Abstract
Purpose
Ultraviolet B (UVB) radiation from sunlight is a known risk factor for human corneal injury. The aim of the present study was to investigate the protective effects of green tea polyphenol epigallocatechin gallate (EGCG) on UVB radiation–induced corneal oxidative damage in male imprinting control region (ICR) mice.
Methods
Corneal oxidative damage was induced by exposure to UVB radiation at 560 μW/cm2. The animals received 0%, 0.1%, and 0.01% EGCG eye drops at a 5 mg/ml dose, twice daily for 8 days. Corneal surface damage was graded according to smoothness and the extent of lissamine green staining. Corneal glutathione (GSH), thiobarbituric acid-reactive substances (TBARS), and protein carbonyl levels, as well as superoxide dismutase (SOD), catalase, glutathione peroxidase (GSH-Px), and glutathione reductase (GSH-Rd) activity in the cornea, were measured to monitor corneal injury.
Results
UVB radiation caused significant damage to the corneas, including apparent corneal ulceration and severe epithelial exfoliation, leading to a decrease in SOD, catalase, GSH-Px, GSH-Rd, and GSH activity in the cornea. However, the corneal TBARS and protein carbonyls increased compared with the control group. Treatment with EGCG eye drops significantly (p<0.05) ameliorated corneal damage, increased SOD, catalase, GSH-Px, GSH-Rd, and GSH activity, and decreased the TBARS and protein carbonyls in the corneas compared with the UVB-treated group.
Conclusions
EGCG eye drops exhibit potent protective effects on UVB radiation–induced corneal oxidative damage in mice, likely due to the increase in antioxidant defense system activity and the inhibition of lipid peroxidation and protein oxidation.
Introduction
Ultraviolet (UV) radiation, one part of the sunlight spectrum, is the most common cause of radiation damage to the eye. The cornea has the physiologic capability of blocking the majority of UVB radiation and protects the inner eye against UVB-induced phototoxicity and oxidative damage. A recent study indicated that 92% of UVB and 60% of UVA radiation are absorbed by the cornea, which is most sensitive to UVB injury [1]. However, the corneal effects of excessive exposure to UVB radiation may include photokeratitis, damage to the epithelium, edema [2], and a number of biochemical changes, including DNA modification, protein cross-linking [3], enzyme inactivation, and the production of excessive reactive oxygen species (ROS) [4].
Many studies have reported that natural antioxidants are efficacious in preventing and curing UVB-induced corneal pathology due to their particular interactions and synergism [5]. A major defense mechanism for preventing and treating corneal damage comprises reducing the production of reactive metabolites by increasing the levels of endogenous antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), and decreasing lipid peroxidation [6].
Green tea (Camellia sinensis, Theaceae) is one of the most popular beverages in the world, and is associated with many Asian countries, particularly China and Japan. Green tea contains abundant bioactive substances and has been reported to have beneficial biologic effects attributed to its polyphenols, mainly catechins and catechin derivatives including (-)-epigallocatechin-3-gallate (EGCG), epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and (-)-gallocatechin gallate (GCG) [7,8]. The functional activities of green tea catechins have been reported in vitro and in vivo; specifically, EGCG has been a research focus in recent years due to its high antioxidant activity and its presence in relatively large amounts in green tea. Many studies have reported that EGCG can prevent certain types of chronic diseases, including cancer, obesity, type 2 diabetes mellitus, lipid metabolism abnormity, atherosclerosis, and other cardiovascular diseases [9-12]. EGCG has been demonstrated to protect the heart, brain, and kidney from oxidative injury [13-15]. However, few studies have investigated the effectiveness of EGCG in vision science and eye diseases, although limited evidence in vitro suggests that EGCG, used as an anti-inflammatory and antioxidant agent, provides short- and long-term protection against strong oxidative stress in multiple human eye epithelial cell types through various mechanisms [16-19]. Additionally, only a few in vivo models are available for EGCG study of eye diseases.
Based on the excellent antioxidant activities of EGCG found in vitro, we were interested in evaluating its protective effects in vivo. In the present study, male imprinting control region (ICR) mice were treated with topical EGCG eye drops two times daily, accompanied by UVB exposure for 8 days. Corneal surface damage was graded according to corneal smoothness and the extent of lissamine green staining. Corneal SOD, catalase, GSH-Px, and GSH-Rd activity, as well as GSH, TBARS, and protein carbonyl levels in the corneal tissues were measured to monitor corneal injury.
Methods
Material
Lissamine green and (-)-EGCG were purchased from Sigma Chemical Company (St. Louis, MO). Deionized water was prepared using a Mill-RQ and Milli Q-UV water purification system (Millipore, Taipei City, Taiwan). All other chemicals and reagents used were obtained from local sources and were of analytical grade.
