Abstract
Polymer gel dosimetry aims to provide three-dimensional images of radiation therapy dose distributions in irradiated aqueous gels. The first gels required manufacture in an oxygen-free environment, but later the MAGIC formulation was introduced, which could be made in normal atmospheric conditions. Here we report our studies of the effects of variations in the composition of the MAGIC gel performed in order to optimize its performance over the useful dose range of 0 to 20 Gy. A new formulation (termed ‘MAGIC-2’) is comprised of 87% water, 4% methacrylic acid, 9% gelatin, 17.38 × 10−6 M Cu2+ and a molar ratio of ascorbic acid to [Cu2+] of 1000:1. MAGIC-2 has a dose–response slope-to-intercept ratio that is 78% greater than the original formulation and other more favorable properties.
1. Introduction
Polymer gel dosimeters are comprised of an aqueous matrix (usually gelatin) in which one or more monomers are dispersed. When exposed to ionizing radiation, polymerization is initiated by radicals that result from radiolysis. Several bulk properties (e.g., the nuclear magnetic resonance transverse relaxation rate R2 and optical density) are sensitive to the molecular weight of the resultant polymer, and measurements of these can be used to determine the absorbed dose. Employing large containers of the gelatin mixture, it is possible to produce a 3D dose map using magnetic resonance imaging or optical scanning that may be used to validate radiation therapy planning or for quality assurance.
The first polymer gel dosimeters (BANG©, or non-commercially, PAG) were based on the monomers acrylamide and bisacrylamide (Maryanski et al 1993, Baldock et al 1998). Although effective, these dosimeters required hypoxic conditions in order to prevent molecular oxygen quenching of the short-lived initiating radicals. This prerequisite dictated that inert atmosphere glove boxes be used in their preparation, and that container materials be limited to oxygen-impermeable plastics and glass. Since most clinical radiation physicists had neither the equipment nor the technical resources to prepare oxygen sensitive formulations, the use of these gels was hampered.
Previously our laboratory introduced a formulation (Fong et al 2001) that permitted gel dosimeters to be prepared under normal atmospheric conditions. The new type of dosimeter was termed methacrylic and ascorbic acid in gelatin initiated by copper, or MAGIC, and is less toxic than acrylamide-based dosimeters. The polyacrylamide gel dosimeter formulation was later adapted for preparation in regular atmospheric conditions through the addition of antioxidants such as tetrakis (hydroxymethyl) phosphonium chloride (De Deene et al 2006, Venning et al 2005). A summary of the different combinations of formulae can be found elsewhere (Senden et al 2006).
A recent study (De Deene et al 2006) has compared the PAG, nPAG (a normoxic PAG) and MAGIC gel formulations for properties such as tissue equivalence, dose sensitivity, spatial integrity, temperature sensitivity and energy and dose-rate dependence. The authors found that the methacrylic acid-based gel was superior in terms of dose sensitivity and stability over time while nPAG performed better in other areas. However, the differences in normoxic gel dosimeters are due to different chemical reaction schemes and both types deserve more in-depth study. The utility of MAGIC dosimeters depends heavily on the ability to measure accurately a significant response to polymerization of some localized property. The precise dependence of the dose response on the composition of the gels has not been described in detail. We present here studies designed to investigate the influences of different components with the aim of optimizing the performance of MAGIC polymer gel dosimeters for practical applications.
2. Methods
2.1. Gel preparation
The formulation of polymer gels studied here contains the same basic ingredients as the previous formulation: gelatin (300 bloom, Aldrich; Milwaukee, WI), ascorbic acid (Mallinckrodt; Paris, KY), CuSO4·5H2O (Aldrich; Milwaukee, WI), methacrylic acid (Sigma; St. Louis, MO) and HPLC grade distilled water. We omit hydroquinone as it is already present in the methacrylic acid, added by the manufacturer.
