Abstract
Previous studies from our laboratory indicate that intratumoral (i.t.) injections of CpG-ODN are the most effective adjuvant strategy to induce an antitumor immune response in tolerant BALB-neuT mice but insufficient for tumor eradication. We evaluated whether this treatment strategy could be enhanced by the presence of anti-OX40 and anti-4-1BB antibodies. Treatment with anti-4-1BB resulted in a greater antitumor response than anti-OX40. The results indicate that anti-4-1BB but not anti-OX40 inhibited the suppressive function of T regulatory cells (Tregs). Through microarray analysis we evaluated the mechanism by which anti-4-1BB inhibits iTregs using the Foxp3-GFP mice. We observed specific transcriptional differences in over 100 genes in iTregs treated with anti-4-1BB, and selected those genes that remained unaffected by exposure to anti-OX40. Interleukin 9 was transcriptionally down-regulated 28-fold by anti-4-1BB treatment, and this was matched by a significant reduction of IL-9 secretion by iTregs. Furthermore, blockade of the common γ-chain receptor resulted in the inhibition of iTreg-suppressive function. More importantly, neutralization of IL-9 plus i.t. injections of CpG-ODN induces tumor rejection in BALB-neuT and MUC-1 tolerant transgenic mice. These results indicate that IL-9 plays a role in iTreg biology during the tumor inflammatory process enhancing/promoting the suppressive function of these cells and that the blockade of IL-9 could serve as a novel strategy to modulate the function of Tregs to enhance the antitumor effect of tumor vaccines.
Keywords: Tregs, 4-1BB, IL-9, Tumor immunotherapy, CpG-ODN
Introduction
T cell immunity is a critical component of the immune response to a growing tumor. Although immunotherapy is a promising approach for the treatment of cancer, enthusiasm for this therapy is tempered by the fact that it is not consistently successful. A major mechanism impairing the optimal activation of an antitumor immune response is the promotion of networks of immune suppression by the tumor [1]. T regulatory cells (Tregs) induce immune cell tolerance by directly inhibiting T cells, NK cells, and dendritic cell functions [2]. The increased numbers of Tregs correlates with poor survival in several malignancies [3]. Tregs can be divided into two principal subsets: natural Tregs (nTregs) and induced Tregs (iTregs) [4]. nTregs are naturally occurring thymus-derived CD4+Foxp3+ cells. iTregs are generated from naïve CD4+ T cells which are stimulated through the T cell receptor, in the presence of IL-2 and TGF-β (transforming growth factor-β) [5]. Both nTregs and iTregs share a similar phenotype expressing Foxp3 with suppressive capabilities making it difficult to distinguish between these populations [2]. Both nTregs and iTregs could contribute to tumor-specific T cell tolerance [6]; however, there is evidence indicating that the high accumulation of Tregs within the tumor are mainly CD4+ T cells that were converted to iTregs by the tumor microenvironment [7]. Depletion of CD4+CD25+ T cells by the administration of anti-CD25 has been shown to suppress the growth of a variety of different syngeneic tumors in mice, significantly improving the efficacy of immune-based therapies [8]. The observation that the removal of immunoregulatory CD4+CD25+ T cells can abrogate unresponsiveness to syngeneic tumors in vivo, leading to the spontaneous development of tumor-specific responses, which indicates that the maintenance of self-tolerance against tumor-self antigens could potentially be lifted [9]. There is cumulative evidence indicating that disrupting the effector function of Tregs is critical for the optimal stimulation of antitumor responses in tolerant hosts [10, 11]. Current methods used to deplete Tregs such as anti-CD4, anti-CD25, IL-2-immunotoxin or cyclophosphamide, have serious drawbacks such as depleting T effector cells [12]. Therefore, new approaches that affect only Tregs without having an effect on effector T cells are needed.
The innate immune response relies on the recognition of the antigen by receptors that recognize specific structures found exclusively in microbial pathogens termed pathogen-associated molecular patterns (PAMPs) [13]. Recognition of PAMPs by toll-like receptors (TLRs) expressed by the innate immune system [14] can regulate the induction of adaptive immune responses [15]. There are currently more than 10 known TLR family members capable of sensing by products of pathogens, such as LPS (TLR-2/4), CpG-DNA (TLR-9), flagellin (TLR-5), as well as other microbial products [16]. TLR signaling triggers maturation and activation of APCs resulting in the induction of adaptive response. Previous studies from our group show that TLRs induce an immune response in BALB-neuT tolerant mice. We found that only intratumoral (i.t.) injections of CpG-ODN were capable of activating an antitumor response in these animals; however, it was not sufficient for tumor eradication [17]. In recent years, several molecules have been identified as having co-stimulatory function in T cell activation. Among these molecules, the members of the TNF receptor superfamily such as CD27, CD30, CD40, 4-1BB, and OX40 have gained importance as co-stimulatory molecules delivering signals that prolong and propagate T cell responses [18]. We have previously shown that anti-OX40 and anti-4-1BB mAbs enhance the immune responses in Her-2/neu transgenic mice [19–21]. In these studies, we tested the antitumor effect of i.t. injections of CpG-ODN plus anti-4-1BB or anti-OX40. Our results show that the combination of CpG-ODN plus anti-4-1BB leads to slower tumor growth and improved tumor rejection, when compared to injections of CpG-ODN plus anti-OX40. Analyses to identify how anti-4-1BB enhances antitumor responses indicated that anti-4-1BB but not anti-OX40 inhibited the suppressive capabilities of Tregs. To evaluate the mechanism by which anti-4-1BB inhibits the suppressive function of Tregs, we employed expression microarray analysis using Tregs derived from Foxp3-GFP mice. We observed that anti-4-1BB treatment down-regulated the expression of IL-9 in Tregs by 28-fold compared to untreated Tregs. In contrast, treatment with anti-OX40 did not alter the expression of IL-9 on Tregs. Furthermore, treatment of Tregs with anti-4-1BB significantly reduced the secretion of IL-9 by Tregs. Blockade of IL-9 signaling through the common γ-chain receptor inhibits the suppressive function of Tregs. More importantly, the combination of i.t. injections of CpG-ODN and neutralization of IL-9 caused tumor rejection in two different tolerant tumor models: BALBneuT and MUC-1. These results demonstrate that IL-9 plays a role in Treg biology during the tumor inflammatory process enhancing/promoting the suppressive function of these cells. We propose that the blockade of IL-9 could serve as a novel strategy to impair the function of Tregs without affecting the effector function of T effector cells, enhancing the antitumor effect of tumor vaccines.
Materials and methods
Mice, cell line, and reagents
BALB-neuT mice were generated as previously described [22] and bred in our facilities. Female BALB-neuT mice 6–8 weeks old were used before autochthonous tumor appearance. MUC1 transgenic (C57BL/6-MUC1.Tg) mice were developed as previously described [23] provided by Dr. Sandy Gendler (Mayo Clinic Arizona). Foxp3-GFP mice [24] were kindly provided by Dr. Alexander Rudensky (Sloan Kettering, New York, NY). Animals were housed under specific pathogen-free conditions. The TUBO and C57MUC1 breast cancer cell lines were obtained from Dr. Guido Forni (University of Torino, Torino, Italy), and Dr. Gendler, respectively. The parental 66.3 cell line (H-2 d)derived from a mouse breast tumor and its transfectant, the A2L2, expressing the rat HER-2/neu was provided by Dr. J.E. Price (M. D. Anderson Cancer Center). The mouse renal cell carcinoma RENCA cells of BALB/c origin were used as a negative control for the cytotoxic assays. Anti-OX40 (OX86) mAb was obtained from the European Cell Culture Collection (Wiltshire, UK), and the anti-4-1BB (3H3) was obtained from Dr. R. Mittler (Emory University, Atlanta, GA). Anti-IL-9 mAb was obtained from Drs. Randolph Noelle (Dartmouth College, Lebanon, New Hampshire) and Jacques Van Snick (Ludwig Institute, Brussels, Belgium). Cells were maintained in complete RPMI 1640 medium supplemented with 10% FCS, 2 mM glutamine, 5 × 10−5 M 2-ME, and 50 μg/ml gentamicin. CpG-ODN (1826) was from Invivogen (San Diego, CA). CD4+ and CD8+ T cells were enriched using negative selection kits (Invitrogen, Carlsbad, CA).
