White | 69 |
| Hispanic/Mexican | 13 |
| African/African American | 4 |
| Pacific Islander | 4 |
| Multi-Racial | 4 |
| Asian | 3 |
| American Indian/Alaskan Native | 3 |
Table 2.
Sample demographics, marital, parity, insurance.
| Study Demographics | % of Sample (n = 100) |
|---|---|
| Marital Status | |
| Married | 64 |
| Living with Partner | 13 |
| Single | 21 |
| Divorced/Separated | 2 |
| Parity | |
| Nulliparous | 36 |
| Multiparous | 64 |
| Health Insurance | |
| Private | 56 |
| State Medicaid | 33 |
| Uninsured/Self Pay | 11 |
3.2. Descriptive Statistics
Descriptive statistics for psychosocial measures are presented in Table 3, and biological measures are presented in Table 4.
Table 3.
Descriptive statistics for psychological distress measures.
| Range | Mean (SD) | |
|---|---|---|
| CES-D (n = 84) | 0 – 49 | 18.5 (12.15) |
| PSS (n = 84) | 2 – 34 | 18.7 (7.84) |
| PSA (n = 85) | 2 – 20 | 10.3 (4.09) |
CES-D: Center for Epidemiologic Studies Depression Scale; PSS: Perceived Stress Scale; PSA: Pregnancy-Specific Anxiety.
Table 4.
Descriptive statistics for inflammatory markers.
| Range | Mean (SD) | |
|---|---|---|
| Body Mass Index (n = 79) | 15.8 – 52.1 | 28.8 (7.01) |
| Interleukin-1β (IL-1β) | ||
| T1 (n = 98) | 0 – 32.4 | 3.537 (5.702) |
| T2 (n = 76) | 0 – 31.0 | 2.886 (6.075) |
| Interleukin-6 (IL-6) | ||
| T1 (n = 97) | 0 – 26.2 | 2.542 (4.321) |
| T2 (n = 75) | 0 – 22.3 | 2.415 (4.925) |
| Interleukin-10 (IL-10) | ||
| T1 (n = 97) | 0 – 29.6 | 2.589 (4.847) |
| T2 (n = 76) | 0 – 31.8 | 3.934 (5.837) |
| Tumor Necrosis Factor-α (TNFα) | ||
| T1 (n = 98) | 0 – 19.8 | 3.476 (2.370) |
| T2 (n = 76) | 0 – 14.6 | 3.420 (2.616) |
| C Reactive Protein (CRP) | ||
| T2 (n = 76) | 0 – 601,931 | 104,095 (111,843) |
| Macrophage Migration Inhibitory Factor (MMIF) | ||
| T2 (n = 75) | 0 – 30.99 | 4.776 (5.453) |
T1: Time one (14 – 20 weeks gestation); T2: Time two (26 – 30 weeks gestation).
3.3. Correlations between Inflammatory Markers and Psychological Distress
Table 5 presents the correlations between inflammatory markers and psychosocial/demographic measures. Negative relationships were identified between IL1β, 6, and 10 and measures of depression (CES-D), perceived stress (PSS), and pregnancy specific anxiety (PSA) at T1 (14 – 20 weeks gestation), but not at T2 (26 – 28 weeks gestation). Negative correlations were also observed between TNFα and PSS and PSA scores at T1, but not at T2. A positive correlation was demonstrated between TNFα and BMI at T1, as well as T2.
Table 5.
Correlations between inflammatory markers and psychological distress measures, maternal age, and body mass index.
| CES-D | PSS | PSA | Maternal Age | Maternal BMI | |
|---|---|---|---|---|---|
| Interleukin-1β | |||||
| T1 | −0.235* | −0.243* | −0.262* | NS | NS |
| T2 | NS | NS | NS | NS | NS |
| Interleukin-6 | |||||
| T1 | −0.257* | −0.250* | −0.274* | NS | NS |
| T2 | NS | NS | NS | NS | NS |
| Interleukin-10 | |||||
| T1 | −0.272* | −0.285** | −0.252* | NS | NS |
| T2 | NS | NS | NS | NS | NS |
| TNF-α | |||||
| T1 | NS | −0.219* | −0.230* | NS | 0.215* |
| T2 | NS | NS | NS | NS | 0.256* |
| CRP | |||||
| T2 | NS | NS | NS | NS | 0.519** |
| MMIF | |||||
| T2 | NS | NS | NS | NS | NS |
p < 0.05;
p < 0.01;
NS: not significant; T1: 14 – 20 weeks gestation; T2: 26 – 30 weeks gestation; BMI: Body Mass Index; GA: gestational age; TNF-α: Tumor Necrosis Factor-alpha; CRP: C Reactive Protein; MMIF: Macrophage Migration Inhibitory Factor.