Animals
Male ICR mice (22 ± 2 g; 5 weeks old) were obtained from the Animal Department of BioLASCO Taiwan (Taipei City, Taiwan). The animals were quarantined and allowed to acclimate for 1 week before the experiment began. They were housed three to four per cage under standard laboratory conditions with a 12 h:12 h light-dark cycle. The animal room temperature was maintained at 25 ± 2 °C with 55 ± 5% relative humidity. Air-handling units in the animal rooms were set to provide approximately 12 fresh air changes per hour. The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee (IACUC), and the animals were cared for in accordance with the institutional ethical guidelines. All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Treatment
The animals were randomly divided into four groups, each consisting of ten mice. Group I served as the untreated normal control. To induce corneal damage in vivo, the eyes of the animals in groups II, III, and IV were exposed to UVB radiation using Tanito and colleagues’ method [20], with slight modifications. After anesthesia was induced by intraperitoneal injection of sodium pentobarbital (60 mg/kg bodyweight), both eyes were exposed to 560 μW/cm2 of UVB light (UVLS-26; UVP, Cambridge, UK) for 180 s in a darkroom. During the radiation course, the mice were confined in an adjustable retaining cage that protected most of the animal, except the head, from the UV light. The wavelength of the light source peaked at 312 nm (range, 280–315 nm), and the energy output was measured with a UV detector (USB4000) with a sensor (CC-3-UV-S; both from Ocean Optics, Dunedin, FL). The entire UVB radiation course was completed in a consecutive 5-day period. After UVB light exposure, Group II served as the UVB control exposed to UVB light and was treated with a drop solution (pH 8.3) without EGCG, twice daily (at 8:00 AM and 8:00 PM), for 8 days. Groups Ш and IV were treated with 0.01% and 0.1% EGCG eye drops, respectively, twice daily (at 8:00 AM and 8:00 PM), for 8 days. The EGCG eye drops were in a 5 mg/ml concentration dissolved in a solution at pH 8.3 that contained 150 mM NaCl and 100 mM Na2CO3. To evaluate the effects of the EGCG eye drops on the corneal surface without UVB damage, the EGCG control mice received the instillation of 0.1% EGCG without radiation.
At the end of the experiment, the animals were anesthetized with phenobarbital sodium (6.0 mg/100g body weight, intraperitoneal injection) and an evaluation of the corneal damage was made using a dissection microscope. After an assessment of the corneal damage, all animals were put in a CO2 box for euthanasia. Eye samples were dissected out, washed immediately with ice-cold saline to remove as much blood as possible, and stored at −70 °C for further analysis.
Evaluation of corneal damage
Seventy-two hours after UVB exposure, digitized images of the mouse corneas were obtained with a dissection microscope (SZ-PT; Nikon, Tokyo, Japan) equipped with a digital camera (Coolpix P5000; Nikon, Tokyo, Japan). To obtain an image of the cornea, a ring-shaped light source (FC100; Meike, Taichung City, Taiwan) was attached to the dissection microscope, and the light was projected to the center of the cornea. To evaluate the corneal surface irregularities caused by UVB exposure, the mire irregularity, which is thought to reflect corneal surface integrity, was quantified based on Tanito and colleagues’ method [20]. Corneal surface irregularities were graded 0–3 as follows: absent (grade 0), mild (grade 1), moderate (grade 2), and severe (grade 3).
After the corneal smoothness was scored, either the right or left eye was randomly selected and stained with 1% lissamine green (Sigma-Aldrich). Digitized images of the lissamine green staining on the corneal surface were taken and scored according to Chen and colleagues’ method [21]. Briefly, the total area of punctuate staining was designated grade 0; grade 1, less than 25% of the cornea was stained with scattered punctuate staining; grade 2, 25–50% of the cornea was stained with diffuse punctuate staining; grade 3, 50–75% of the cornea was stained with punctuate staining and apparent epithelial defects; and grade 4, more than 75% of the cornea was stained with abundant punctuate staining and large epithelial defects. The final numerical score was calculated by dividing the sum of the numbers per grade of the affected mice by the total number of examined mice. Two observers without prior knowledge of the UVB exposure and study groups performed all scoring.
Superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione measurements
Corneal homogenates were prepared in cold Tris-HCl (5 mmol/l, containing 2 mmol/l EDTA, pH 7.4) using a homogenizer. The unbroken cells and cell debris were removed by centrifugation at 10,000 ×g for 10 min at 4 °C. The supernatant was used immediately for the assays for SOD, catalase, GSH-Px, GSH-Rd, and GSH. The activities of all of these enzymes, and the GSH levels, were determined following the instructions in the Randox Laboratories kit (Antrim, UK).
Lipid peroxidation measurement
Lipid peroxidation was quantitatively measured and used to calculate the TBARS concentration in the cornea, using Berton and colleagues’ method [22]. The samples were mixed with a TBA reagent consisting of 0.375% TBA and 15% trichloroacetic acid in 0.25 N hydrochloric acid. The reaction mixtures were placed in a boiling water bath for 30 min and centrifuged at 1,811 ×g for 5 min. The supernatant was collected, and its absorbance was measured at 535 nm. The results are expressed as nmole/mg protein using the molar extinction coefficient of the chromophore (1.56 × 10−5 M−1cm−1).
Protein carbonyl measurement
Oxidative damage to the proteins was quantified with the carbonyl protein assay, which is based on the reaction with dinitrophenylhydrazine, as described previously [8]. The proteins were precipitated by adding 20% trichloroacetic acid and redissolved in 10 mM dinitrophenylhydrazine to give a final protein concentration of 1–2 mg/ml, with 2 N hydrogen chloride added to the corresponding sample aliquot reagent blanks. The absorbance was measured at 370 nm with an enzyme-linked immunosorbent assay (ELISA) plate reader (Quant, BioTek, Winooski, VT). The data are expressed as the nmol of carbonyls/mg protein.
Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Comparison between any two groups was performed using a chi-square or one-way ANOVA (ANOVA) followed by Dunnett multiple comparison tests using the statistical software SPSS (DR Marketing, New Taipei City, Taiwan). Statistically significant differences between groups were defined as p<0.05.
