Abstract
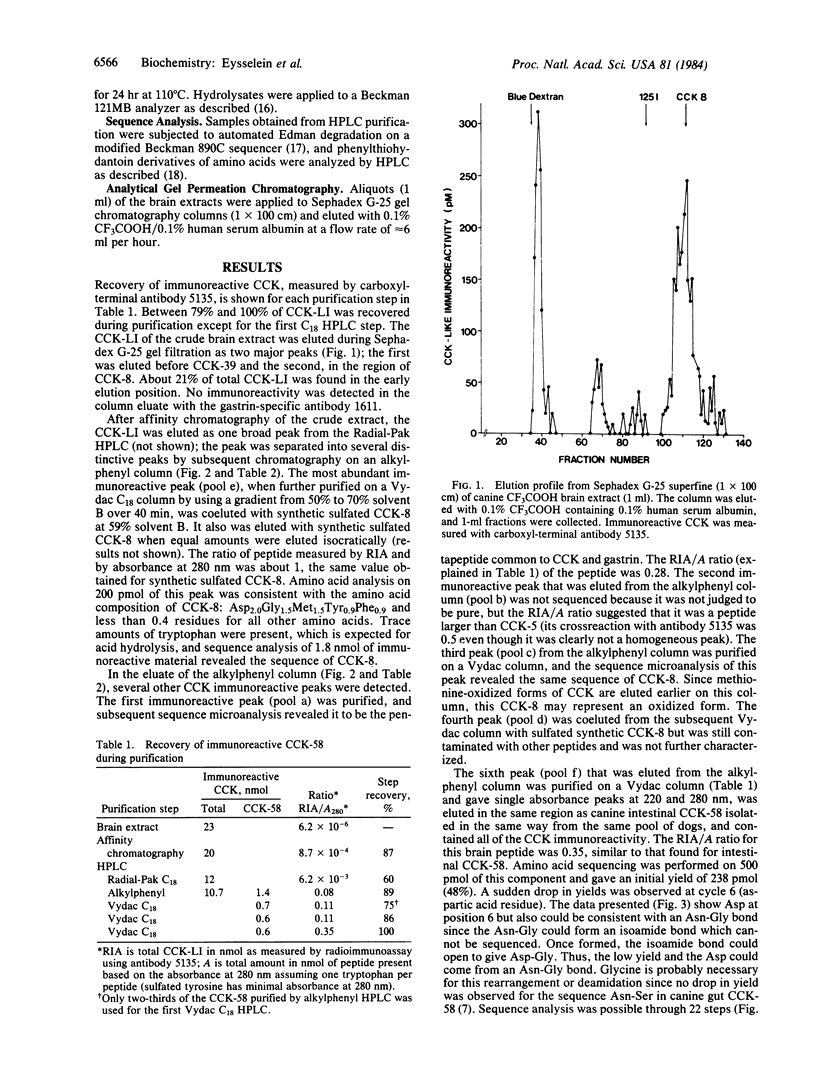
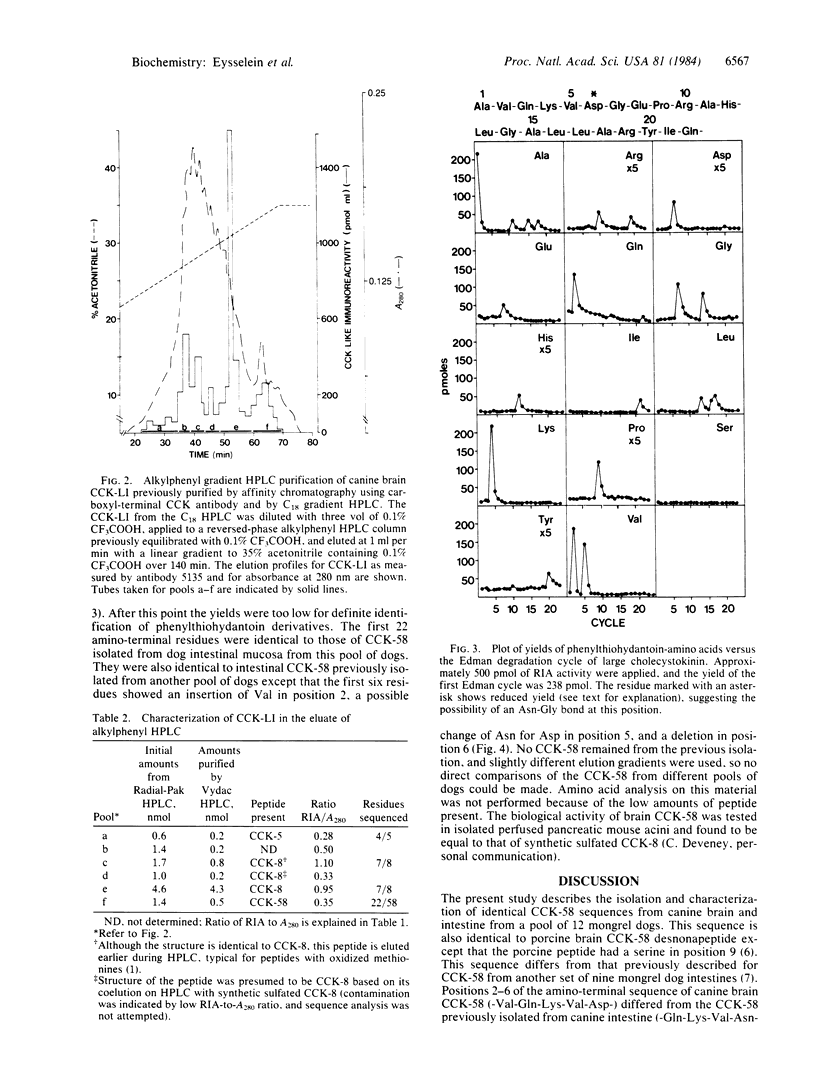
Cholecystokinin (CCK)-like immunoreactivity (CCK-LI) in a pool of 12 dog brains was extracted sequentially into boiling water and cold 2% trifluoroacetic acid. Gel filtration on Sephadex G-50 revealed three main molecular forms detected by a carboxyl-terminal antibody; one was eluted in the position of CCK-58 (58 amino acid residues long); a second, in the position of CCK-8; and a third, near the radioactive iodide marker. When the CCK-LI was purified by affinity chromatography using carboxyl-terminal CCK antibody followed by three steps of reversed-phase high-pressure liquid chromatography, three components were isolated and characterized by sequence microanalysis. The smallest component was the pentapeptide common to gastrin and CCK. The second peak was eluted in the same region as synthetic CCK octapeptide, and sequence analysis showed that the chemical structure of this biologically active region of canine CCK is identical to that found in sheep and pig brains. The 22-residue amino-terminal sequence of brain CCK-58 was: Ala-Val-Gln-Lys-Val-Asp-Gly-Glu-Pro-Arg-Ala-His-Leu-Gly -Ala-Leu-leu-Ala-Arg-Tyr-Ile-Gln-, the same as the sequence found for canine intestinal CCK-58 from this pool of dogs. This is the same sequence others have reported for porcine brain CCK-58 lacking nine amino acid residues (CCK-58 desnonapeptide) except that the porcine peptide had a serine in position 9. The canine CCK amino-terminal sequence differed from the sequence Ala-Gln-Lys-Val-Asn-Ser previously reported for intestinal CCK-58 purified from another pool of dog tissue, but the rest of the residues identified were identical in the two peptides. CCK-58 may be a molecular precursor of the smaller forms of CCK in brain as well as in gut.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinfeld M. C., Meyer D. K., Brownstein M. J. Cholecystokinin octapeptide in the rat hypothalamo-neurohypophysial system. Nature. 1980 Nov 27;288(5789):376–378. doi: 10.1038/288376a0. [DOI] [PubMed] [Google Scholar]