As expected, CRP was also positively correlated with body mass Index (BMI), but no other correlations with CRP were demonstrated. No associations were observed between MMIF and any psychosocial measure at T1 or T2.
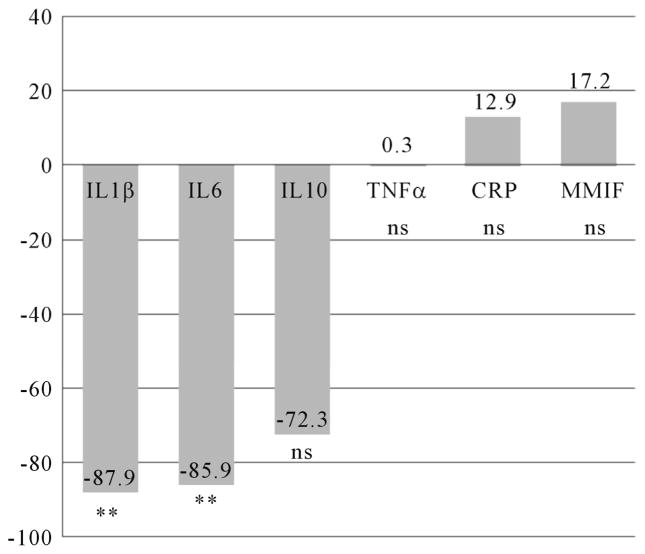
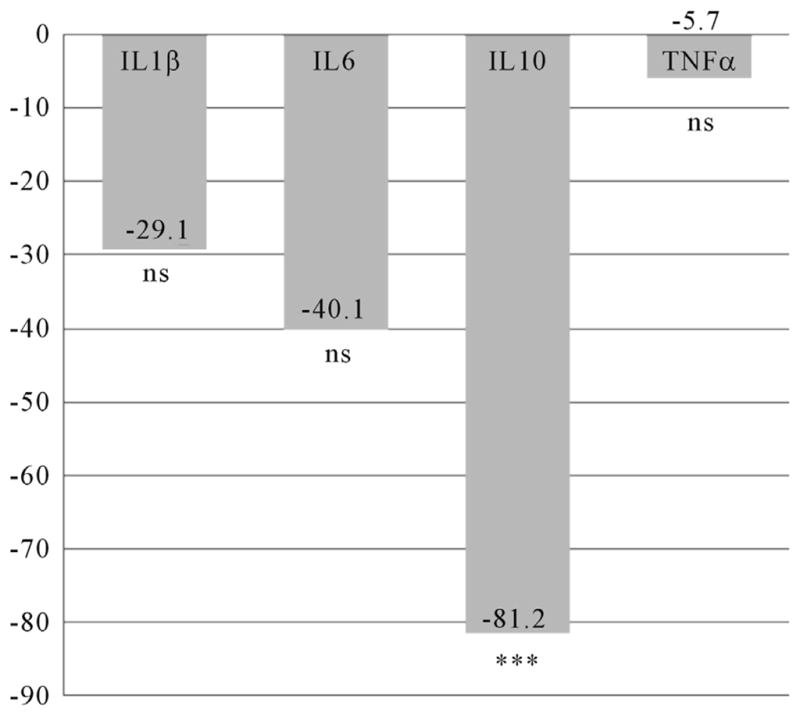
The mean IL10 level was significantly lower (t = 3.67; df 95; p = 0.000) in women with pCRH levels in the 4th quartile (n = 24) at time 1 (Figure 2), but no differences were found at time 2 (Figure 3). For women who took an SSRI during pregnancy (n = 13), mean IL1β (t = 2.77; df 44; p = 0.008) and IL10 (t = 2.29; df 34; p = 0.029) levels were significantly decreased at time 1 (Figure 4), while mean IL1β (t = 3.35; df 73; p = 0.001) and IL6 (t = 3.13; df 59; p = 0.003) levels were significantly decreased at time two (Figure 5).
Figure 2.