Gels for all experiments were prepared in the following manner: a flask containing water was placed in an equilibrated water bath at 48 °C. The gelatin, ascorbic acid solution (AA) and copper sulfate solution (Cu2+) were all added and the solution stirred with a magnetic bar for 2 min. Methacrylic acid (MAA) was then added and the solution stirred for an additional 90 s. The gel was immediately poured into glass test tubes, sealed with screw-cap tops and centrifuged at 15.4g for 15 s plus ramp time. The gels were taken out of the centrifuge and placed in a refrigerator for storage overnight, approximately 18 h.
The effects of variations of the gelatin, monomer and copper concentrations were investigated, as described below.
2.2. Gel irradiation
In each experiment, one gel dosimeter for each concentration variation was reserved unirradiated, and one was irradiated. Samples to be irradiated were placed in a room temperature water bath for approximately 2 h to equilibrate temperature, and irradiated to 20 Gy using a Therapax orthovoltage x-ray unit with a dose rate of 1.844 Gy min−1, 180 kVp and 17 mA.
2.3. Relaxation measurements
The measurements of relaxation times were performed on a standard clinical MRI scanner. The ultimate goal of gel dosimetry is to provide high resolution maps of radiation doses for practical applications. To that end, the main criterion used in the development of the MR imaging protocol was that the method should be reasonable for the widest range of clinical MR scanners. Hardware and software limitations of the three most popular MR scanner vendors were taken into consideration, and the protocol tailored to suit all of them. Thus, other approaches may provide more accurate data for specific purposes and choice of equipment.
The echo train length was the most notable limitation. For some scanners, the maximum number of echoes in a multi-echo (CPMG-type) spin-echo is four. Theoretically, it is possible to calculate T2 from the signal measured at two echo times, but the accuracy and reproducibility suffer if stringent T2-dependent criteria are not met. Since a gel dosimeter in a practical application will undoubtedly have regions of greatly differing T2 values, it is necessary to sample a range of TE values.
The limitation of the four echoes was dictated by the scanner software, so the echo times become the most important parameter to optimize. Linear echo spacing is also a limitation in practice, so an echo spacing of 30 ms was chosen, yielding echo times of 30, 60, 90 and 120 ms. These echo times ensure that an appropriate range of T2 values was adequately measured. For example, if a minimum echo spacing of 15 ms were selected, the high dose range would be more optimally sampled while the lose dose range would not be adequately sampled, and vice versa for echoes longer than 30 ms.
To optimize the signal-to-noise ratio, a TR of at least five times the longest T1 is necessary. For MAGIC gel dosimeters we have found T1 to be in the range of approximately 0.9–1.2 s, and chose TR to be 7 or 8 s, as detailed below. The matrix size was chosen to produce the resolution necessary for most dosimetry applications.
Twenty-four hours following irradiation, samples were placed in a custom-made holder, immersed in mineral oil, and imaged with a GE Signa 3 T MRI system with a multi-echo spin-echo pulse sequence with the following parameters: TR = 8 s (7 s for the optimization of monomer concentration), TE = 30 ms, 4 echoes, slice thickness = 10 mm, 256 × 128 matrix, 140 × 140 mm2 field of view and bandwidth = 15.64 kHz. T 2 images were calculated by performing a least-squares fit to a single exponential for each pixel of the transaxial echo images. R2 values were taken as the inverse of the average T 2 value of a circular region of interest for each sample. Dose sensitivity (s−1 Gy−1) was calculated as the slope of the linear portion of the R2-dose response between 0 and 20 Gy. Dose resolution (Gy) was calculated using a previously published method (Baldock et al 2001) for 95% confidence. It is desirable to maximize dose sensitivity while also optimizing dose resolution.