Generation of CTL bulk cultures and cytotoxic activity
BALB-neuT tumor-bearing mice were injected i.t. three times a week with CpG-ODN (30 μg/injection) for 2 weeks. Groups of animals were also injected with anti-4-1BB or anti-OX40 on days 9 and 16 (100 μg/injection) after tumor challenge. After 1 week, the last injection with CpG-ODN, animals were sacrificed. Spleen cells (6 × 106) from primed animals were restimulated in vitro with 5 × 105 irradiated (3,000 rads) TUBO cells plus 1 × 106 irradiated BALB/c spleen cells as feeders. After 5 days, cultures were assayed for lytic activity in a 51Cr release assay against TUBO, 66.3, A2L2, and RENCA cells. Supernatants were recovered after 6 h of incubation at 37°C, and the percentage of lysis was determined by the formula: percent specific lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Analysis of CD4+, CD8+, and Tregs
Evaluation of CD4+ and CD8+ T cells, as well as Treg cell numbers in spleen and tumor draining lymph nodes (LN), derived from tumor baring BALB-neuT mice was done using the Foxp3 staining buffer set (eBioscience) following the manufacturer’s protocol.
CD4+GFP(Foxp3)− and CD4 + GFP(Foxp3)+ cell sorting, conversion, and suppression assays
CD4+GFP(Foxp3)− cells were sorted from Foxp3-GFP mice (≥95% purity) and seeded on plates pretreated with 2 μg/ml anti-CD3 (BD Pharmingen) and incubated for 3 days with IL-2 (100 U/ml, Roche), TGF-β (R&D Systems) at 5 ng/ml. The percent of induced Tregs was evaluated by flow cytometry on the third day. For inhibition of conversion, anti-4-1BB and anti-OX40 were added at 10 μg/ml. Pure populations of nTregs for suppression assays were derived by cell sorting CD4+GFP(Foxp3)+ splenocytes using Foxp3-GFP–mice. Pure populations of iTregs for suppression and microarray assays were isolated after conversion, as described above by sorting CD4+Foxp3+ converted cells using FACS Aria (BD Bioscience) (≥95% purity). For suppression assays, sorted nTregs or iTregs (2.5 × 104 cells) were incubated with freshly isolated CD8+ cells (1 × 105 cells) in a 1:4 ratio, on plates pretreated with 2 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 (BD Bioscience). After 2 days coculture, 1 μCi (3H)thymidine was added and cells were incubated for an additional 16 h. Incorporated (3H)thymidine was measured using the Top Count Instrument (PerkinElmer, Shelton, CT). For inhibition of suppression anti-4-1BB and anti-OX40 were added, as previously described. For CD132 suppression assays, serum free media (X-VIVO-20, Lonza) was used.
Microarray analysis
Sorted iTregs from Foxp3-GFP mice were cultured under the following conditions: (1) anti–CD3 + IL-2 + TGF-β; (2) anti–CD3 + IL-2 + TGF-β + anti-4-1BB; (3) anti–CD3 + IL-2 + TGF-β + anti-OX40; On day 3, cells were collected. RNA was isolated according to manufacturer’s instructions and further purified with RNeasy mini columns (Qiagen, Valencia, CA). RNA quantity and integrity were verified with the Nanodrop (Thermo Scientific, Wilmington, DE) and Bioanalyzer (Agilent, Wilmington, DE) using the nano and pico chips (Agilent). RNA (200 ng per sample) was labeled using the low-input linear amplification kit, two-color (Agilent). Each matched set of cells for each condition was labeled independently with cy3 and cy5. Dye incorporation and amplified RNA amounts were verified with the Nanodrop (Thermo Scientific). Each sample (850 ng) was hybridized to a 4 × 44 K mouse whole genome slide, washed, and feature extracted according to the manufacturer’s (Agilent) instructions. Data analysis was performed using GeneSpring (GX7) software (Agilent). Briefly, an ANOVA was performed to select genes with significant variance between iTregs treated with anti—CD3 + IL-2 + TGF-β; anti–CD3 + IL-2 + TGF-β + anti-4-1BB; anti–CD3 + IL-2 + TGF-β + anti-OX40. This gene list was filtered through a series of criteria designed to yield only those genes that are differentially expressed with anti-4-1BB as compared to control or anti-OX40 treated iTregs.
IL-9 QRTPCR
iTregs were in culture for 3 days in the following conditions: (1) anti-CD3 + IL2 + TGF-β (5 ng/ml); (2) anti-CD3 + IL2 + TGF-β+anti-4-1BB; and (3) anti-CD3 + IL2 + TGF-β+anti-OX40. Cells were harvested and treated with RLT. RNA was isolated according to manufacturer’s instructions and further purified with RNeasy min columns (Qiagen). RNA quality was verified by Nanodrop and Bioanalyzer (Agilent, Wilmington, DE) using the nano and pico chips (Agilent). Reverse transcription was done using Superscript III RT kit (Invitrogen) according to manufacturer’s instructions. The expression levels of IL-9 were measured by real-time PCR using ABI Power SYBR Green (ABI, Foster City, CA, USA) on ABI 7900. All samples were normalized to GAPDH expression.
IL-9 secretion
Sorted nTregs and iTregs were in culture for 3 days with anti-CD3 mAb + IL2 in the presence or absence of anti-4-1BB, anti-OX40, or IgG. Supernatants were collected and ELISA was performed according to manufacturer’s instructions using mouse IL-9 ELISA Ready-SET-Go Set (eBioscience). Concentrations for supernatants were quantified from absorbance values read by FlexStation 3 Microplate Reader (Molecular Devices).
In vivo tumor studies
Tolerant BALB-neuT and MUC-1 mice were implanted s.c. with 1 × 106 TUBO or C57MUC1 cells, respectively and treated with i.t. of CpG-ODN as previously described [17] with the addition of weekly anti-4-1BB, anti-OX40, anti-IL-9 mAB or IgG (100 μg each) i.p. injections. Mice were treated for 3 weeks, measured twice weekly and sacrificed when the tumors reached 1.5 cm3 or showed signs of external necrosis.
Statistical analysis
Statistical significance of data was determined in most cases using the Student t test, and the χ2 test was used to evaluate significant differences in survival.
Results
Antitumor effect of CpG-ODN is enhanced by anti-4-1BB in BALB-neuT mice
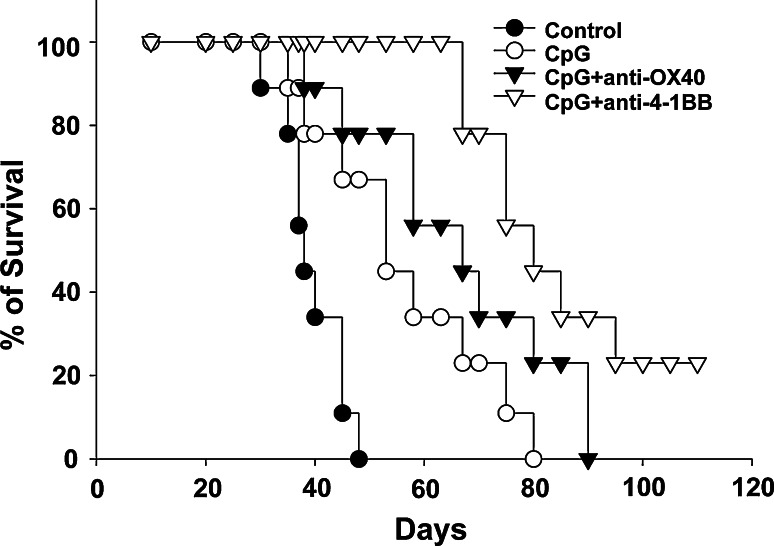
We have previously shown that i.t. injections of CpG-ODN is the most effective TLR ligand in modulating antitumor responses in tolerant BALB-neuT mice implanted with TUBO cells [17]. However, immune stimulation with CpG-ODN treatment alone is not sufficient to eliminate tumors [17]. Furthermore, treatment with agonistic antibodies against 4-1BB and OX40 enhances the immune responses in BALB-neuT mice [19–21]. We tested the antitumor effect of i.t. injections of CpG-ODN plus anti-4-1BB or anti-OX40. As shown in Fig. 1, i.t. injections of CpG-ODN delayed the tumor growth and prolonged the survival of the animals when compared to the control group. The addition of anti-OX40 slightly prolonged survival of the animals, however, treatment with anti-4-1BB resulted in 2/9 animals rejecting the tumor and an overall increase in survival. The antitumor responses were dependent on the activation of CD4 and CD8 T cell responses and NK cells since depletion of these populations abrogated the antitumor immune responses (data not shown).