Differences in Inflammatory Cytokines at 14 – 20 Weeks Gestation for Women with pCRH Levels in the 4th Quartile (measured at 14 – 20 weeks). n = 24. Differences expressed as a percentage of the reference group (CRH levels below the 4th quartile). IL = Interleukin TNFα = Tumor Necrois Factor alpha ***p < 0.001; s ns = non significant.
Figure 3.
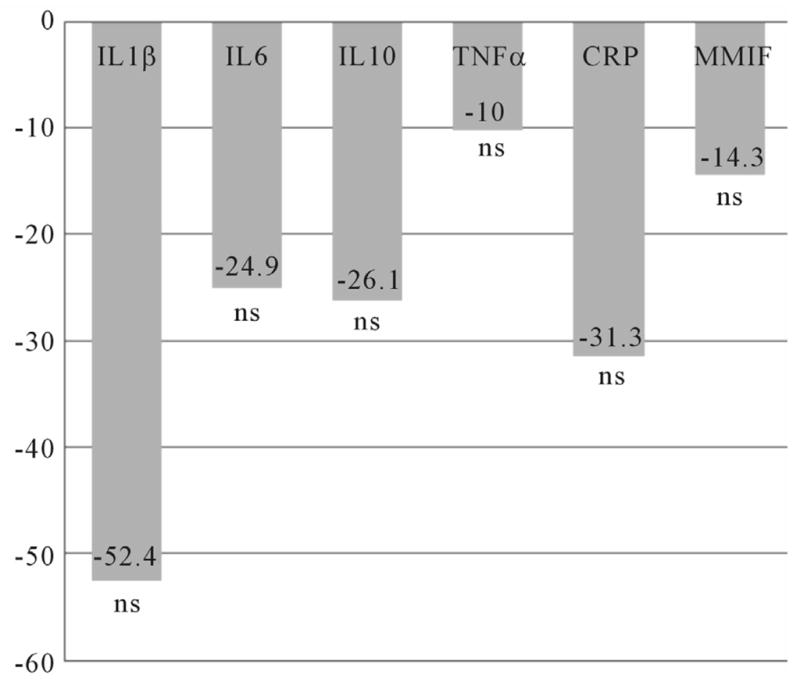
Differences in Inflammatory Cytokines at 26 – 30 Weeks Gestation for Women with pCRH Levels in the 4th Quartile (measured at 14–20 weeks). n = 24. Differences expressed as a percentage of the reference group (CRH levels below the 4th quartile). IL = Interleukin; TNFα = Tumor Necrosis Factor alpha; CRP: C Reactive Protein; MMIF: Macrophage Migration Inhibitory Factor; ns = non significant.
Figure 4.
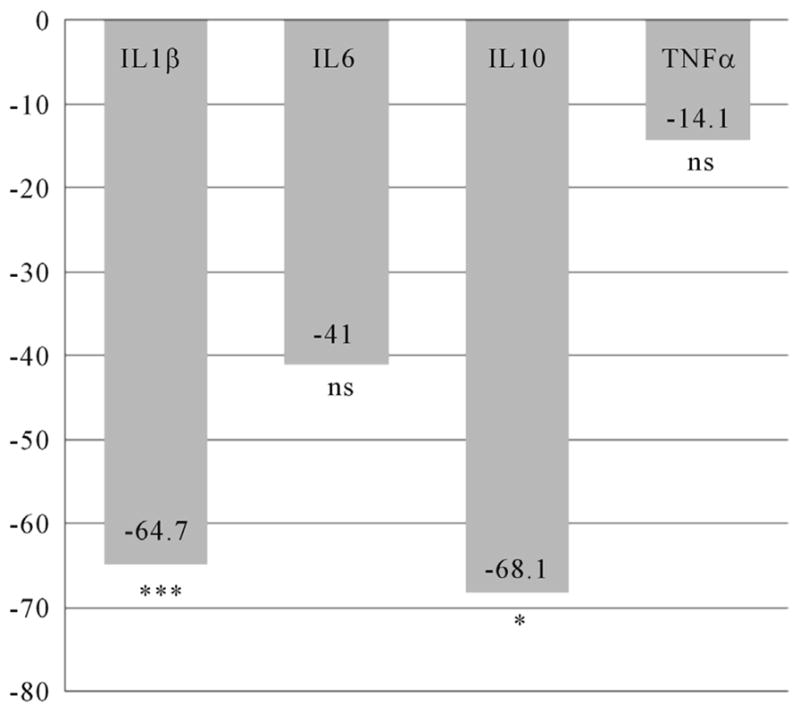
Differences in Inflammatory Cytokines at 14 – 20 Weeks Gestation for Women Using SSRIs during Pregnancy. n = 13. Differences expressed as a percentage of the reference group (women without SSRI use during pregnancy). IL = Interleukin; TNFα = Tumor Necrosis Factor alpha; *p < 0.05; ***p < 0.001; ns = non significant.