Results
Effect of epigallocatechin gallate eye drops on ultraviolet B–induced corneal damage
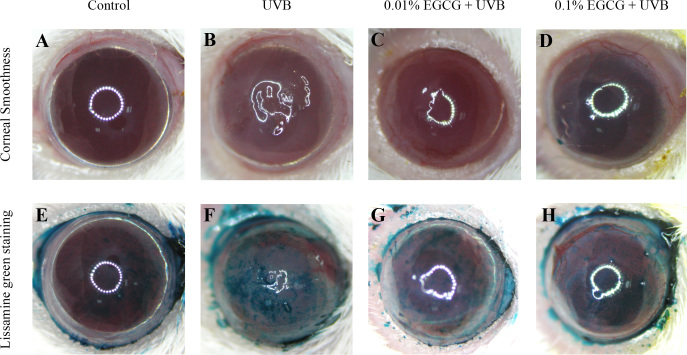
The corneal surface examination provided important evidence of the corneal damage caused by UVB radiation. In clinical diagnosis and experimental examination, lissamine green staining has been shown to be a routine staining technique for evaluating corneal damage. The corneas of the normal control mice were smooth with an integral surface in most of the animals (Figure 1A), although one out of ten showed a mild degree of corneal surface irregularity. The UVB radiation caused serious damage to the corneal surface (Figure 1B), including apparent corneal ulcers, severe epithelial exfoliation, and deteriorated corneal smoothness. Moderate and severe degrees of corneal surface irregularity (30% and 70%, respectively) were observed in the corneas of the UVB-treated mice.
Figure 1.
Effects of epigallocatechin gallate drops on ultraviolet B radiation–induced corneal damage. Comparison of corneal smoothness and lissamine green staining among the (A, E) control, (B, F) ultraviolet B (UVB), and (C, G; D, H) UVB/epigallocatechin gallate (EGCG; UVB exposure with 0.01% and 0.1% EGCG eye drops, respectively) groups.
In contrast, significant amelioration was observed in the corneal surface examination of the groups treated with 0.01% and 0.1% EGCG eye drops (Figure 1C,D), compared with those observed in the UVB-treated group. Approximately 50% and 90% of the mice treated with 0.01% and 0.1% EGCG eye drops, respectively, had trace to mild degrees of corneal surface irregularity. Similar results were found in the lissamine green staining evaluation. With lissamine green staining, the dark-blue devitalized epithelial areas on the ocular surface were obvious in the corneas from the UVB-treated group (Figure 1F), where up to 80% of the mice had more than 50% of their corneas stained with abundant punctuate staining and large epithelial defects, indicating that UVB induced serious damage on the corneal surface.
In contrast, no dark-blue devitalized epithelial areas were found in the corneas from the normal control group (Figure 1E). Compared with the lesions observed in the UVB-treated group, trace to mild degrees of corneal ulcers, epithelial exfoliation, and deteriorated corneal smoothness were observed on the ocular surfaces of the EGCG eye drop–treated mice at 0.01% and 0.1% doses, respectively (Figure 1G,H).
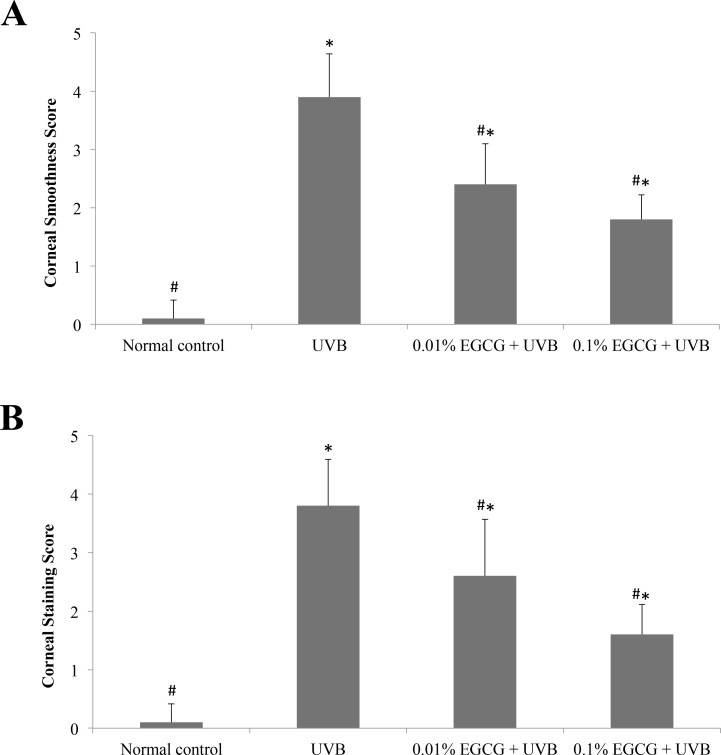
Corneal surface examinations for corneal smoothness and lissamine green staining were recorded and scored, as shown in Figure 2. Through semiquantitative assessment, all corneal smoothness and lissamine green staining scores in the UVB-treated group were significantly higher than those of the normal control (p<0.05), indicating that UVB had induced severe damage to the cornea. All of the tested doses of the EGCG eye drops significantly decreased (p<0.05) the corneal surface scores, when compared to the UVB-treated group, suggesting that the EGCG eye drops ameliorated the UVB-induced corneal damage.
Figure 2.