Fig. 1.
Anti-4-1BB induces a stronger antitumor response than anti-OX40. BALB-neuT mice were implanted with 1 × 106 TUBO cells on day zero. On day ten, animals were treated with i.t. injections of CpG-ODN. Animals were treated three times a week (30 μg/injection) for three weeks. Anti-4-1BB or anti-OX40 was injected once week for three weeks i.p. (100 μg/injection). Kaplan–Mayer survival plot, cumulative data from two independent experiments (n = 9 mice)
Analysis of T cell populations and CTL activity following treatment of tumor-bearing mice
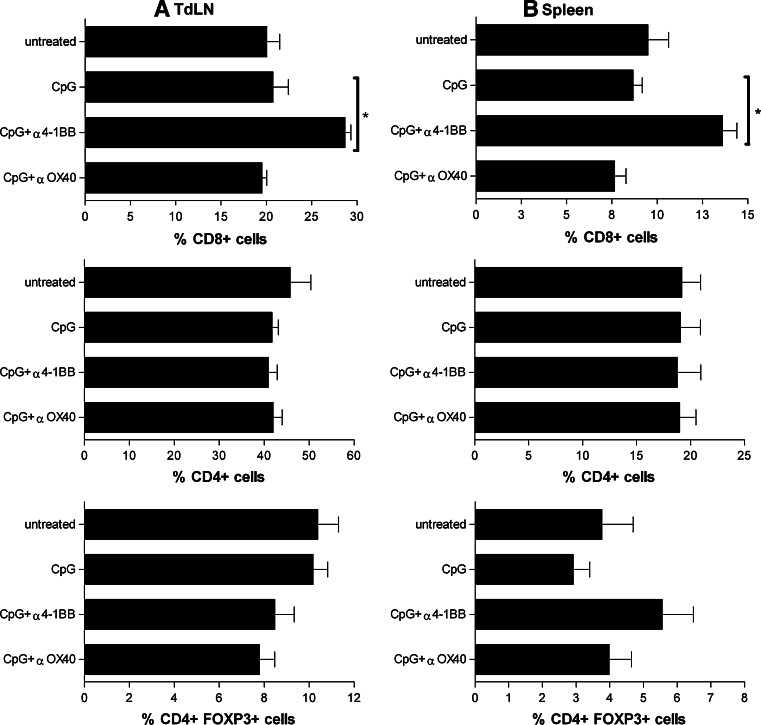
We evaluated whether the antitumor responses observed in mice treated with CpG-ODN + anti-4-1BB and CpG-ODN + anti-OX40 correlated with changes in CD4+, CD8+ T cells, and Treg cell number or function. Analysis of cell populations present in the peripheral lymphoid organs showed a significant increase in the percent of CD8+ T cells in tumor draining lymph nodes (TdLN) (Fig. 2a) and spleen (Fig. 2b) after treatment with CpG-ODN + anti-4-1BB. However, there were no significant changes in the percent of CD8+ cells with CpG-ODN or CpG-ODN + anti-OX40 treatment compared to untreated tumor-bearing mice (Fig. 2a, b). Levels of CD4+ cells (Fig. 2a, b) and Tregs (Fig. 2a, b) did not significantly change among any of the treatments.
Fig. 2.
Changes in T cell populations after CpG + anti-4-1BB treatment. The percentages of CD4+, CD8+, and CD4+Foxp3+ T cells were evaluated in tumor draining lymph nodes (TdLN) (a) and spleen (b) from tumor-bearing animals treated with CpG, CpG + anti-OX40, CpG + anti-4-1BB or untreated. BALB-neuT mice were implanted with 1 × 106 TUBO cells on day zero. On day ten, animals were treated with i.t. injections of CpG-ODN. Animals were treated three times a week (30 μg/injection) for a week. Anti-4-1BB or anti-OX40 was injected once (100 μg/injection) or not treated. Peripheral lymphoid organs were harvested. Bar graphs are lymphocyte gated cells showing percentage of CD8+, CD4+, and CD4+ Foxp3+ cells from TdLN and spleen. Percentages shown are the average number of cells derived from 6 to 9 mice ± SD. Addition of CpG-ODN + anti-4-1BB significantly increase TdLN and spleen CD8+ numbers (* CpG-ODN compared to CpG-ODN + anti-4-1BB P = 0.0008 in TdLN, and *CpG-ODN compared to CpG-ODN + anti-4-1BB P = 0.001 in spleen)
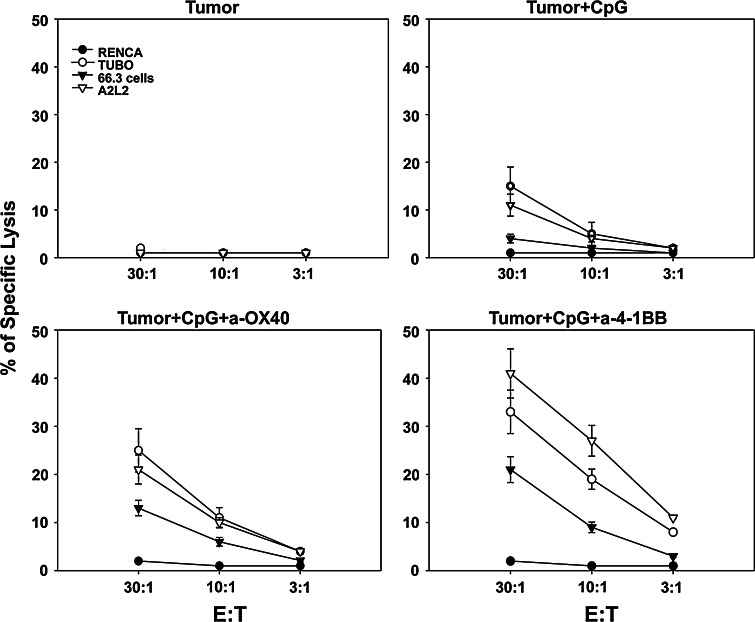
Next, we asked whether addition of anti-4-1BB or anti-OX40 to CpG-ODN enhances the cytotoxic responses. Combination treatments of CpG-ODN and anti-4-1BB or anti-OX40 results in enhanced cytotoxic responses able to kill the breast cancer lines TUBO (a breast cell line expressing the rat Her-2/neu), A2L2 (a H2d 66.3 breast cell line transfected with the rat Her-2/neu), and to a lesser extent 66.3 cells (a breast cell line that is Her-2/neu negative) indicating that treatment with CpG-ODN induced immune responses to Her-2/neu and other antigens (Fig. 3). However, treatment with anti-4-1BB induced the strongest CTL responses. No cytolytic activity was observed against the RENCA cells (haplotype irrelevant control cell line) indicating that the CTL responses were predominantly Her-2/neu specific (Fig. 3).
Fig. 3.