3. Results
3.1. Gelatin concentration
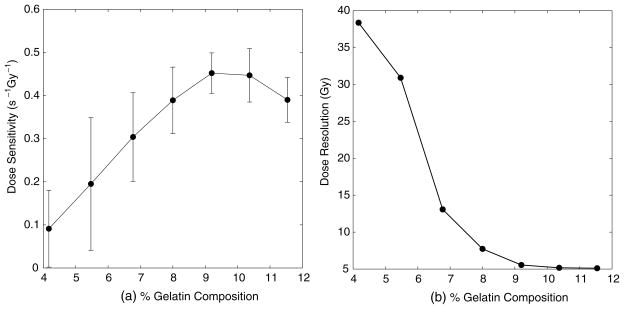
In order to determine the optimum concentration of gelatin in the MAGIC gels over a useful dose range, the experiment above was performed, with the concentration of gelatin being varied while all other formulation components were kept constant. The dose sensitivity and dose resolution values are displayed in figure 1.
Figure 1.
Dose sensitivity (a) and resolution (b) versus per cent gelatin composition. A concentration of 9% is chosen as optimal.
A concentration of 9% was chosen as optimal, because neither the dose sensitivity nor the dose resolution improves past this point. These results are slightly different from those found in a recent report (De Deene et al 2006), where the authors found no significant change in dose sensitivity for gelatin compositions above 8% in methacrylic-acid-based gels.
3.2. Monomer concentration
In order to determine the optimum monomer concentration of the MAGIC gels over a useful dose range, seven sets of gels were prepared identically, each with a different concentration of methacrylic acid. The dose sensitivity and dose resolution values are displayed below in figure 2.
Figure 2.
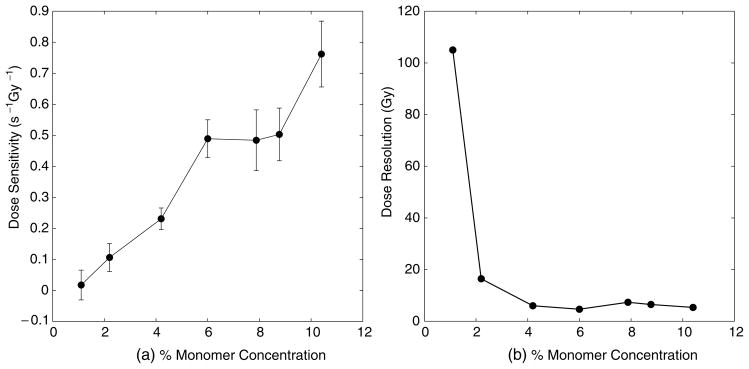
Dose sensitivity (a) and resolution (b) versus monomer concentration. A concentration of 4% is chosen as optimal.
The optimal concentration of methacrylic acid is chosen to be 4%. While the dose sensitivity is higher for greater concentrations, the uncertainty of dose sensitivity also increases and the dose resolution is relatively unchanged. Lower dose sensitivity is useful for a wider range of doses. Additionally, as previously reported (Fong et al 2001), increasing the amount of methacrylic acid increases the intercept or background of the response curve, reducing the slope-to-intercept ratio (another determinant of how well small changes in dose may be detected). Note however, that larger dose sensitivities can be obtained at a higher per cent monomer, and such a response may be desirable under circumstances in which the dose resolution and dynamic range are less important.
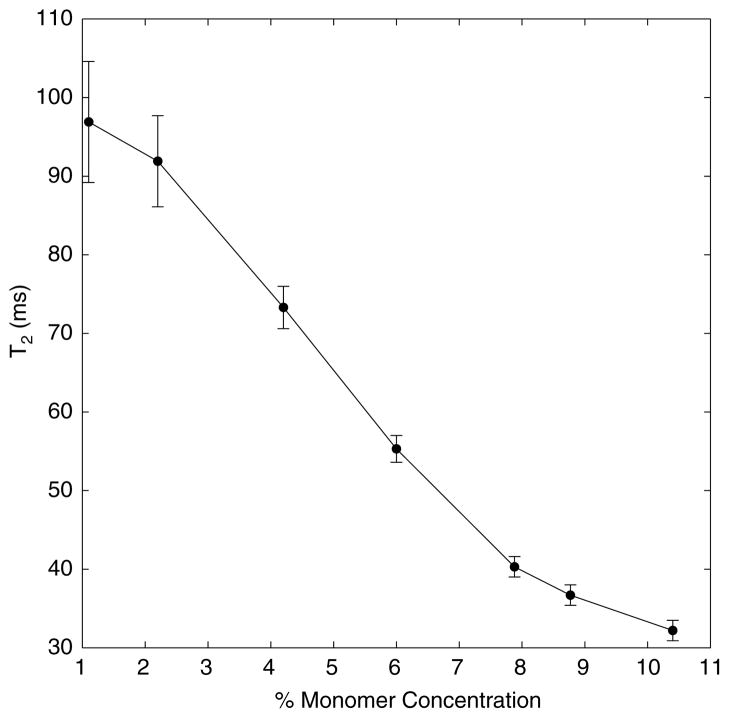
Figure 3 provides further explanation for the choice of 4% monomer concentration. Greater concentrations of monomer shorten the T2 of the unirradiated gel. The range of T2 values for a 4% gel is approximately 70 and 50 ms for doses of 0 and 20 Gy, respectively, which matches the choice of TE values on clinical scanners well.