- Beinfeld M. C., Meyer D. K., Eskay R. L., Jensen R. T., Brownstein M. J. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981 May 11;212(1):51–57. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Bunge R. P. Neurological mutants affecting myelination. Nature. 1980 Jul 10;286(5769):106–107. doi: 10.1038/286106b0. [DOI] [PubMed] [Google Scholar]
- Del Valle U., Shively J. E. Two-column system for determination of glucosamine, galactosamine, and amino acids on a Beckman 121MB amino acid analyzer: separation of the anomers of glucosamine and galactosamine. Anal Biochem. 1979 Jul 1;96(1):77–83. doi: 10.1016/0003-2697(79)90556-6. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunoreactive component resembling cholecystokinin octapeptide in intestine. Nature. 1977 Nov 24;270(5635):359–361. doi: 10.1038/270359a0. [DOI] [PubMed] [Google Scholar]
- Eng J., Shiina Y., Pan Y. C., Blacher R., Chang M., Stein S., Yalow R. S. Pig brain contains cholecystokinin octapeptide and several cholecystokinin desoctapeptides. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6381–6385. doi: 10.1073/pnas.80.20.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J., Shiina Y., Straus E., Yalow R. S. Post-translational processing of cholecystokinin in pig brain and gut. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6060–6064. doi: 10.1073/pnas.79.19.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysselein V. E., Reeve J. R., Jr, Shively J. E., Hawke D., Walsh J. H. Partial structure of a large canine cholecystokinin (CCK58): amino acid sequence. Peptides. 1982 Jul-Aug;3(4):687–691. doi: 10.1016/0196-9781(82)90171-1. [DOI] [PubMed] [Google Scholar]