Cytotoxic activity after treatment. On day ten, animals were treated with i.t. injections of CpG-ODN. Animals were treated three times a week (30 μg/injection) for a week. Anti-4-1BB or anti-OX40 was injected once (100 μg/injection) or not treated. After treatment, splenocytes were isolated and stimulated with irradiated TUBO cells plus feeder cells for 5 days in complete media. Cytotoxic activity of restimulated cultures was measured against three breast cancer cell lines: TUBO (a rat HER-2/neu positive cell line), 66.3(a rat Her-2/neu negative line), A2L2 (a transfectant of 66.3 cells expressing rat HER-2/neu), and RENCA a renal cell carcinoma line (haplotype irrelevant control cells) in standard 6-h 51Cr release assay at 30:1, 10:1, and 3:1 effector/target (E:T) ratios. Four animals were included per group ± SD
Anti-4-1BB but not anti-OX40 inhibits the suppressive function of Tregs
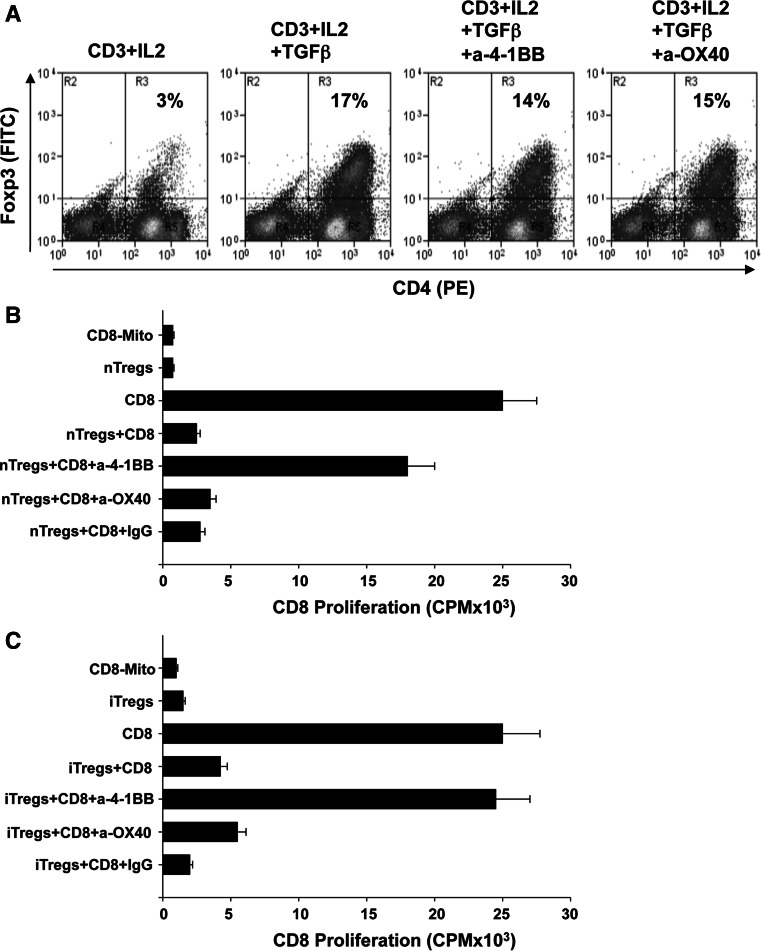
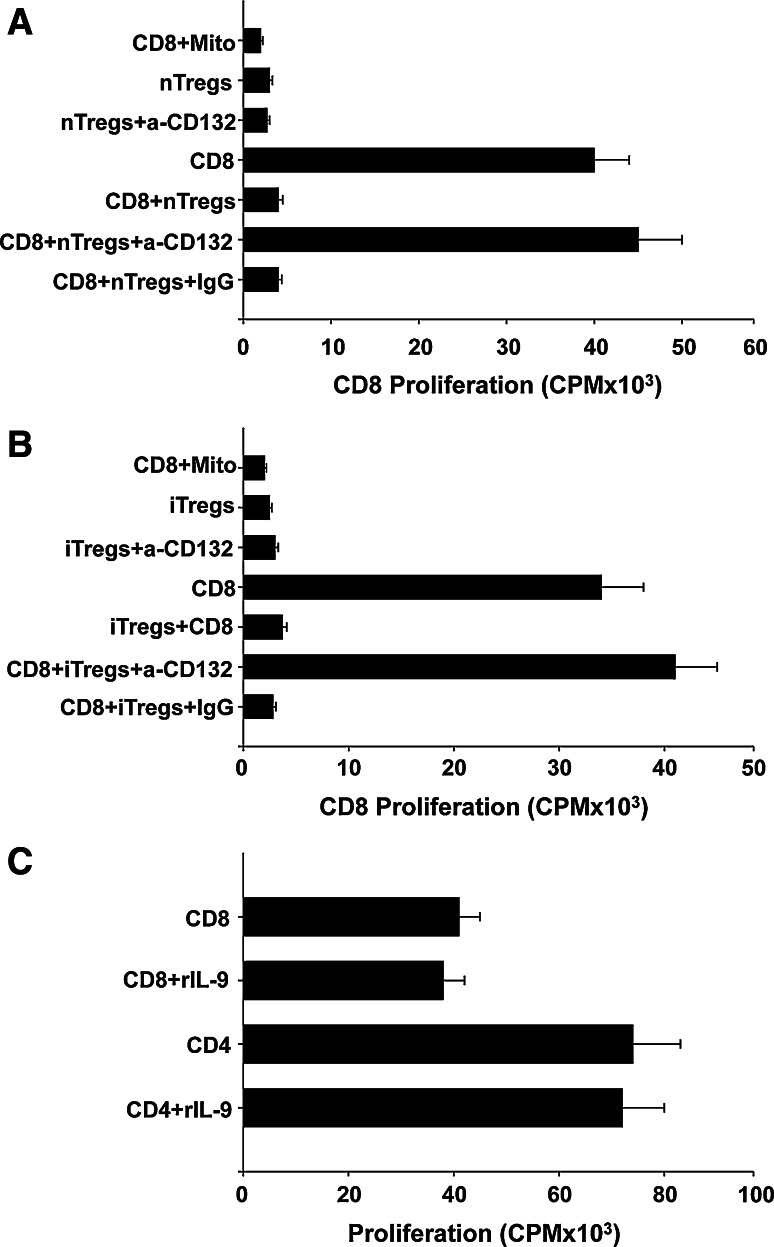
Although treatment with anti-4-1BB induces a stronger CD8+ T cell response when compared to anti-OX40, this difference is not profound enough to account for the significant differences in the antitumor responses. Previous studies have shown that blockade of TNF-receptors such as GITR, OX40, and 4-1BB, could interfere with Treg function [25, 26]. We asked whether treatment with anti-OX40 or anti-4-1BB influences de novo conversion or the suppressive function of Tregs. Using CD4+ T cells from Foxp3-GFP mice [24], we found that neither anti-4-1BB or anti-OX40 inhibited TGF-β-dependent Treg conversion (Fig. 4a). Next, we evaluated whether anti-4-1BB or anti-OX40 inhibits the suppressive function of nTregs or iTregs. nTregs were isolated by sorting CD4+GFP(Foxp3)+ cells from Foxp3-GFP mice. iTregs were induced by stimulating CD4+GFP(Foxp3)− with anti-CD3 in the presence of IL-2 + TGF-β and after stimulation CD4+GFP(Foxp3)+ cells were sorted. For the inhibition assays, CD8+GFP(Foxp3)− (T effectors) were sorted and plated at 1:0.25 effector/Treg ratio on plates coated with anti-CD3/anti-CD28 in the presence or absence of anti-OX40, anti-4-1BB, or IgG1 isotype control Ab (10 μg/ml) for 72 h, and proliferation of effector cells were measured. As expected, nTregs (Fig. 4b) and iTregs (Fig. 4c) inhibited the proliferation of CD8+ T cells. More importantly, our results show that nTregs or iTregs treated with anti-4-1BB could not inhibit the proliferation of effector T cells when compared to effectors without Tregs (Fig. 4b, c). These results indicate that targeting 4-1BB in nTregs or iTregs blocks the suppressive activity of these cells. nTregs or iTregs treated with anti-OX40 or isotype control Ab inhibited the proliferation of the effector cells indicating that anti-OX40 did not alter the suppressive function of these cells. As a control of inhibition of CD8+ T cell proliferation, mitomycin was added to the wells (CD8-Mito Fig. 4b, c). In addition, the proliferation of CD8+ T cells in the presence of anti-OX40 and anti-4-1BB was evaluated, confirming that anti-OX40 or anti-4-1BB do not directly promote CD8+ T cell proliferation (Supplement 1). The same experiments were executed using CD4+GFP(Foxp3)− effector T cells obtaining identical results (Supplement 2). These results indicate that anti-4-1BB, but not anti-OX40, inhibit the suppressive function of Tregs and this could be the reason why stronger antitumor responses were observed in the presence of anti-4-1BB.
Fig. 4.