Corneal mire grade and the lissamine green staining index. Effects of epigallocatechin gallate (EGCG) eye drops on corneal mire grade (A) and the lissamine green staining index (B) in ultraviolet B (UVB)-induced corneal oxidative damage in mice. * p<0.05 compared with the normal control. # p<0.05 compared with the UVB-treated alone group. All data are expressed as the mean ± standard deviation (SD; n = 10).
Effect of epigallocatechin gallate eye drops on antioxidant enzyme activities after ultraviolet B exposure
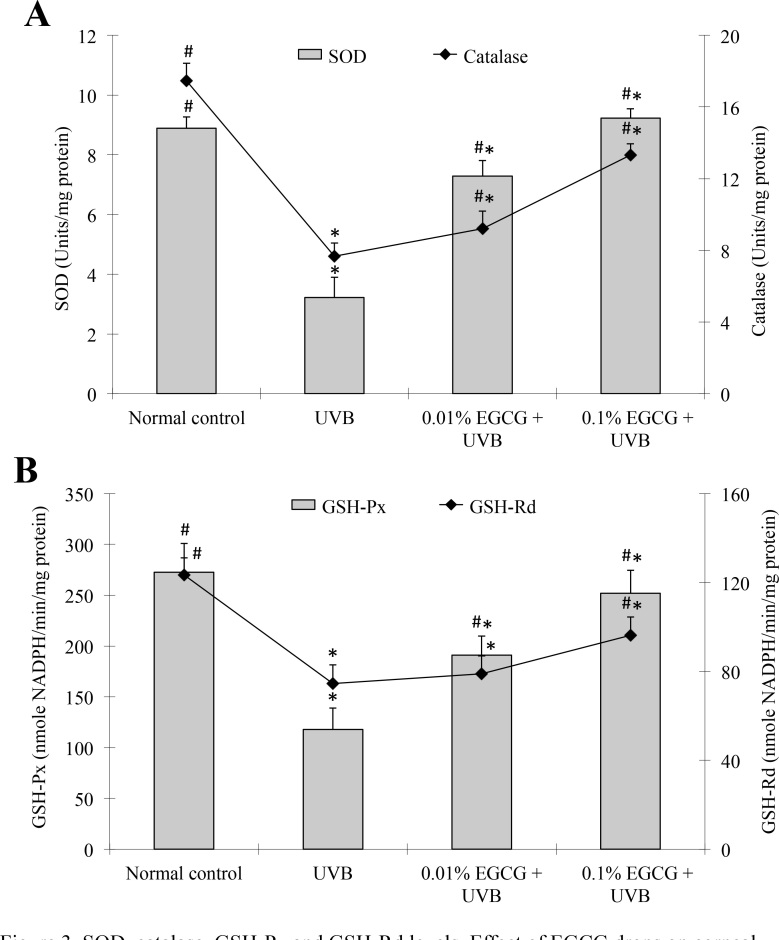
The decrease in enzymatic antioxidant activity was related to the increase in lipid peroxide or free radical production following the UVB-induced corneal damage. To further elucidate the decrease in TBARS accumulation in the UVB-exposed corneas, we measured the status of the antioxidant enzymes, SOD, catalase, GSH-Px, and GSH-Rd, in the corneas. The results are shown in Figure 3. Corneal SOD, catalase, GSH-Px, and GSH-Rd activity in the UVB-treated group was significantly decreased (p<0.05) by 64%, 56%, 57%, and 40%, respectively, when compared with the normal control group. In contrast, the mice treated with the 0.1% EGCG eye drops showed a significant increase (p<0.05) in SOD, catalase, GSH-Px, and GSH-Rd activity, up to 187%, compared to the UVB-treated group. Administering 0.01% EGCG eye drops also significantly increased (p<0.05) SOD, catalase, and GSH-Px activity by 126%, 20%, and 62%, respectively, but did not significantly affect GSH-Rd activity, compared to the UVB-treated group (Figure 3B).
Figure 3.
Superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase levels. Effect of epigallocatechin gallate (EGCG) eye drops on corneal antioxidant enzymes superoxide dismutase (SOD), catalase (A), glutathione peroxidase (GSH-Px), and glutathione reductase (GSH-Rd) (B) in ultraviolet B (UVB)-induced corneal oxidative damage in mice. *p<0.05 compared with the normal control. # p<0.05 compared with the UVB-treated alone group. All data are expressed as the mean ± standard deviation (SD; n = 10).
Effect of epigallocatechin gallate drops on thiobarbituric acid-reactive substances and glutathione levels after ultraviolet B exposure
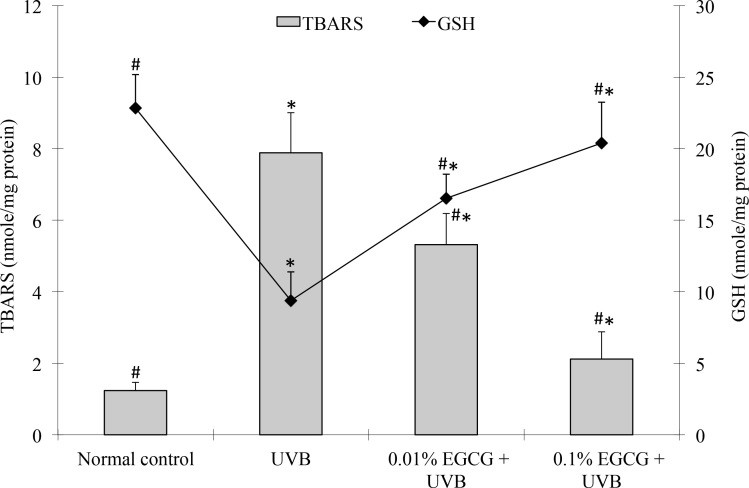
Measuring TBARS is a well-established method for screening and monitoring lipid peroxidation. We measured the TBARS levels in the corneas, and the results are shown in Figure 4. The TBARS levels in the UVB-treated group (7.88 ± 1.13 nmol/mg protein) were significantly higher than those in the control group (1.24 ± 0.22 nmol/mg protein, p<0.05). The TBARS levels in the EGCG eye drop–treated group (5.32 ±0.87 and 2.12 ± 0.76 nmol/mg protein at 0.01% and 0.1% doses, respectively) were significantly lower than those in the UVB-treated group (p<0.05).
