Abstract
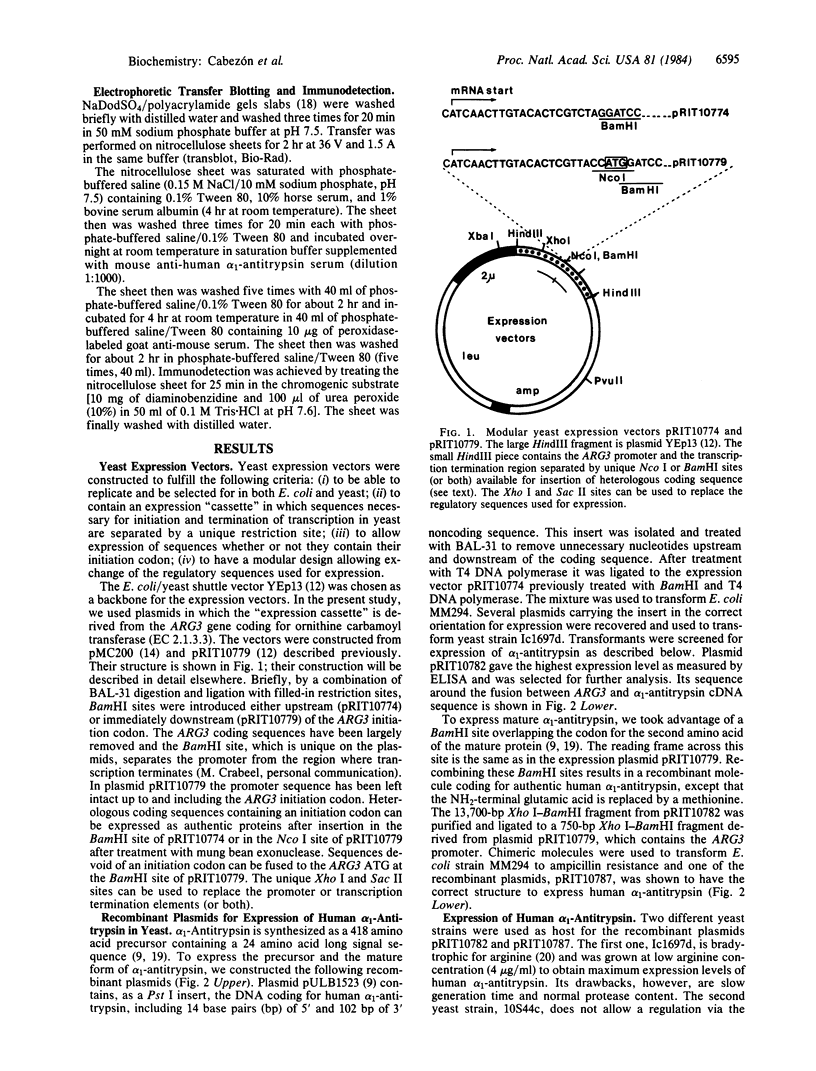
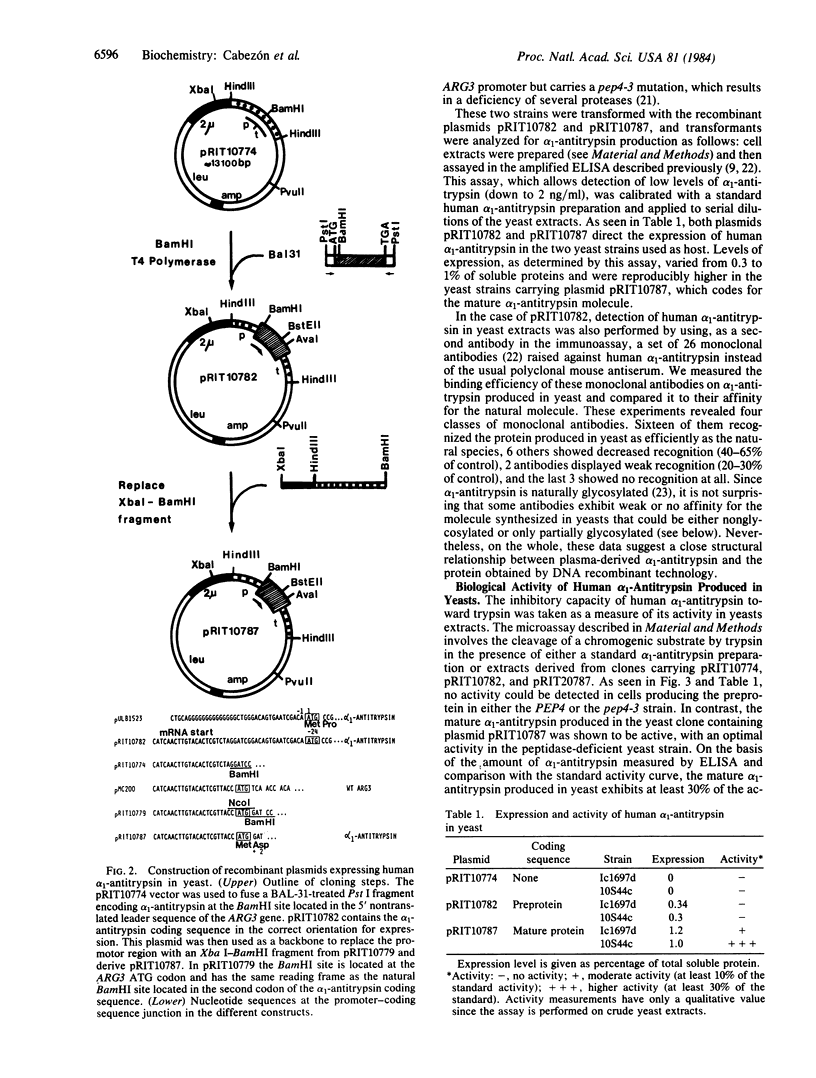
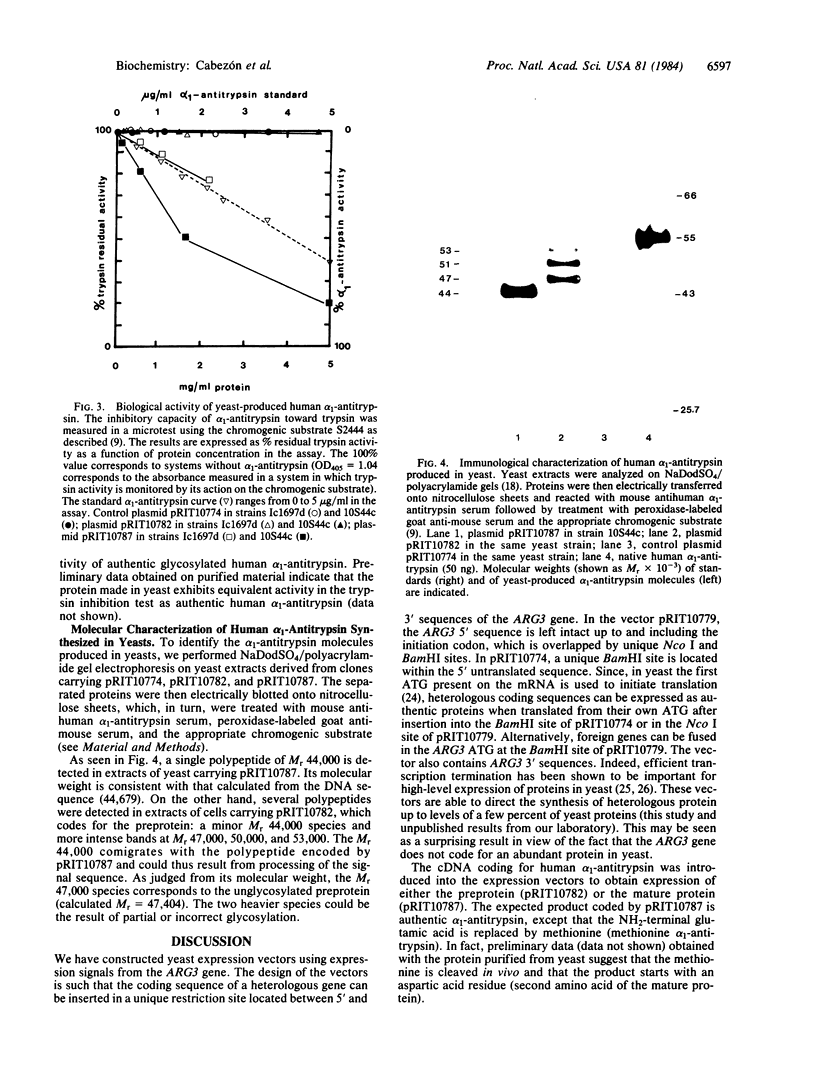
Nucleotide sequences coding either for the precursor or the mature form of human alpha 1-antitrypsin have been placed under the control of the yeast ARG3 expression signals. Recombinant plasmids pRIT10782 and pRIT10787 express the precursor or the mature alpha 1-antitrypsin species, respectively, in two different yeast strains, with yields ranging between 0.3 and 1% of total soluble proteins. The alpha 1-antitrypsin synthesized in yeasts was specifically recognized by polyclonal and monoclonal antibodies raised against human alpha 1-antitrypsin. In addition, it was shown to be biologically active in its mature form only, with optimal activity in a peptidase-deficient yeast strain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Bollen A., Herzog A., Cravador A., Hérion P., Chuchana P., Vander Straten A., Loriau R., Jacobs P., van Elsen A. Cloning and expression in Escherichia coli of full-length complementary DNA coding for human alpha 1-antitrypsin. DNA. 1983;2(4):255–264. doi: 10.1089/dna.1983.2.255. [DOI] [PubMed] [Google Scholar]