Anti-4-1BB, but not anti-OX40 treatment abrogates Treg-mediated suppression of CD8+ T cell proliferation. a Agonistic antibodies to 4-1BB and OX40 do not alter de novo conversion of Tregs. Enriched 1 × 106 CD4+ T cells were cultured in the presence under the specified conditions as indicated and Treg conversion (number of CD4+Foxp3+ cells) was measured by flow cytometry. Values are representative of three independent determinations. Proliferation assay measuring the capacity of nTregs and iTregs to suppress CD8 + T cell proliferation. Sorted nTregs (b) and iTregs (c) were isolated from Foxp3-GFP mice as indicated in “Materials and methods” and cocultured with purified CD8+ T cells with or without the addition of agonist antibodies against OX40, 4-1BB or IgG (control) (10 μg/ml). As a control of inhibition of CD8 T cell proliferation, mitomycin was added to the wells (CD8-Mito). The effect of antibodies on proliferation/suppression was evaluated by measuring levels of 3H-thymidine incorporation (cpm), and values are averages of quadruplicate wells ± SD from a representative experiment using three independent replicates. Presence of anti-4-1BB reduces the capacity of nTregs or iTregs to suppress CD8+ T cell proliferation (*P = 0.0001)
Evaluation of the mechanism by which anti-4-1BB inhibits Tregs
We were interested in evaluating the mechanism by which anti-4-1BB inhibits the suppressive function of Tregs. To identify the molecular mechanisms by which anti-4-1BB treatment modulates Treg suppressive function, we performed expression microarray analysis. Since our data show that anti-4-1BB inhibits the suppressive function of nTregs and iTregs, for these experiments we used sorted iTregs from Foxp3-GFP mice because in the tumor microenvironment the majority of Tregs are iTregs and we are able to obtain a larger number of iTregs for these experiments. iTregs were: (1) untreated; (2) treated with anti-OX40; and (3) treated with anti-4-1BB. We designed a data analysis algorithm to filter genes to identify those that are only differentially expressed with anti-4-1BB as compared to control or anti-OX40 treated Tregs. This algorithm is as follows: (1) removed genes which had less than a 2 fold change in relative fluorescence units between anti-4-1BB treated Tregs and untreated Tregs; (2) removed genes with less than twofold change in relative fluorescence units between anti-4-1BB treated Tregs and anti-OX40 treated Tregs allowing us to identify anti-4-1BB specific gene candidates; and (3) removed genes with less than a value of 50 relative fluorescence units to remove genes with signals near background fluorescence. This generated a list of 116 genes specific to anti-4-1BB treatment in Tregs (Supplement 3). A selection of the most differentially regulated and biologically interesting genes are listed in Table 1. The values shown are gene expression-fold changes of iTregs treated with either anti-4-1BB or anti-OX40, as compared to untreated iTregs. One of the genes which showed the highest differential regulation in iTregs treated with anti-4-1BB, as compared to iTregs without treatment or anti-OX40, was IL-9; which was down-regulated 28-fold.
Table 1.
Gene list of most differentiated genes after anti-4-1BB and anti-OX40 treatment
| iTregs | |||
|---|---|---|---|
| Symbol | Gene name | IL2 + TGFb + α4-1BB | IL2 + TGFb + αOX40 |
| IL9 | Interleukin-9 | −28.6 | +1.0 |
| S1pr1 | Sphingosine-1-phosphate receptor 1 | +6.7 | +3.2 |
| Ms4a4b | Membrane-spanning 4-domains, subfamily A, member 4B | +6.9 | +2.0 |
| Lta | Lymphotoxin-alpha | −2.6 | +1.2 |
| Ghrn | Ghrelin | +4.6 | +3.2 |
| IL17ra | Inteleukin-17 receptor alpha chain | +5.7 | +2.7 |
| Hes5 | Hairy and enhancer of split 5 | +4.0 | +1.8 |
| Tcf7 | Transcription factor 7, T cell-specific | +10.2 | +4.0 |
| Paqr9 | Progestin and adipoQ receptor family member IX | +6.7 | −3.7 |
| Lrig1 | Leucine-rich repeats and immunoglobulin-like domains 1 | +3.8 | +1.6 |
Anti-4-1BB inhibits IL-9 production by Tregs
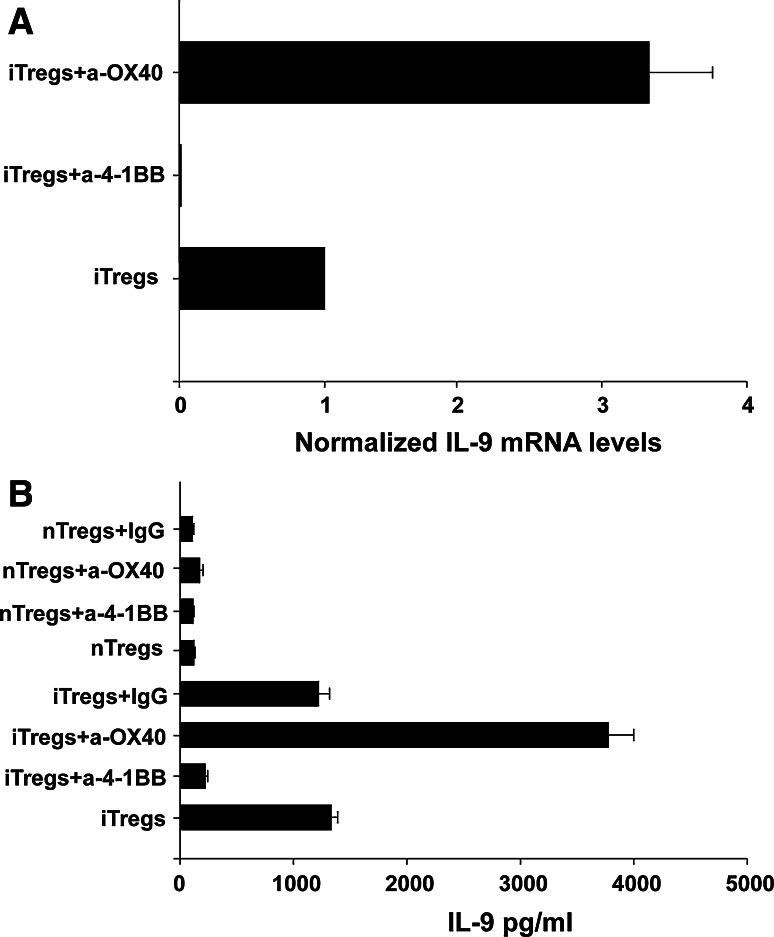
Next, we confirmed IL-9 mRNA expression and secretion since this cytokine has been recently associated with Treg function [27]. To validate the differential expression of IL-9 seen in the microarray analysis, we confirmed the reduction of IL-9 mRNA levels in iTregs with anti-4-1BB treatment by QRTPCR. IL-9 mRNA levels were normalized to housekeeping gene GAPDH mRNA levels and expression of each condition was expressed relative to the control untreated iTreg population. IL-9 mRNA levels are down-regulated in iTregs treated with anti-4-1BB treatment by 1190-fold relative to the untreated population (P = 0.0001) (Fig. 5a). iTregs treated with anti-OX40, IL-9 mRNA levels are up-regulated 3.25-fold relative to the untreated population (P = 0.0005). Secretion of IL-9 was confirmed to further validate the microarray results. Cell sorted nTregs and iTregs were cultured in the presence or absence of either anti-4-1BB or anti-OX40 and IL-9 levels in supernatants were tested by ELISA. Untreated iTregs secrete 1,200 pg/ml of IL-9, while iTregs treated with anti-4-1BB secrete 200 pg/ml (Fig. 5b). Interestingly, iTregs treated with anti-OX40 secreted the highest amount of IL-9 at close to 4,000 pg/ml. nTregs secrete very low levels of IL-9 (~100 pg/ml) and treatment with anti-4-1BB or anti-OX40 minimally modify the secretion of this cytokine (Fig. 5b). These results confirmed that anti-4-1BB treatment strongly reduces IL-9 mRNA transcription and secretion particularly in iTregs.
Fig. 5.