Figure 3.
T2 of unirradiated gels versus concentration for optimization of monomer.
3.3. Cu2+ concentration
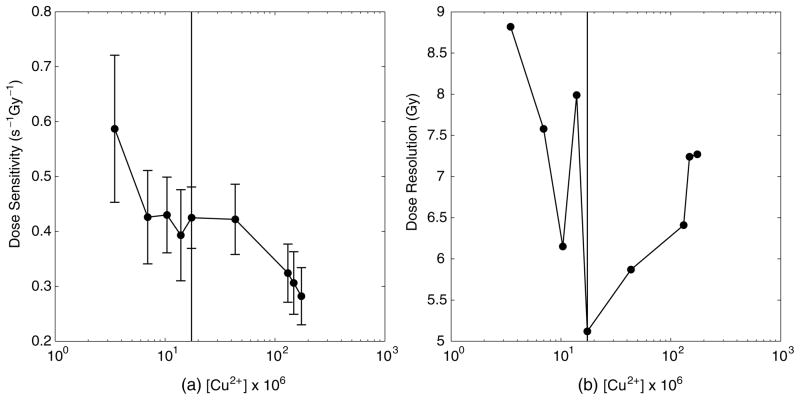
The above experiment was repeated, with the concentration of copper being varied. Nine sets of gels were prepared identically, each with a different concentration of copper sulfate. The dose sensitivity and dose resolution values are displayed in figure 4.
Figure 4.
Dose sensitivity (a) and resolution (b) versus [Cu2+]. The vertical line in both plots indicates the chosen concentration of 17.38 × 10−6 M. Note the semilog plot.
The optimum concentration of Cu2+ was determined to be 17.38 × 10−6 M, beyond which the dose sensitivity and resolution deteriorate as [Cu] increases. It is interesting to note from our data that the optimal dose sensitivity does not arise when the concentration of copper is maximal, when (presumably) the level of oxygen is minimized.
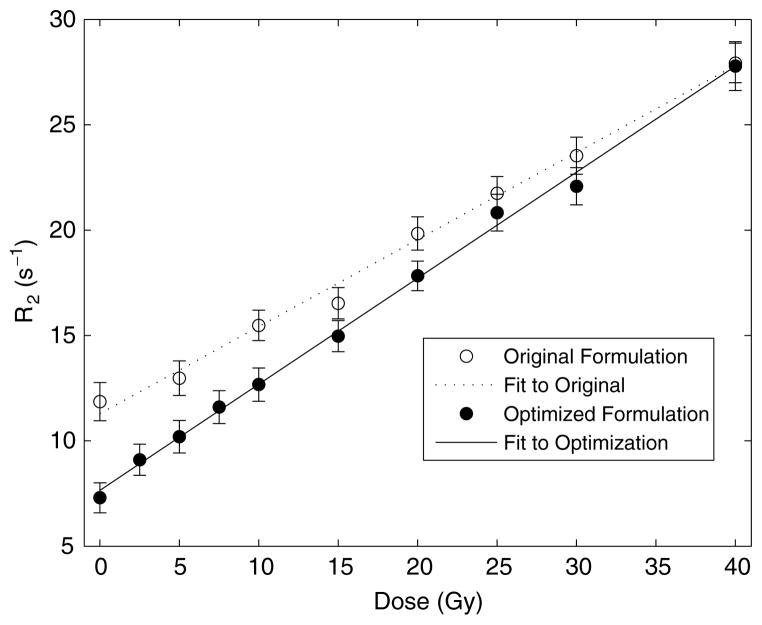
When compared to the original MAGIC gel formulation, this new formulation, which we call ‘MAGIC-2,’ has a 22% higher (0.503 versus 0.413 s−1 Gy−1) dose sensitivity than the original, as shown in figure 5.