Figure 4.
Thiobarbituric acid-reactive substances and GSH levels. Effect of epigallocatechin gallate (EGCG) eye drops on corneal thiobarbituric acid-reactive substances (TBARS) and glutathione (GSH) levels in ultraviolet B (UVB)–induced corneal oxidative damage in mice. *p<0.05 compared with the normal control. #p<0.05 compared with the UVB-treated alone group. All data are expressed as the mean ± standard deviation (SD; n = 10).
GSH is an extremely efficient intracellular antioxidant against ROS that protects cells from UVB radiation damage. Therefore, the intracellular GSH level is an important marker for cellular antioxidative status. The results of the present study demonstrate that UVB radiation caused a significant decrease (p<0.05) in the GSH levels in the cornea (9.38 ± 2.01 nmole/mg protein) compared to the normal control group (22.83 ± 2.36 nmole/mg protein). In contrast to the UVB-treated group, the mice treated with 0.01% and 0.1% doses of EGCG eye drops showed significantly increased (p<0.05; by 76% and 117%, respectively) GSH levels (Figure 4). These findings indicate that the free radicals being released in the cornea were effectively scavenged when treated with EGCG eye drops.
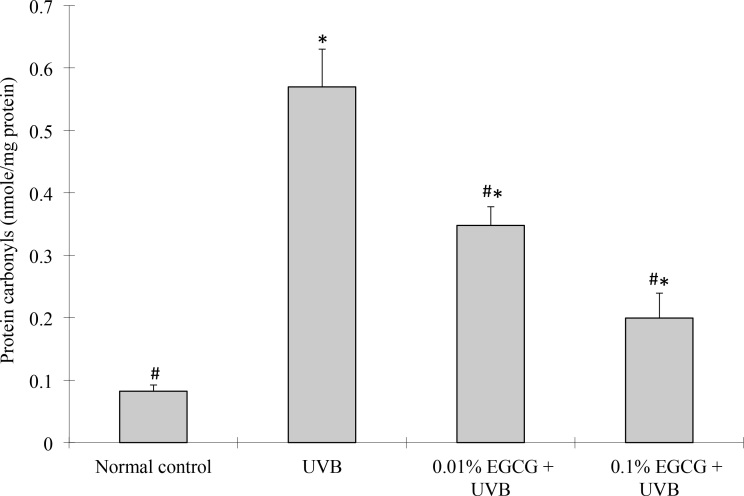
Effect of epigallocatechin gallate drops on protein carbonyl levels after ultraviolet B exposure
Protein carbonyl levels are the most frequently used indicator of the oxidative modification of proteins. To evaluate the effects of EGCG on UVB-induced oxidative damage in the cornea, protein carbonyl levels were determined in this study, and the results are presented in Figure 5. The protein carbonyl levels were significantly higher in the corneas of the UVB control group than in the normal control group (p<0.05). In contrast, the EGCG eye drop–treated groups (at 0.01% and 0.1% doses) showed a significantly decreased percentage of UVB-induced oxidative modification of the proteins in the corneas, by 39% and 65%, respectively, compared to the UVB-treated group (p<0.05).
Figure 5.
Protein carbonyl levels. Effect of epigallocatechin gallate (EGCG) eye drops on corneal protein carbonyl levels in ultraviolet B (UVB)-induced corneal oxidative damage in mice. *p<0.05 compared with the normal control. #p<0.05 compared with the UVB-treated alone group. All data are expressed as the mean ± standard deviation (SD; n = 10).
Discussion
The results of this study demonstrate that EGCG eye drops significantly ameliorate damage on the corneal surface, including apparent corneal ulcers, severe epithelial exfoliation, and deteriorated corneal smoothness, caused by UVB radiation. Additionally, the EGCG eye drops significantly increased SOD, catalase, GSH-Px, GSH-Rd, and GSH activity and decreased the TBARS in the corneas. This study shows that topical EGCG eye drops can ameliorate UVB-induced photokeratitis in mice. Ultraviolet radiation is an environmental agent that can lead to ocular inflammation and pathological changes, particularly in the cornea. The acute response of the cornea to excessive UV radiation can cause photokeratitis, which commonly occurs after exposure to UV radiation reflected from snow, ice, sea, and sand [23,24]. UVB-induced reactive oxygen species, such as singlet oxygen, superoxide anions, and hydroxyl radicals, initiate peroxidation [25] and react to proteins or lipids, leading to membrane lipid peroxidation, and finally cell necrosis [26,27]. High exposure to UVB radiation has been reported to generate high-level ROS in the cornea [2,5]. Therefore, the corneal epithelium is the first layer of UVB filtering that can absorb UVB radiation [28,29].