Anti-4-1BB modulates mRNA levels and secretion of IL9 in iTregs. a IL-9 mRNA levels in iTregs from each treatment condition were measured by QRTPCR and normalized to levels of a housekeeping (GAPDH). Levels of IL9 in the untreated iTreg control population are set to one, and the levels in the treatments samples are expressed relative to that. Experiments were performed in triplicate. b Sorted nTregs and iTregs were cultured in the presence or absence of antibodies against OX40, 4-1BB or IgG (control) (10 μg/ml). After three days, supernatants were collected to measure IL-9 secretion by ELISA. Values ± SD are a representative experiment of three independent replicates. A significant P ≤ 0.005 difference was found between iTregs and iTregs + anti-4-1BB
Blockade of γ-chain receptor inhibits suppressive function of Tregs
We evaluated whether neutralization of IL-9 in vitro inhibits Tregs. For these experiments, T effector cells and nTregs or iTregs were mixed at different ratios and treated with anti-IL-9 mAb. Under these conditions, treatment with anti-IL-9 minimally inhibited the suppressive function of Tregs (data not shown). Perhaps the reason why we did not observe a strong inhibition of Tregs after treatment with anti-IL-9 is because other cytokines (e.g., IL-2, IL-4, and IL-7) produced by the effector T cells or Tregs were present in the cocultures and able to signal through the γ-chain activating the Tregs. To evaluate whether the signal provided by IL-9 and possibly other cytokines through the cytokine γ-chain receptor is critical for the suppressive function of Tregs, we treated nTregs and iTregs with a neutralizing anti-CD132 (γ-chain receptor) mAb. In the presence of this antibody, nTregs (Fig. 6a) and iTregs (Fig. 6b) lost their ability to inhibit the proliferation of T cells. These results indicate that the signals provide by IL-9 and other cytokines through the γ-receptor are critical to maintain the suppressive function of Tregs [28]. The inhibitory effect observed in the presence of Tregs is not due to the secretion of IL-9, since in the presence of high concentrations of IL-9 (1 ng/ml), the proliferation of CD8+ T cells was not altered (Fig. 6c).
Fig. 6.
Blockade of common γ-chain receptors inhibits suppressive function of Treg. Sorted nTregs (a) and iTregs (b) were cocultured with purified CD8+ T cells in the presence or absence of anti-CD132 or IgG (control) (10 μg/ml). The proliferation/suppression was evaluated by measuring levels of 3H-thymidine incorporation (cpm), and values shown as a percent of CD8+ T cell proliferation and are averages of quadruplicate wells ± SD of a representative experiment of three independent replicates. A significant P ≤ 0.001 difference was found between nTregs or iTregs and iTregs + anti-CD132. c Enriched CD4+ or CD8+ T cells were stimulated wih anti-CD3 + anti-CD28 in the presence or absence of IL-9 (1 ng/ml). Values ± SD are a representative experiment of three independent replicates
Neutralizing IL-9 enhances the antitumor effect of CpG-ODN vaccination promotes antitumor responses and provides long-term survival in tolerant models
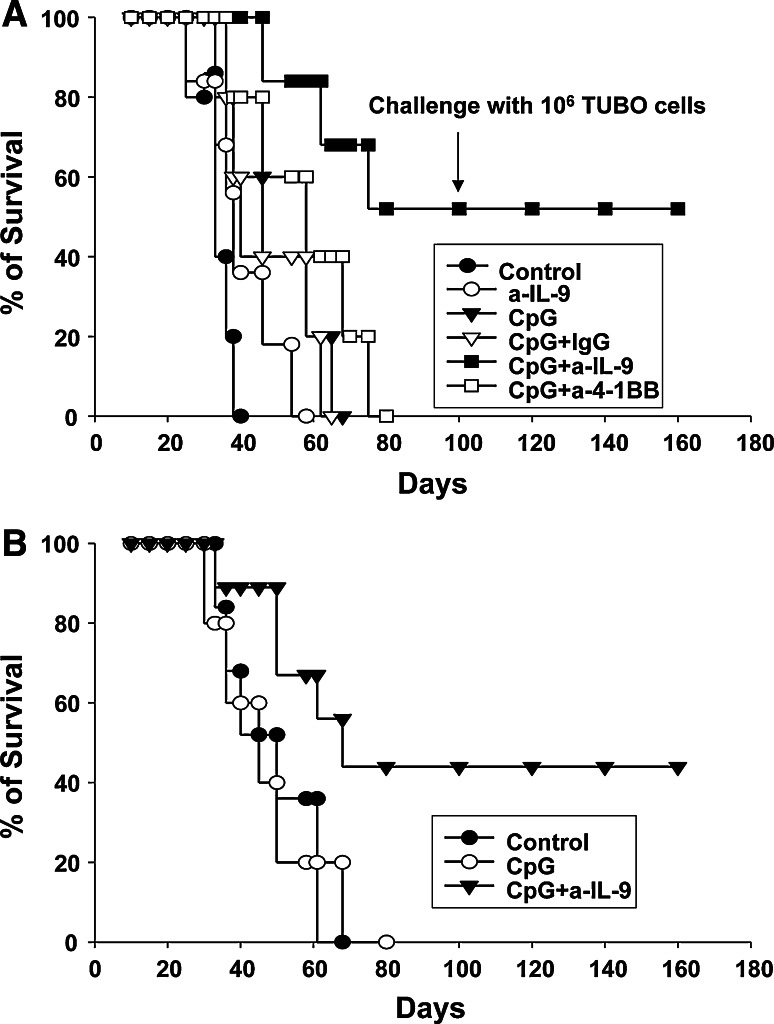
Since we observed that anti-4-1BB treatment reduces IL-9 secretion in Tregs, we asked whether reduction of IL-9 is necessary for anti-4-1BB’s ability to enhance tumor rejection. To test this, we neutralized the effect of IL-9 in vivo. BALB-neuT tolerant mice were implanted with TUBO cells and then treated with CpG alone or in combination anti-IL-9 or anti-4-1BB. Fifty percent of CpG-ODN + anti-IL-9 treated mice survived > 200 days, whereas mice treated with CpG-ODN, CpG + IgG control (1A7) or CpG-ODN + anti-4-1BB died within 100 days (Fig. 7a). Untreated mice survived a maximum of 48 days post-tumor implantation. Treatment with anti-IL-9 alone was not sufficient to enhance survival. Animals treated with CpG + anti-IL-9 and rejected the tumor were evaluated for the development of memory responses. Animals were challenged on day 100 with a second dose of TUBO cells and the results show that all animals that rejected the primary tumor developed a protective memory response capable of rejecting a second tumor challenge. The mice were followed for more than12 months, and no reoccurrence of tumors, or formation of autochthonous tumors were observed in these mice. These results indicate that treatment with anti-IL-9 is more effective than anti-4-1BB. To confirm our results that anti-IL-9 enhances antitumor responses, we also tested the antitumor effect of combining CpG-ODN plus anti-IL-9 in MUC-1 transgenic mice which is another tolerant tumor model [23]. MUC1 transgenic mice were implanted with C57MUC1 breast cancer cell line and subsequently treated with CpG-ODN + anti-IL-9. This treatment also showed an enhanced survival time over control populations (P = 0.05) (Fig. 7b). CpG-ODN treatment does not confer enhanced survival in this model. These results confirm that addition of anti-IL-9 to CpG-ODN treatment enhances antitumor responses and increases survival of tumor-bearing tolerant mice.
Fig. 7.