- Geola F. L., Hershman J. M., Warwick R., Reeve J. R., Walsh J. H., Tourtellotte W. W. Regional distribution of cholecystokinin-like immunoreactivity in the human brain. J Clin Endocrinol Metab. 1981 Aug;53(2):270–275. doi: 10.1210/jcem-53-2-270. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Lamers C. B., Morley J. E., Poitras P., Sharp B., Carlson H. E., Hershman J. M., Walsh J. H. Immunological and biological studies on cholecystokinin in rat brain. Am J Physiol. 1980 Sep;239(3):E232–E235. doi: 10.1152/ajpendo.1980.239.3.E232. [DOI] [PubMed] [Google Scholar]
- Mutt V. Further investigations of intestinal hormonal polypeptides. Clin Endocrinol (Oxf) 1976;5 (Suppl):175S–183S. doi: 10.1111/j.1365-2265.1976.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Reeve J. R., Jr, Dimaline R., Shively J. E., Hawke D., Chew P., Walsh J. H. Unique amino terminal structure of rat little gastrin. Peptides. 1981 Winter;2(4):453–458. doi: 10.1016/s0196-9781(81)80104-0. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I. The predominating molecular form of gastrin and cholecystokinin in the gut is a small peptide corresponding to their COOH-terminal tetrapeptide amide. Acta Physiol Scand. 1979 Jan;105(1):117–119. doi: 10.1111/j.1748-1716.1979.tb06320.x. [DOI] [PubMed] [Google Scholar]
- Shively J. E. Sequence determinations of proteins and peptides at the nanomole and subnanomole level with a modified spinning cup sequenator. Methods Enzymol. 1981;79(Pt B):31–48. doi: 10.1016/s0076-6879(81)79011-6. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Species specificity of cholecystokinin in gut and brain of several mammalian species. Proc Natl Acad Sci U S A. 1978 Jan;75(1):486–489. doi: 10.1073/pnas.75.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Lamers C. B., Valenzuela J. E. Cholecystokinin-octapeptidelike immunoreactivity in human plasma. Gastroenterology. 1982 Mar;82(3):438–444. [PubMed] [Google Scholar]
- Yamada T., Brecha N., Rosenquist G., Basinger S. Cholecystokinin-like immunoreactivity in frog retina: localization, characterization, and biosynthesis. Peptides. 1981;2 (Suppl 2):93–97. doi: 10.1016/0196-9781(81)90018-8. [DOI] [PubMed] [Google Scholar]