Several studies have reported that an important mechanism of the protective effects in the cornea may be related to the capacity of antioxidants to scavenge reactive oxygen species [27,30]. In the present study, EGCG, a high antioxidant activity component of the polyphenols in green tea, significantly reduced UVB-induced phototoxic effects in the cornea, as evidenced by corneal surface examination (Figure 1 and Figure 2). The results of the corneal surface examination show that EGCG eye drops ameliorated UVB radiation–induced corneal damage. These results are in agreement with an in vitro study that demonstrated EGCG exhibits potent antioxidant activity in human corneal epithelial cells, inhibiting reactive oxygen species generated by the prooxidant glucose oxidase [16].
The balance of intracellular ROS depends on production within the cells during normal aerobic metabolism and removal by the antioxidant defense system, which includes nonenzymatic antioxidants (e.g., GSH, bilirubin, and vitamins E and C) and enzymatic antioxidants such as SOD, catalase, GSH-Px, and GSH-Rd in mammalian cells [31,32]. Therefore, enzymatic antioxidant activity and inhibition of free-radical generation are important in terms of protecting the cornea from UVB-induced oxidative damage [27].
A decrease in antioxidant enzyme activity is related to an increase in free radical production in UVB exposure. SOD converts the dismutation of superoxide anions into hydrogen peroxide (H2O2) [33] and catalase decomposes H2O2 to oxygen and water. GSH-Px metabolizes H2O2 and hydroperoxides to nontoxic products, and terminates the chain reaction of lipid peroxidation by removing lipid hydroperoxides from the cell membrane. GSH-Rd is involved in detoxifying a range of xenobiotic compounds in conjugation with GSH [34,35]. These antioxidant enzymes are easily deprived of their activity by lipid peroxides or free radicals, resulting in decreased activity in UVB radiation exposure [33]. The results of the present study indicate that the SOD, catalase, GSH-Px, and GSH-Rd activity was significantly decreased in the cornea in response to UVB radiation treatment alone, when compared with normal control mice, implying increased oxidative damage to the cornea. In contrast, the EGCG eye drops treatment significantly increased the SOD, catalase, GSH-Px, and GSH-Rd levels, suggesting the ability to restore and/or maintain these enzymes’ activity in UVB radiation–damaged corneas (Figure 3).
The cornea is the first protector of the optic axis, and because the cornea is rich in lipids, it may show more striking changes with UVB radiation exposure. Lipid peroxidation by the free radical derivatives of UVB radiation is one of the principal mechanisms of UVB radiation–induced corneal injury [31]. In experiments, the TBARS level is widely used as a marker of free radical-mediated lipid peroxidation injury. An increase in TBARS levels in the cornea suggests enhanced peroxidation, leading to tissue damage and the failure of the antioxidant defense mechanisms to prevent the formation of excessive free radicals [36,37]. In the present study, UVB radiation–induced oxidative damage caused an increase in the corneal TBARS levels, compared to the normal control group. The treatment with EGCG eye drops significantly reversed these changes (Figure 4), and caused a significant decrease in the TBARS levels when compared to the UVB radiation–induced oxidative damage group.
Previous studies on the mechanism of UVB radiation–induced corneal damage showed that GSH acts as a nonenzymatic antioxidant that reduces H2O2, hydroperoxides (ROOH), and photooxidation [27]. In particular, the amount of GSH depletion is substantially correlated with the degree of corneal damage [24]. Therefore, GSH conjugation is required for attenuating UVB-induced corneal injury. GSH is readily oxidized to glutathione disulfide (GSSG) upon reaction with xenobiotic compounds, which may then cause a decrease in GSH levels. GSSG is either rapidly reduced by GSH-Rd and NADPH, or used in the endoplasmic reticulum to aid protein-folding processes. Eventually, GSSG is recycled by protein disulfide isomerase to form GSH. Because of these recycling mechanisms, GSH is an extremely efficient intracellular buffer for oxidative stress [38]. In the present study, the corneal content of GSH was significantly decreased in the UVB radiation-exposed mice compared with the control mice. Conversely, treatment with topical EGCG eye drops significantly elevated the GSH content in the cornea, compared to the untreated group (see Figure 4), indicating that EGCG eye drops can protect against the UVB radiation–induced depletion of corneal GSH.
Protein carbonyl groups are an important biomarker of the oxidative modification of proteins, and the accumulation of protein carbonyls has been found in several human diseases [39]. Protein carbonyl groups can be induced by almost all types of ROS, including radical species such as superoxide, hydroxyl, peroxyl, alkoxyl, and hydroperoxyl, and nonradical species such as H2O2, hypochlorous acid, ozone, singlet oxygen, and peroxynitrite. Carbonyl groups are produced on protein side chains when they are oxidized by ROS; therefore, protein carbonyl content is essentially the most general indicator of protein oxidation [8]. In the present study, the corneal protein carbonyl content significantly increased in the UVB-treated group; however, the EGCG eye drop–treated group had significantly decreased protein carbonyl levels (Figure 5). These findings are consistent with those of previous in vitro and in vivo reports that showed EGCG consumption could protect cells and tissues from oxidative damage by scavenging oxygen free radicals and significantly reducing the levels of the carbonyl groups caused by UVB [16,18,40].