Neutralization of IL-9 enhances antitumor responses. Kaplan–Meier survival plot showing that CpG-ODN + anti-IL9 mAb treatment has the greatest impact on survival. a BALB-neuT mice were implanted with 1 × 106 TUBO cells and treated for 3 weeks three times a week with CpG-ODN i.t. (30 μg/ml) and once a week with 1A7 (control IgG), neutralizing anti-IL9, or anti-4-1BB i.p. (100 μg/ml). A Kaplan–Meier survival plot shows that addition of neutralizing IL-9 to CpG-ODN enhances the survival of mice. At day 100, CpG-ODN + anti-IL-9 mice that rejected the tumors were challenged with 1 × 106 TUBO cells and animals remained tumor free > 8 months after the rechallenge demonstrating they were capable of generating a memory response. b Kaplan–Meier survival plot of MUC1.tg mice bearing C57MUC1 tumors. MUC1.tg mice were implanted with 1 × 106 C57MUC1 cells and treated with CpG or CpG + anti-IL-9 as described above. Cumulative data from 2 independent experiments comprising 7–10 mice per cohort. A significant P < 0.001 difference was found between control mice and CpG + anti-IL-9 treated BALB-neuT mice
Discussion
The premise of cancer immunotherapy is to stimulate the host’s immune system to destroy the tumor. However, most immunotherapies are unsuccessful because the tumor creates networks of immune suppression, which is capable of inhibiting the immune response. Suppressive cells include Tregs, which are normally found within the immune system as a critical component for immune homeostasis, but within the tumor they foster a pro-tumor microenvironment inhibiting the immune response. These studies evaluated the additive effect of agonist antibodies against 4-1BB and OX40 in conjunction with CpG-ODN injections. Our studies indicate that agonist antibodies against 4-1BB in combination with i.t. injections of CpG-ODN induces a stronger antitumor response than agonist antibodies against OX40. As part of this response, we observed an increase in CD8 numbers with 4-1BB and CpG + ODN treatment. 4-1BB has been described as being able to control expansion and survival of CD8 T cells, but does not increase cytotoxicity [29]. It is likely that the combined effects of Treg functional blockage and CD8 T cells expansion signals, both provided by anti-4-1BB led to the enhancement of CD8 numbers observed in our in vivo experiments. While greater CTL responses were observed in the presence of anti-4-1BB than with anti-OX40, this cannot completely explain the potent antitumor responses observed after anti-4-1BB treatment. Since previous studies have shown antibodies against OX40 and 4-1BB could interfere with Treg biology [24, 25], we evaluated whether treatment with anti-OX40 or anti-4-1BB influence Treg function. Using Foxp3-GFP as a method to isolate Tregs, our results show that these antibodies do not alter de novo conversion of iTregs after TGF-β stimulation. However, anti-4-1BB but not anti-OX40 inhibited the suppressive activity of Tregs. Our results are in agreement with those of Choi et al. [25] in which they demonstrated that anti-4-1BB inhibits the suppressive function of Tregs. On the other hand, our results contradict previous studies indicating that targeting OX40 blocks the inhibitory activity of Tregs [26, 30]. A major difference between our studies and theirs is that in the other studies Tregs were isolated by selecting CD4+CD25+ cells while in ours Tregs were sorted based on the expression of CD4+GFP(Foxp3). In Colombo et al. [31] define Tregs as CD4+CD25+ cells, which is a broader group of cells that include activated CD4 T cells and Tregs. We use the transcription factor FOXP3 that more accurately defines only the suppressive subpopulation of CD4+CD25+ cells. Additionally, the work presented in this paper characterizes the role of nTregs in lymphopenia driven colitis, whereas our studies focus on the role of inhibiting Treg function in the context of Tregs induced by the tumor micro-environment. Perhaps this may account for the discrepancies in the role of OX40 in each population. Recent studies by Ruby et al. [32] indicated that anti-OX40 expanded Tregs rather than inhibiting Tregs. These studies support our results indicating that anti-OX40 does not always inhibit Tregs. Future studies might determine the opposing effects of blocking OX40 on Tregs.
We sought to determine the mechanism in which anti-4-1BB inhibits the suppressive function of Tregs. The biological outcome of the presence of anti-4-1BB on Tregs was examined by microarray analysis. The goal of this analysis was to identify factors that modulate 4-1BB signaling which may be important in the suppression function of Tregs and could be exploited as a specific anti-Treg therapy. The analysis reveals that many genes were differentially regulated in iTregs after anti-4-1BB treatment when compared to anti-OX40 treatment. We looked for gene candidates that were related to Treg biology, and which could be manipulated to elicit changes in Treg behavior. IL-9 was selected because it has an important role in Treg biology [27], and was most down-regulated (28-fold) after anti-4-1BB treatment whereas anti-OX40 did not reverse its expression. We do not see changes in IL-9 production with anti-4-1BB treatment in T effector cells (data not shown). Paradoxically, OX40 stimulation of iTregs induces even greater secretion of IL-9. However, OX40 is required for efficient IL2R signaling, for example, OX40 null Tregs were hyporesponsive to IL-2, and unable to suppress inflammation in a colitis model [31]. Since IL-2 and IL-9 signal through dimeric receptors, which contain the common-γ-chain, it is possible that OX40 stimulation also enhances IL-9 signaling, and that this stimulation is reinforced through secretion of IL-9.
Our data confirm that Tregs secrete large amounts of IL-9 [33] and indicate that iTregs secrete higher levels of IL-9 than nTregs, which is in agreement with previous studies indicating that TGF-β is a critical factor in promoting IL-9 production [34]. However, Elyaman et al. [35] reported that nTreg and iTregs do not secrete or express IL-9. This discrepancy could be due to technical aspects. We used highly purified nTregs or iTregs that were subsequently incubated for an additional 3 days in the presence of anti-CD3 + anti-CD28 and then supernatants and cells were collected, while the work from Elyaman et al. [35] is based on CD4+Foxp3− cells incubated in serum-free medium. They indicated that Tregs rapidly die in serum free medium. Therefore, the number of nTregs and iTregs present in the cultures is different between our system and theirs, which might account for the difference in IL-9 production by Tregs.
How signals transmitted by 4-1BB and not by OX40 regulate the production of IL-9 is not fully understood. OX40 and 4-1BB associate with TNFR-associated factors (TRAFs), which are adaptor molecules for signaling pathways [18]. Although both 4-1BB and OX40 interact with TRAF2, 4-1BB can also interact with TRAF1 and OX40 interacts with TRAF3 and TRAF5 [18]. Perhaps the signals transmitted by these molecules induce different signals in Tregs altering the function of these cells. This is exemplified in previous reports indicating that engagement of 4-1BB is able to inhibit Th2 responses [36]. Sun et al. [37] reported that treatment with anti-4-1BB reduces the expression of GATA-3 which is a critical transcription factor for regulating Th2 responses. How 4-1BB signaling down regulates the expression of GATA-3 needs to be determined. Currently, we are examining the effect of 4-1BB and OX40 agonist antibodies on these signaling pathways on Tregs. Based on this information, therefore, it is not surprising that anti-4-1BB was able to inhibit the production of IL-9 in Tregs.
The studies of Elyaman et al. [35] also demonstrated that blockade of IL-9 inhibits the suppressive function of Tregs, and addition of recombinant IL-9 enhances their suppressive function. We evaluated the effect of blocking IL-9 in vitro and our results indicate that treatment with anti-IL-9 minimally inhibits Tregs (data not shown). We do not understand the differences among the studies but it could be due to methodologies used. Most probably the reason why we did not observe a complete inhibition of Tregs after treatment with anti-IL-9 is because other cytokines (e.g., IL-2, IL-4, IL-7), which are able to signal through the γ-chain, are produced by effector T cells or Tregs in culture. These interleukins are, in turn, capable of activating Tregs through the common γ-chain. Since it is not feasible to block every interleukin signaling through the common γ-chain, we chose to block the common γ-chain by using a neutralizing antibody. When cultures were treated with anti-γ-chain antibody, the suppressive function of Tregs was inhibited. These results support our data indicating that blocking IL-9 in vitro is not sufficient to inhibit the suppressive function of Tregs when T effector cells are present, since other cytokines can maintain the effector function of Tregs. The γ-chain family of cytokines all signal through the JAK-STAT pathway [38]. IL-2, IL-7, IL-9 and IL-15 primarily activate STAT5. Interestingly, mice lacking IL-2Rα or IL-2Rβ do not alter Foxp3 expression or result in the complete loss of Tregs [38], in contrast, mice-deficient of γ-chain or Jak3 are devoid of Foxp3+ Tregs [39]. Additionally, several studies have demonstrated that STAT5 directly regulates Foxp3 [40] and improper signals from STAT5 result in a lower number of Tregs and a marked decrease of Foxp3 expression [39, 40]. Taken together, these results suggest that the signals transmitted through the γ-chain are critical for Treg function.