Figure 5.
Differences in Inflammatory Cytokines at 26 – 30 Weeks Gestation for Women Using SSRIs during Pregnancy. n = 13. Differences expressed as a percentage of the reference group (women without SSRI use during pregnancy). IL = Interleukin; TNFα = Tumor Necrosis Factor alpha; CRP: C Reactive Protein; MMIF: Macrophage Migration Inhibitory Factor; **p < 0.01; ns = non significant.
3.4. Multiple Linear Regression Modeling
Table 6 shows the standardized betas and p-values for each cytokine. For IL1β the regression model showed modest fit (F = 2.63, p = 0.06); psychological distress was the only significant predictor variable (t = 2.33, p = 0.02). Results for IL6 were similar, overall model fit (F = 2.70, p = 0.05) with psychological distress as the only significant predictor (t = 2.55, p = 0.01). The overall model fit for IL10 was good (F = 4.10, p = 0.01), again psychological distress was a significant predictor (t = 2.33, p = 0.02). The model fit for TNFα was poor (F = 1.60, p = 0.20), with psychological distress significant (t = 2.14, p = 0.04).
Table 6.
Results of regression modeling for measures at 14 – 20 weeks gestation.
| Model Fit | IL1 | IL6 | IL10 | TNFα | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| F = 2.63, p = 0.06 | F = 2.70, p = 0.05 | F = 4.10, p = 0.01 | F = 1.60, p = 0.20 | |||||
|
| ||||||||
| Std β | p value | Std β | p value | Std β | p value | Std β | p value | |
| pCRH (high/low)* | 0.03 | 0.83 | −0.02 | 0.88 | −0.18 | 0.10 | −0.01 | 0.92 |
| SSRI (yes/no) | −0.11 | 0.33 | −0.04 | 0.69 | −0.08 | 0.47 | 0.03 | 0.78 |
| Psych. Distress | −0.26 | 0.02 | −0.29 | 0.01 | −0.26 | 0.02 | −0.25 | 0.04 |
High = 4th quartile; Low = below the 4th quartile; pCRH = Placentally-Produced Corticotrophin Releasing Hormone; IL = Interleukin; TNFα: Tumor Necrosis Factor-alpha.
4. DISCUSSION
In initial univariate analyses, maternal psychological distress, pCRH, and SSRI use all appear to be associated with alterations in inflammatory cytokines during pregnancy, particularly at 14 – 20 weeks of gestation. However, multiple linear regression results indicate that only psychological distress remains as a predictor of inflammatory markers, specifically interleukins 1β, 6, 10, and TNFα, after controlling for maternal BMI and age. The study is unique in that it included consideration for SSRI use, as well as pCRH levels when evaluating maternal inflammatory markers during pregnancy. Notably, higher levels of psychological distress predicted lower levels of plasma IL-1, 6, 10, and TNFα at 14 – 20 weeks of gestation.
The study findings regarding IL-10 are consistent with another study which also reported lower levels of IL-10 in association with maternal “stress” or “distress” [25]. Recall that a normal immune status during pregnancy includes a predominantly elevated IL10 (anti-inflammatory cytokine), and decreased levels of IL1β, IL6, and TNFα (pro-inflammatory cytokines) during much of the pregnancy [26]. The final weeks of pregnancy are marked with an increasingly pro-inflammatory environment, including increases in pro inflammatory cytokines (IL1β, IL6, and TNFα) and decreased anti inflammatory cytokines (i.e. IL-10), all of which interact with high levels of pCRH [8] to effect a normal progression towards fetal maturity and parturition. A reduction in IL-10 levels early in pregnancy could perturb the inflammatory balance and is one plausible explanation for links between inflammation and poor pregnancy outcome [27]. Furthermore, perturbations earlier in pregnancy could logically set the stage for a cascade of events that are out of synchrony with normal physiologic pregnancy progression.