EGCG plays an important role in protecting cells and organisms against the harmful effects of light, air, chemicals, and sensitizer pigments. The primary mechanism of action of this phenomenon appears to be the antioxidative ability of EGCG to quench excited sensitizer molecules and ROS [16,41]. Furthermore, EGCG protects against chromosomal damage induced by ROS, as well as against hydroxyl radicals and lipid peroxidation caused by Fe2+-generated radicals [42,43]. Additionally, EGCG has been reported to protect corneal, lens, and retinal epithelial cells from UV-induced oxidation and inflammation in vitro [16,18,44]. Although the anti-inflammatory and antioxidant properties of EGCG are well described in multiple ocular and non-ocular cell types, this is the first study demonstrating that EGCG prevents UVB-induced corneal oxidative damage in vivo, to our knowledge.
In conclusion, the results of this study demonstrate that EGCG eye drops were effective in preventing UVB radiation–induced corneal oxidative stress in vivo. Our results show that the protective effects of EGCG may be due to an increase in the activity of the antioxidant defense system and inhibition of lipid peroxidation and protein oxidative modification. Topical EGCG eye drops may be useful as a protective agent against UVB radiation–induced corneal damage in vivo.
Acknowledgments
Dr. Yu-Wen Hsu (yayen0619@gmail.com) and Dr. Fung-Jou Lu (fjlu@csmu.edu.tw) are the co-corresponding authors for this paper. We thank the National Science Council, Taiwan, for financial support (Grants NSC 102-2320-B-265-001-MY2)
References
- 1.Kolozsvári L, Nogradi A, Hopp B, Bor Z. UV absorbance of the human cornea in the 240- to 400-nm range. Invest Ophthalmol Vis Sci. 2002;43:2165–8. [PubMed] [Google Scholar]
- 2.Downes JE, Swann PG, Holmes RS. Differential corneal sensitivity to ultraviolet light among inbred strains of mice. Correlation of ultraviolet B sensitivity with aldehyde dehydrogenase deficiency. Cornea. 1994;13:67–72. doi: 10.1097/00003226-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Pourzand C, Tyrrell RM. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem Photobiol. 1999;70:380–90. [PubMed] [Google Scholar]
- 4.Genestra M. Oxyl radicals, redox-sensitive signaling cascades and antioxidants. Cell Signal. 2007;19:1807–19. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Shimmura S, Suematsu M, Shimoyama M, Tsubota K, Oguchi Y, Ishimura Y. Subthreshold UV radiation-induced peroxide formation in cultured corneal epithelial cells: the protective effects of lactoferrin. Exp Eye Res. 1996;63:519–26. doi: 10.1006/exer.1996.0142. [DOI] [PubMed] [Google Scholar]
- 6.Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 1994;32:671–83. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Provan GJ, Helliwell K. HPLC determination of catechins in tea leaves and tea extracts using relative response factors. Food Chem. 2003;81:307–12. [Google Scholar]
- 8.Tsai CF, Hsu YW, Ting HC, Huang CF, Yen CC. The in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae). Food Chem. 2013;136:1337–44. doi: 10.1016/j.foodchem.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea – a review. J Am Coll Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 10.Moore RJ, Jackson KG, Minihane AM. Green tea (Camellia sinensis) catechins and vascular function. Br J Nutr. 2009;102:1790–802. doi: 10.1017/S0007114509991218. [DOI] [PubMed] [Google Scholar]
- 11.Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2011;64:146–54. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–31. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Koo MW. EGCG protects HT-22 cells against glutamate-induced oxidative stress. Neurotox Res. 2006;10:23–30. doi: 10.1007/BF03033331. [DOI] [PubMed] [Google Scholar]
- 14.Hirai M, Hotta Y, Ishikawa N, Wakida Y, Fukuzawa Y, Isobe F, Nakano A, Chiba T, Kawamura N. Protective effects of EGCg or GCg, a green tea catechin epimer, against postischemic myocardial dysfunction in guinea-pig hearts. Life Sci. 2007;80:1020–32. doi: 10.1016/j.lfs.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Itoh Y, Yasui T, Okada A, Tozawa K, Hayashi Y, Kohri K. Examination of the anti-oxidative effect in renal tubular cells and apoptosis by oxidative stress. Urol Res. 2005;33:261–6. doi: 10.1007/s00240-005-0465-7. [DOI] [PubMed] [Google Scholar]
- 16.Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011;17:533–42. [PMC free article] [PubMed] [Google Scholar]
- 17.Yao K, Ye PP, Zhang L, Tan J, Tang XJ, Zhang YD. Epigallocatechin gallate protects against oxidative stress-induced mitochondria-dependent apoptosis in human lens epithelial cells. Mol Vis. 2008;14:217–23. [PMC free article] [PubMed] [Google Scholar]
- 18.Heo J, Lee BR, Koh JW. Protective effects of epigallocatechin gallate after UV irradiation of cultured human lens epithelial cells. Korean J Ophthalmol. 2008;22:183–6. doi: 10.3341/kjo.2008.22.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao G, Chen M, Song Q, Liu Y, Xie L, Han Y, Liu Z, Ji Y, Jiang Q. EGCG protects against UVB-induced apoptosis via oxidative stress and the JNK1/c-Jun pathway in ARPE19 cells. Mol Med Rep. 2012;5:54–9. doi: 10.3892/mmr.2011.582. [DOI] [PubMed] [Google Scholar]