Our results showed that vaccination with i.t. injections of CpG plus anti-IL-9 induces a stronger antitumor response than treatment with anti-4-1BB. When CpG-ODN vaccination was combined with neutralization of IL-9, it resulted in the rejection of the primary tumor and the generation of a protective memory response. Taken together, these results indicate that tumor vaccination and modulation of Treg function by blocking IL-9 optimally activate a primary response and generate a protective memory response against self-tumor antigens. In contrast to the weak effects of blocking IL-9 in vitro (data not shown), we believe that IL-9 is critical in the tumor inflammatory process regulating the function of Tregs in vivo. This is due to the different cytokine combinations found under culture conditions and in vivo. We do not think that IL-9 is critical for the development of Tregs since IL-9 KO mice have normal T cell development and the percentages of Tregs in these animals is similar as wild type mice [41], which is compatible with our in vitro inhibition assays. Our hypothesis regarding the role of IL-9 in regulating Tregs to promote tumor growth is as follows: CD4+ T cell effector cells are converted into iTregs by the tumor microenvironment. Previous studies from our group indicate that the levels of IL-2, IL-4, and IL-15 within the tumor are minimal [42] indicating that other mechanisms might exist within the tumor to maintain/expand the number and suppressive function of Tregs. Since iTregs secrete large quantities of IL-9 (as shown in Fig. 5) and the presence of IL-9 could help in expanding and maintaining the suppressive function of Tregs [35], we believe that IL-9 secreted by iTregs creates an autocrine feedback loop mechanism to maintain the number and suppressive function of Tregs. This might suggest that IL-9 is the cytokine needed in a tumor environment to maintain the survival and suppressive function of Tregs. This hypothesis is supported by our results indicating that following neutralization of IL-9, stronger antitumor immune responses can be activated. Therefore, the IL-9 secreted by Tregs within the tumor is critical to maintain/expand the number and suppressive function of these cells. This is in agreement with the recent report of Eller et al. [43] which indicated that Tregs lacking IL-9 failed to suppress nephrotoxic serum nephritis in vivo. Although IL-9 attracts mast cells [27], we do not observe an accumulation of mast cells within the tumor (data not shown). Taken together, these results suggest that IL-9 plays an important role during the tumor inflammatory process sustaining/enhancing the number and suppressive function of Tregs [35], in an environment that lacks other common γ-chain signaling interleukins. Future studies are needed to better determine the role of IL-9 in tumor growth. Currently, we are crossing the IL-9 KO with BALB-neuT mice, and this model will allow us to examine whether IL-9 is critical for tumor growth, involved in the inflammatory process and how this cytokine influences the function of Tregs or other cell types in tumor-bearing hosts.
It is advantageous to employ the neutralization of IL-9 as a strategy that will specifically target and disrupt Treg function without compromising the function of effector T cells, in contrast, the use of strategies such as anti-CD25 can deplete T effector cells [17]. Additionally, it has been suggested that IL-9 is an important factor driving Th17 differentiation and by neutralizing IL-9 as a mechanism of Treg suppression, it may be more beneficial to patients in the long term by preventing autoimmunity that often results from immunotherapy [44]. We tested two different tolerant models and the results indicate that i.t. injection with CpG-ODN plus anti-IL-9 results in tumor rejection in both models as compared to control mice receiving CpG-ODN injection only. We have reported the construction of anti-neu-CpG and anti-MUC1-CpG conjugated molecules to target localized and metastatic tumors anywhere in the body [17]. Currently, we are investigating the combination of these hybrid molecules and other vaccination modalities (e.g., dendritic cells and adenovirus) with anti-IL-9 to evaluate the effectiveness of these strategies to control tumor growth.
Translation to the clinic is feasible since a phase I clinical trial using humanized neutralizing anti-IL-9 (MEDI-528) for treatment of asthma was recently published. MEDI-528 was given intravenously or subcutaneously to healthy volunteers without serious adverse events in both delivery modes [45]. There are large-scale phase 2 trials under way to further evaluate safety and determine MEDI-528 efficacy in atopic asthma. This establishes that using anti-IL9 in the clinic is feasible and has (to date) no serious side effects, making it a possible addition to cancer immunotherapy tools.
In summary, these studies show for the first time that activating 4-1BB signaling alters the suppressive function of Tregs by reducing the production of IL-9. Furthermore, neutralization of IL-9 significantly enhances the antitumor effect of tumor vaccines indicating that: 1) IL-9 plays an important role in the inflammatory process of tumors by promoting Treg function; and 2) neutralization of IL-9 could be used as an alternative approach to interfere with Treg function and enhance antitumor responses without compromising effector T cell function.
Acknowledgments
This work was supported by Grants CA 114336 and AG287510 from the National Institutes of Health and American Federation for Aging Research (AFAR) to J.L.
References
- 1.Gross S, Walden P. Immunosuppressive mechanisms in human tumors: why we still cannot cure cancer. Immunol Lett. 2008;116:7–14. doi: 10.1016/j.imlet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4 + CD25− naive T cells to CD4 + CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou G, Levitsky HI. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 7.Wada J, Suzuki H, Fuchino R, Yamasaki A, Nagai S, Yanai K, Koga K, Nakamura M, Tanaka M, Morisaki T, Katano M. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009;29:881–888. [PubMed] [Google Scholar]
- 8.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 9.Kretschmer K, Apostolou I, Verginis P, von Boehmer H. Regulatory T cells and antigen-specific tolerance. Chem Immunol Allergy. 2008;94:8–15. doi: 10.1159/000154846. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez AL, Lustgarten J. Implications of aging and self-tolerance on the generation of immune and antitumor immune responses. Cancer Res. 2008;68:5423–5431. doi: 10.1158/0008-5472.CAN-07-6436. [DOI] [PubMed] [Google Scholar]
- 11.Hoelzinger DB, Smith SE, Mirza N, Dominguez AL, Manrique SZ, Lustgarten J. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J Immunol. 2010;184:6833–6842. doi: 10.4049/jimmunol.0904084. [DOI] [PubMed] [Google Scholar]
- 12.Couper KN, Lanthier PA, Perona-Wright G, Kummer LW, Chen W, Smiley ST, Mohrs M, Johnson LL. Anti-CD25 antibody-mediated depletion of effector T cell populations enhances susceptibility of mice to acute but not chronic Toxoplasma gondii infection. J Immunol. 2009;182:3985–3994. doi: 10.4049/jimmunol.0803053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton G, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/S0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 14.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophy Acta. 2002;1589:1–13. doi: 10.1016/S0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Liu D, Majewski P, Schulte L, Korn J, Young R, Lander E, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 16.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 19.Cuadros C, Dominguez AL, Frost GI, Borgstrom P, Lustgarten J. Cooperative effect between immunotherapy and antiangiogenic therapy leads to effective tumor rejection in tolerant Her-2/neu mice. Cancer Res. 2003;63:5895–5901. [PubMed] [Google Scholar]
- 20.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 21.Lustgarten J, Dominguez AL, Cuadros C. The CD8 + T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34:752–761. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 22.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 23.Xia J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, Gong J. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170:1980–1986. doi: 10.4049/jimmunol.170.4.1980. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot J, Rasmussen J, Williams L, Dooley J, Farr A, Rudensky A. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, Kwon BS. 4-1BB-dependent inhibition of immunosuppression by activated CD4 + CD25 + T cells. J Leukoc Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 26.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg A, Colombo M. Triggering of OX40 (CD134) on CD4(+)CD25 + T cells blocks their inhibitory activity: a novel regulatory role for OX40 and the comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 27.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 29.Cooper D, Bansal-Pakala P, Croft M. 4-1BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. Eur J Immunol. 2002;32:521–529. doi: 10.1002/1521-4141(200202)32:2<521::AID-IMMU521>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piconese S, Pittoni P, Burocchi A, Gorzanelli A, Care A, Tripodo C, Colombo MP. A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. Eur J Immunol. 2010;40:2902–2913. doi: 10.1002/eji.201040505. [DOI] [PubMed] [Google Scholar]
- 32.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, Offner H, Weinberg AD. Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 34.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 35.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3 + natural regulatory T cells. Proc Natl Acad Sci USA. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukushima A, Yamaguchi T, Ishida W, Fukata K, Mittler RS, Yagita H, Ueno H. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J Immunol. 2005;175:4897–4903. doi: 10.4049/jimmunol.175.8.4897. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y, Blink SE, Liu W, Lee Y, Chen B, Solway J, Weinstock J, Chen L, Fu YX. Inhibition of Th2-mediated allergic airway inflammatory disease by CD137 costimulation. J Immunol. 2006;177:814–821. doi: 10.4049/jimmunol.177.2.814. [DOI] [PubMed] [Google Scholar]
- 38.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3 + regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 41.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/S1074-7613(00)00056-X. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Dominguez AL, Hoelzinger DB, Lustgarten J. CpG-ODN but not other TLR-ligands restore the antitumor responses in old mice: the implications for vaccinations in the aged. Cancer Immunol Immunother. 2008;57:549–561. doi: 10.1007/s00262-007-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 2011;186:83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White B, Leon F, White W, Robbie G. Two first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteers. Clin Ther. 2009;31:728–740. doi: 10.1016/j.clinthera.2009.04.019. [DOI] [PubMed] [Google Scholar]