Our study is incongruent with others which report elevated pro-inflammatory cytokines in relation to increased maternal stress or distress. Our study results indicate consistently decreased levels of pro inflammatory cytokines in association with measures of psychological distress early in pregnancy, and no such associations later in pregnancy. This could be the result of differing study populations and measures of psychological stress/distress. For example, while our study included an extensively used measure for depression (CES-D) and perceived stress (PSS), others have used measures of “global stress” or identification of life events and stressors, thus perhaps reflecting evaluations of different latent variables [25,28,29]. Furthermore, our study population was predominantly Caucasian, whereas others were predominantly Latinas [28]. There is evidence to indicate variations in inflammatory markers between ethnic/racial groups, for example in CRP [30]. Obese and lean women also demonstrate differences in inflammatory markers, and based on normal or complicated pregnancy [31]. Perhaps most importantly, most studies did not exclude participants with conditions that are known or suspected to contribute to a pro inflammatory response. We eliminated these potential confounders by excluding all women with hypertension, diabetes, cardiovascular disease, systemic corticosteroid therapy, immune or autoimmune disorders, threatened abortion, etc.
5. SUMMARY
Our study results are consistent with and bolster current evidence that psychological distress adversely alters inflammatory-mediated pathways during pregnancy, thus perhaps contributing to poor pregnancy outcome. After controlling for maternal age and BMI, regression modeling indicates that psychological distress (not pCRH or SSRI use) remains to predict variation in inflammatory cytokines at 14 – 20 weeks of gestation, particularly IL10.
Most previous studies have focused on pro-inflammatory cytokines, not anti-inflammatory, (i.e. IL10), and have included limited measures of maternal psychological distress. This study is the first to document biobehavioral predictors of alterations in inflammatory markers in pregnant women, including the use of an SSRI, psychological distress, and pCRH. A reduction in IL-10 levels suggests that a pro-inflammatory environment may exist in association with psychological distress, and contribute to pregnancy complications such as preterm birth.
5.1. Study Limitations
Given the total study sample size (n = 100) and the small number of women who took SSRIs (n = 13) or had pCRH levels in the 4th quartile (n = 24) in this study, the results must be interpreted with caution. Until prospective studies are undertaken in a larger population of women, with consideration for SSRI dosage and therapeutic levels, and using appropriate comparison groups, we can only speculate about the pathophysiologic mechanisms involved. Future studies would also require pre pregnancy, pregnancy, and post pregnancy measures to more fully evaluate any associations. Regardless of the study limitations, the study results are thought provoking. These results may call into question whether the current approaches to use of SSRIs during pregnancy could be a factor in dysregulation of the prenatal inflammatory milieu and associated adverse pregnancy outcomes, such as preterm birth.
5.2. Clinical Implications
In our study, psychological distress remained to predict alterations in cytokines, particularly for decreased IL-10 levels, after controlling for pCRH levels, BMI, maternal age, and SSRI use. A reduction in IL-10 (an anti-inflammatory cytokine) levels suggests that a pro-inflammatory environment may exist in association with psychological distress, and contribute to pregnancy complications such as preterm birth. These findings might also suggest that pregnant women are being undertreated for psychological distress (i.e. depression and anxiety) during the prenatal period. This could be a result of the increased metabolism of SSRIs during pregnancy [32] and/or because health providers and pregnant women themselves are reluctant to initiate or increase SSRI dosage during pregnancy [33]. Undertreatment by way of subtherapeutic maternal SSRI levels could be a contributor to ongoing psychological distress and associated increases in the maternal pro inflammatory environment. Indeed, we have previously reported that women who use SSRIs during pregnancy still score significantly higher on measures of perceived stress and depression [18]. A pro inflammatory environment despite pharmacotherapeutic interventions for depression, anxiety, and perceived stress could provide some explanation for increased risk of adverse pregnancy outcome, but this deserves further study.
Acknowledgments
Funding Support: NRSA-NIH/NINR (F31 NR010046-01) March of Dimes Foundation, Inc. American College of Nurse-Midwives Foundation, Inc. University of Utah College of Nursing Research Committee Grant.
