Abstract
Purpose
Cidofovir (CDV) is a FDA approved nucleoside antiviral agent used to treat severe human cytomegalovirus (HCMV) infection. Until now, no clear therapeutic effects of CDV have been reported outside of the setting of viral infection, including a potential role for CDV as an antineoplastic agent for the treatment of brain tumors.
Experimental Design
We investigated CDV cytotoxicity against glioblastoma (GBM) cells, U87MG and primary SF7796, in vitro and in vivo, using an intracranial xenograft model. Standard techniques for cell culturing, immunohistochemistry, Western blotting, and real-time PCR were employed. The survival of athymic mice (n= 8–10 per group) bearing GBM tumors, treated with CDV alone or in combination with radiation, was analyzed by the Kaplan-Meier method and evaluated with a two-sided log-rank test.
Results
CDV possesses potent antineoplastic activity against HCMV infected GBM cells. This activity is associated with inhibition of HCMV gene expression and with activation of cellular apoptosis. Surprisingly, we also determined that CDV induces GBM cell death in the absence of HCMV infection. CDV is incorporated into tumor cell DNA, which promotes double-stranded DNA breaks and induces apoptosis. In the setting of ionizing radiation treatment (RT), the standard of care for GBM in humans, CDV augments radiation-induced DNA damage and further promotes tumor cell death. Combined CDV and RT treatment significantly extended the survival of mice bearing intracranial GBM tumors.
Conclusion
We have identified a novel anti-glioma property of the FDA approved drug CDV, which heightens RT cytotoxic effect, the standard of care therapy for GBM.
Keywords: Brain/central nervous system cancers, DNA-reactive agents, Cidofovir, Glioblastoma, Cytomegalovirus
Introduction
Glioblastoma (GBM) is the most common primary brain tumor in adults, and despite advances in our understanding of GBM biology, and the use of these molecular insights to test innovative approaches for treating GBM, mean GBM patient survival remains 12–15 months (1). We hypothesized that persistent viral infection plays a role in GBM pathogenesis, given the extensive results from our laboratory and others demonstrating that human cytomegalovirus (HCMV) infection is present in a majority of human GBMs (2). Several HCMV gene products that are expressed in human GBMs are thought to contribute to tumor pathogenesis, including the expression of HCMV IE1 protein which has been associated with length of patient survival (3–7). Furthermore, improved survival was recently reported for patients with GBM who were treated with the oral antiviral drug valganciclovir, when used in combination with standard of care treatment for this cancer, radiation and temozolomide, suggesting that anti-HCMV therapy might provide clinical benefit to GBM patients (8).
To determine whether inhibition of HCMV gene expression impacts the biology of GBM, we used the antiviral agent cidofovir (CDV), an acyclic nucleoside phosphonate analog with a broad spectrum anti-viral DNA synthesis activity that has been approved for the treatment of CMV retinitis in patients with AIDS (9, 10). CDV has also proved effective for the treatment of other DNA viral related diseases including the central nervous system disease progressive multifocal leukoencephalophathy (PML) (11). The antiviral activity of CDV is thought to result from the selective inhibition of viral DNA polymerase by its diphosphate metabolite (CDVpp). CDVpp acts as a competitive inhibitor and alternative substrate for dCTP. CDV is also converted to CDVp-choline, which has a half-life of over 80 hours and may serve as a reservoir from which the active metabolite of CDVpp can be produced (12, 13). Although nephrotoxicity can be a limitation of using high doses of CDV in up to 15% patients (14), new lipophilic CDV derivatives administered orally causing minimum toxicity are already being tested (15).
In the setting of virus-driven oncogenesis, CDV has been shown to inhibit cancer growth in various in vivo models, including those involving human tumor xenografts (Epstein-Barr virus-associated nasopharyngeal carcinoma and human papillomavirus-induced cervical carcinoma) and polyomavirus-induced rodent tumors (hemangiomas in rats as well as hemangiosarcoma development in mice) (16–20). In addition, hemangioma tumors are inhibited by CDV, independent of viral infection, by induction of endothelial cell apoptosis (13, 21). This effect was associated with S-phase arrest, PARP activation, p53 up-regulation, and caspase activation (22). The latter study results therefore suggest that CDV can inhibit cellular DNA synthesis and inhibit tumor cell proliferation independent of endogenous viral presence, potentially by incorporation into cellular DNA as a substitute for dCTP, as demonstrated in the case of HPV-infected human keratinocytes (23).