- Bollen A., Loriau R., Herzog A., Hérion P. Expression of human alpha 1-antitrypsin in Escherichia coli. FEBS Lett. 1984 Jan 23;166(1):67–70. doi: 10.1016/0014-5793(84)80046-0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Huygen R., Cunin R., Glansdorff N. The promoter region of the arg3 gene in Saccharomyces cerevisiae: nucleotide sequence and regulation in an arg3-lacZ gene fusion. EMBO J. 1983;2(2):205–212. doi: 10.1002/j.1460-2075.1983.tb01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delforge J., Messenguy F., Wiame J. M. The regulation of arginine biosynthesis in Saccharomyces cerevisiae. The specificity of argR- mutations and the general control of amino-acid biosynthesis. Eur J Biochem. 1975 Sep 1;57(1):231–239. doi: 10.1111/j.1432-1033.1975.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Eriksson S. Studies in alpha 1-antitrypsin deficiency. Acta Med Scand Suppl. 1965;432:1–85. [PubMed] [Google Scholar]
- Fagerhol M. K., Laurell C. B. The Pi system-inherited variants of serum alpha 1-antitrypsin. Prog Med Genet. 1970;7:96–111. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N., Cabezon T., Crabeel M., Simoen E., Rutgers A., De Wilde M. Expression of hepatitis B surface antigen in yeast. Dev Biol Stand. 1983;54:125–130. [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Levine H. L., Goeddel D. V., Ammerer G., Hall B. D. Expression of a human gene for interferon in yeast. Nature. 1981 Oct 29;293(5835):717–722. doi: 10.1038/293717a0. [DOI] [PubMed] [Google Scholar]
- Hitzeman R. A., Leung D. W., Perry L. J., Kohr W. J., Levine H. L., Goeddel D. V. Secretion of human interferons by yeast. Science. 1983 Feb 11;219(4585):620–625. doi: 10.1126/science.6186023. [DOI] [PubMed] [Google Scholar]
- Hérion P., Siberdt D., Francotte M., Urbain J., Bollen A. Monoclonal antibodies against plasma protease inhibitors: II. Production and characterization of 25 monoclonal antibodies against human alpha 1-antitrypsin. Correlation between antigenic structure and functional sites. Biosci Rep. 1984 Feb;4(2):139–147. doi: 10.1007/BF01120310. [DOI] [PubMed] [Google Scholar]
- Jones E. W., Zubenko G. S., Parker R. R. PEP4 gene function is required for expression of several vacuolar hydrolases in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):665–677. doi: 10.1093/genetics/102.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Chandra T., Degen S. J., White T. T., Marchioro T. L., Woo S. L., Davie E. W. Cloning and sequence of cDNA coding for alpha 1-antitrypsin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6826–6830. doi: 10.1073/pnas.78.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. II. Structure of oligosaccharides. J Biol Chem. 1980 May 10;255(9):4057–4061. [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]