References
- 1.Glynn LM, et al. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychology. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hobel C, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clinical Obstetrics and Gynecology. 2008;51:333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 3.Behrman RE, Stith Butler A. Preterm Birth: Causes, Consequences, and Prevention. National Academy Press; Washington DC: 2007. [PubMed] [Google Scholar]
- 4.Karimi K, Arck PC. Natural Killer cells: Keepers of pregnancy in the turnstile of the environment. Brain, Behavior, and Immunity. 2010;24:339–347. doi: 10.1016/j.bbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Latendresse G. The interaction between chronic stress and pregnancy: preterm birth from a biobehavioral perspective. J Midwifery Womens Health. 2009;54(1):8–17. doi: 10.1016/j.jmwh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challis JR, et al. Endocrine and paracrine regulation of birth at term and preterm. Encodrine Reviews. 2000;21:514–550. doi: 10.1210/er.21.5.514. [DOI] [PubMed] [Google Scholar]
- 7.Norwitz ER, Robinson JN, Challis JR. The control of labor. New England Journal of Medicine. 1999;341:660–666. doi: 10.1056/NEJM199908263410906. [DOI] [PubMed] [Google Scholar]
- 8.Snegovskikh V, Park JS, Norwitz ER. Endocrinology of parturition. Endocrinology and Metabolism Clinics of North America. 2006;35:173–191. doi: 10.1016/j.ecl.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Challis JR, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 10.Pearce BD, et al. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. American Journal of Obstetrics and Gynecology. 2008;199:e1–e6. doi: 10.1016/j.ajog.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christian LM, et al. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain, Behavior, and Immunity. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christian LM, et al. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain, Behavior, and Immunity. 2010;24:49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright RJ, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso, et al. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosomatic Medicine. 2004;66:762–769. doi: 10.1097/01.psy.0000138284.70670.d5. [DOI] [PubMed] [Google Scholar]
- 15.Latendresse G, Ruiz RJ. Maternal coping style and perceived adequacy of income predict CRH levels at 14–20 weeks of gestation. Biological Research for Nursing. 2010:20798157. doi: 10.1177/1099800410377111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobel C, et al. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180:S257–S263. doi: 10.1016/S0002-9378(99)70712-X. [DOI] [PubMed] [Google Scholar]
- 17.Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 18.Latendresse G, Ruiz RJ. Maternal corticotropin-releasing hormone and the use of selective serotonin reuptake inhibitors independently predict the occurrence of preterm birth. Journal of Midwifery Womens Health. 2011;56:118–126. doi: 10.1111/j.1542-2011.2010.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suri R, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. American Journal of Psychiatry. 2007;164:1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 20.Dayan J, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosomatic Medicine. 2006;68:938–946. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 21.Wisner KL, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. American Journal of Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Anna-Hernandez KL, et al. Acculturation, maternal cortisol, and birth outcomes in women of Mexican descent. Psychosomatic Medicine. 2012;74:296–304. doi: 10.1097/PSY.0b013e318244fbde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrousos G. Stressors, stress, and neuroendocrine integration of the adaptive response. Annual New York Academic Science. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 24.Kudielka BM, Wust S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- 25.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Kalantaridou SN, et al. Corticotropin-releasing hormone, stress and human reproduction: an update. Journal of Reproductive Immunology. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Gotsch F, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/ inflammation, and spontaneous parturition at term: a role for interleukin-10. Journal of Maternal Fetal and Neonatal Medicine. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce BD, et al. Interrelationship of cytokines, hypothalamic-pituitary-adrenal axis hormones, and psychosocial variables in the prediction of preterm birth. Gynecology and Obstetrics Investigation. 2010;70:40–46. doi: 10.1159/000284949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Entringer S, et al. Influence of prenatal psychosocial stress on cytokine production in adult women. Developmental Psychobiology. 2008;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picklesimer AH, et al. Racial differences in C-reactive protein levels during normal pregnancy. American Journal of Obstetrics and Gynecology. 2008;199:e1–e6. doi: 10.1016/j.ajog.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Founds SA, et al. A comparison of circulating TNF-alpha in obese and lean women with and without preeclampsia. Hypertension in Pregnancy. 2008;27:39–48. doi: 10.1080/10641950701825838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hostetter A, et al. Dose of selective serotonin uptake inhibitors across pregnancy: Clinical implications. Depression and Anxiety. 2000;11:51–57. doi:10.1002/(SICI)1520-6394(2000)11:2<51::AID-DA1> 3.0.CO;2-R. [PubMed] [Google Scholar]
- 33.Patil AS, Kuller JA, Rhee EH. Antidepressants in pregnancy: A review of commonly prescribed medications. Obstetrics and Gynecology Survey. 2011;66:777–87. doi: 10.1097/OGX.0b013e31823e0cbf. [DOI] [PubMed] [Google Scholar]