Figure 5.
Comparison of dose response for original and optimized formulations, measured at 3 T.
3.4. Density measurements and tissue equivalence
To determine the density of the unirradiated formulation, the dosimeter was manufactured and poured into a flask of known mass and volume. Weight fractions and the effective atomic number were calculated to determine the formulation’s comparison to human muscle tissue and water. Results for the optimized MAGIC gel formulation are listed in table 1, along with data from other formulations for comparison.
Table 1.
Comparison of elemental composition, electron densities (mass density ρ and relative electron density ) and average atomic numbers for various normoxic gel dosimeter formulations, human muscle tissue and water (weight fractions denoted as wk).
| Material | wH | wC | wN | wO | wS | wCu(ii) | ρ(g cm−3) |
|
Zeffa | |
|---|---|---|---|---|---|---|---|---|---|---|
| MAGIC-2 (this work) | 0.1066 | 0.0604 | 0.0129 | 0.8202 | 7.732 × 10−7 | 1.532 × 10−6 | 1.017 | 1.015 | 7.12 | |
| MAGIC (Fong et al 2001) | 0.1062 | 0.0751 | 0.0139 | 0.8021 | 2.58 × 10−6 | 5.08 × 10−6 | 1.060 | 1.055 | 7.07 | |
| nPAGb (De Deene et al 2006) | 0.1073 | 0.0625 | 0.0218 | 0.8080 | 0.0002 | – | 1.035 | 1.033 | 7.11 | |
| PAGATc (Venning et al 2005) | 0.1059 | 0.0681 | 0.0242 | 0.8008 | – | – | 1.026 | 1.027 | 7.10 | |
| Muscle | 0.1020 | 0.1230 | 0.0350 | 0.7298 | – | – | 1.030 | 1.014 | 6.92 | |
| Water | 0.1111 | – | – | 0.8889 | – | – | 1.000 | 1.000 | 7.22 |
Calculated as Z = Σk wk Zk.
wP = 0.0003.
wP = 0.0002, wCl = 0.0002.
4. Discussion
4.1. Comparison with other normoxic polymer gel dosimeters
As mentioned previously, several authors have reported the dose–response characteristics of other normoxic polymer gel dosimeters. Table 2 summarizes the dose–response characteristics of these various formulations, including the optimized MAGIC formulation.
Table 2.
Dose–response characteristics for various normoxic polymer gel formulations. Values are quoted as published except where noted.
| Formulation | Dose sensitivity (s−1 Gy−1) | Intercept | Calculated slope-intercept ratio | Field strength of measurement (T) | Linear region (Gy) |
|---|---|---|---|---|---|
| MAGIC-2 | 0.503 | 7.653 | 0.066 | 3 | 0–20 |
| MAGIC | 0.413 | 11.290 | 0.037 | 3 | 0–30 |
| nPAGa | 0.19b | 0.9b | 0.211 | 1.5 | |
| PAGAT | 0.183 | 1b | 0.183 | 1.5 | 0–7 |
Dose sensitivity and intercept estimated in the dose range of 0–10 Gy.
From inspection.
In comparison to other normoxic formulations, the MAGIC-type gels both have significantly higher dose sensitivities, while acrylamide-based formulations have the advantage of lower intercepts, indicative of less pre-irradiation polymerization.