- 20.Tanito M, Takanashi T, Kaidzu S, Yoshida Y, Ohira A. Cytoprotective effects of rebamipide and carteolol hydrochloride against ultraviolet B–induced corneal damage in mice. Invest Ophthalmol Vis Sci. 2003;44:2980–5. doi: 10.1167/iovs.02-1043. [DOI] [PubMed] [Google Scholar]
- 21.Chen YT, Li S, Nikulina K, Porco T, Gallup M, McNamara N. Immune profile of squamous metaplasia development in autoimmune regulator-deficient dry eye. Mol Vis. 2009;15:563–76. [PMC free article] [PubMed] [Google Scholar]
- 22.Berton TR, Conti CJ, Mitchell DL, Aldaz CM, Lubet RA, Fischer SM. The effect of vitamin E acetate on ultravioletinduced mouse skin carcinogenesis. Mol Carcinog. 1998;23:175–84. doi: 10.1002/(sici)1098-2744(199811)23:3<175::aid-mc6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Pitts DG, Cullen AP, Hacker PD. Ocular effects of ultraviolet radiation from 295 to 365 nm. Invest Ophthalmol Vis Sci. 1977;16:932–9. [PubMed] [Google Scholar]
- 24.Chen BY, Lin DPC, Wu CY, Teng MC, Sun CY, Tsai YT, Su KC, Wang SR, Chang HH. Dietary zerumbone prevents mouse cornea from UVB-induced photokeratitis through inhibition of NF-κB, iNOS, and TNF-α expression and reduction of MDA accumulation. Mol Vis. 2011;17:854–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Riley MV, Susan S, Peters MI, Schwartz CA. The effects of UV-B irradiation on the corneal endothelium. Curr Eye Res. 1987;6:1021–33. doi: 10.3109/02713688709034873. [DOI] [PubMed] [Google Scholar]
- 26.Podskochy A, Gan L, Fagerholm P. Apoptosis in UV-exposed rabbit corneas. Cornea. 2000;19:99–103. doi: 10.1097/00003226-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Tsai CF, Lu FJ, Hsu YW. Protective effects of Dunaliella salina – a carotenoids-rich alga – against ultraviolet B-induced corneal oxidative damage in mice. Mol Vis. 2012;18:1540–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Ringvold A. Corneal epithelium and UV-protection of the eye. Acta Ophthalmol Scand. 1998;76:149–53. doi: 10.1034/j.1600-0420.1998.760205.x. [DOI] [PubMed] [Google Scholar]
- 29.Podskochy A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta Ophthalmol Scand. 2004;82:714–7. doi: 10.1111/j.1600-0420.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 30.Rubowitz A, Assia EI, Rosner M, Topaz M. Antioxidant protection against corneal damage by free radicals during phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44:1866–70. doi: 10.1167/iovs.02-0892. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Antioxidants and human disease: a general introduction. Nutr Rev. 1997;55:44–49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal irons in human disease: an overview. Methods Enzymol. 1990;186:59–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 33.Cejková J, Típek S, Crkovská J, Ardan T, Pláteník J, Čejka Č, Midelfart A. UV rays, the prooxidant/antioxidant imbalance in the cornea and oxidative eye damage. Physiol Res. 2004;53:1–10. [PubMed] [Google Scholar]
- 34.Reiter RJ, Tan D, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 35.Naik SR. Antioxidants and their role in biological functions: an overview. Indian Drugs. 2003;40:501–16. [Google Scholar]
- 36.Buege JA, Aust SD. Microsomal lipid peroxidation. In: Sidney Fleischer, Leister Packer (Eds.), Methods in Enzymology, vol. 52. Academic Press, New York, San Francisco, London, 1978. pp. 302–10. [DOI] [PubMed] [Google Scholar]
- 37.Vaca CE, Wilhelm J, Harms-Rihsdahl M. Interaction of lipid peroxidation product with DNA. A Rev. Mutat Res Rev Genet Toxicol. 1988;195:137–49. doi: 10.1016/0165-1110(88)90022-x. [DOI] [PubMed] [Google Scholar]
- 38.Cantin AM, White TB, Cross CE, Forman HJ, Sokol RJ, Borowitz D. Antioxidants in cystic fibrosis Conclusions from the CF Antioxidant Workshop, Bethesda, Maryland, November 11–12, 2003. J Free Radic Biol Med. 2007;42:15–31. doi: 10.1016/j.freeradbiomed.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–6. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 40.Vayalil PK, Elmets CA, Katiyar SK. Treatment of green tea polyphenols in hydrophilic cream prevents UVB-induced oxidation of lipids and proteins, depletion of antioxidant enzymes and phosphorylation of MAPK proteins in SKH-1 hairless mouse skin. Carcinogenesis. 2003;24:927–36. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 41.Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (-)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–6. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 42.Sugisawa A, Umegaki K. Physiological concentrations of (-)-epigallocatechin- 3-O-gallate (EGCG) prevent chromosomal damage induced by reactive oxygen species in WIL2-NS cells. J Nutr. 2002;132:1836–9. doi: 10.1093/jn/132.7.1836. [DOI] [PubMed] [Google Scholar]
- 43.Lee SR, Im KJ, Suh SI, Jung JG. Protective effect of green tea polyphenol (-)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother Res. 2003;17:206–9. doi: 10.1002/ptr.1090. [DOI] [PubMed] [Google Scholar]
- 44.Xu JY, Wu LY, Zheng XQ, Lu JL, Wu MY, Liang YR. Green tea polyphenols attenuating ultraviolet B-induced damage to human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 2010;51:6665–70. doi: 10.1167/iovs.10-5698. [DOI] [PubMed] [Google Scholar]