4.2. General discussion
The new formulation performs better as a dosimeter than the original MAGIC gel formulation. Although we report the results of varying only one ingredient at a time, in practice we have also explored other combinations and have not found better dose responses.
These results indicate a clear benefit to using a higher gelatin composition than that originally reported. Although the dose resolution does not decrease by a substantial amount after approximately 8% composition, the dose response continues to increase beyond that level. Using a higher concentration of gelatin appears to improve the dose response, presumably because the gel facilitates grafting or propagation of the polymerization.
There does not seem to be an appreciable benefit to using greater than 4% monomer concentration. Although the dose sensitivity is higher, the uncertainty of dose measurements may also be higher, and the overall dose resolution is about the same.
The slope-to-intercept ratio of the dose response of polymer gels is another index for quantifying the dose response and comparing different formulations. The new formulation has a ratio of 0.066, compared to the original formulation’s ratio of 0.037, an increase of 78%.
Finally, dose resolutions for the original MAGIC gel formulation and MAGIC-2 were calculated. Over a range of 40 Gy, the original formulation has a dose resolution of 6.4 Gy while the MAGIC-2 formulation has a dose resolution of 5.1 Gy for the parameters discussed above, an improvement of 20%.
5. Conclusion
By comparing the effects of different compositions, we have optimized the formulation for making MAGIC gel dosimeters, producing a dosimeter with greater dose sensitivity while maintaining the desirable qualities of less toxicity, normoxic manufacture and tissue equivalence. In addition, we anticipate that studies of the effects of different compositions will help to better understand the mechanisms of the response of polymer gel dosimeters.
Acknowledgments
This work was supported by NIH grants R01 EB000214 and R01 CA090844.
References
- Baldock C, Burford RP, Billingham N, Wagner GS, Patval S, Badawi RD, Keevil SF. Experimental procedure for the manufacture and calibration of polyacrylamide gel (PAG) for magnetic resonance imaging (MRI) radiation dosimetry. Phys Med Biol. 1998;43:695–702. doi: 10.1088/0031-9155/43/3/019. [DOI] [PubMed] [Google Scholar]
- Baldock C, Lepage M, Back SAJ, Murry PJ, Jayasekera PM, Porter D, Kron T. Dose resolution in radiotherapy polymer gel dosimetry: effect of echo spacing in MRI pulse sequence. Phys Med Biol. 2001;46:449–60. doi: 10.1088/0031-9155/46/2/312. [DOI] [PubMed] [Google Scholar]
- De Deene Y, Vergote K, Claeys C, De Wagter C. The fundamental radiation properties of normoxic polymer gel dosimeters: a comparison between a methacrylic acid based gel and acrylamide based gels. Phys Med Biol. 2006;51:653–73. doi: 10.1088/0031-9155/51/3/012. [DOI] [PubMed] [Google Scholar]
- Fong PM, Keil DC, Does MD, Gore JC. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys Med Biol. 2001;46:3105–13. doi: 10.1088/0031-9155/46/12/303. [DOI] [PubMed] [Google Scholar]
- Maryanski MJ, Gore JC, Kennan RP, Schulz RJ. NMR relaxation enhancement in gels polymerized and cross-linked by ionizing radiation: a new approach to 3D dosimetry by MRI. Magn Res Imaging. 1993;11:253–8. doi: 10.1016/0730-725x(93)90030-h. [DOI] [PubMed] [Google Scholar]
- Senden RJ, De Jean P, Mcauley KB, Schreiner LJ. Polymer gel dosimeters with reduced toxicity: a preliminary investigation of the NMR and optical dose-response using different monomers. Phys Med Biol. 2006;51:3301–14. doi: 10.1088/0031-9155/51/14/001. [DOI] [PubMed] [Google Scholar]
- Venning AJ, Hill B, Brindha S, Healy BJ, Baldock C. Investigation of the PAGAT polymer gel dosimeter using magnetic resonance imaging. Phys Med Biol. 2005;50:3875–88. doi: 10.1088/0031-9155/50/16/015. [DOI] [PubMed] [Google Scholar]