While most cytosine nucleoside analogs (e.g., ara-C and gemcitabine) block tumor growth by inducing DNA chain termination, CDV appears to act by inhibiting secondary rounds of DNA synthesis (24, 25). Because of this, CDV may have unique potential as a radiation-sensitizing agent (26, 27). CDVpp incorporation into radiation damaged DNA may activate tumor cell apoptotic response mechanisms. Indeed the literature supports a “radio-sensitiser” role for CDV in the setting of ionizing radiation (28).
In order to determine whether CDV inhibits GBM cell growth, as well as to address viral dependency of observed anti-tumor effects, we conducted experiments to evaluate CDV effects on GBM cell lines and primary explant cultures, both in the presence or absence of HCMV infection. To determine how growth-inhibitory effects of CDV interact with ionizing radiation therapy (RT), we extended our experiments to include in vivo analysis of combined CDV + RT treatment, using orthotopic GBM xenograft models. Our data indicate a previously unrecognized potent anti-glioma effect of CDV, which acts in combination with RT for increased tumor cell kill through increased apoptotic response.
Materials and Methods
Primary GBM samples and neurosphere growth assays
Tissue samples were obtained during surgery from patients diagnosed with GBM using an IRB-approved protocol. Fresh tumor tissues were subjected to enzymatic digest, mechanically dissociated, and cultured as neurospheres as previously described by our group (29). For growth assays, cells were cultured in neurosphere media (Neural Basal Media + EGF/FGF2) at 1000 cells/well in 96-well plates. Media was replaced every other day, with or without CDV (100 μM, where indicated) and tumor cells monitored using an inverted microscope fitted with a camera. 96–120 hours following initial culturing, spheres were photographed and counted. All sample incubations were in quadruplicate. Experiments were repeated twice for each primary culture tested.
Apoptosis Antibody Arrays
Proteins were extracted from GBM cultures, treated with or without CDV for 48 hr, and assayed with the Human Apoptosis Array Kit (R&D Systems) according to the manufacturer’s protocol.
Protein extraction for Western blot and Western blot analysis are described in the Supplementary Methods.
Cell culture, infection, treatment, and viability assays are described in the Supplementary Methods.
RNA extraction and real-time PCR are described in the Supplementary Methods.
Incorporation of 14C-CDV into cellular DNA
U87MG cells and MRC-5 fibroblasts were grown in MEM supplemented with 10% FBS and penicillin/streptomycin. Cells were seeded into T-25 flasks and allowed to reach ~ 50% confluence. One variant with MRC-5 was carried with the cells at 100% confluency (contact inhibition – no mitoses). Cells were incubated for 24, 48, and 72 hours with 2 nCi/5ml of 14C-labeled CDV. In addition, three log-phase cultures of U87MG were irradiated with 1.5 Gy using a cesium-137 source (J.L Shepherd & Associates, San Fernando, CA) at a dose rate of 3.2 Gy/min. Immediately following irradiation, medium was exchanged with fresh medium and 14C-labeled CDV. Irradiated cells with corresponding control cultures were harvested after 2, 6, and 24 hours. Cultures were harvested at each time point and after 3 washes frozen cell pellets were shipped to Accium Biosciences (Seattle, WA) where DNA was isolated using a Qiagen PureGene Tissue Kit to remove unbound 14C-CDV. DNAs were dissolved in 100 μl of hydration buffer, and incorporation of 14C into cellular DNA was quantified by ultrasensitive accelerator mass spectrometry (AMS) (Accium BioSciences, Seattle, WA).
GBM xenografts
U87MG cells were cultured as described above. Human GBM primary tissue SF7796 was established and maintained as serially passaged subcutaneous xenografts in athymic mice. Both U87MG and SF7796 were modified by lentiviral infection for stable expression of firefly luciferase to enable in vivo bioluminescence imaging, as previously described (30). To prepare tumor cells from subcutaneous xenografts for intracranial injection, previously described protocols were used (31). Both SF7796 and U87MG cells were resuspended in DMEM at 1 × 108 cells/mL.
Intracranial tumor establishment in athymic mice
Five to six-week-old female athymic mice (nu/nu, homozygous: Simonsen laboratories, Gilroy, CA) were housed under aseptic conditions, and received intracerebral tumor cell injection as previously described (31). Briefly, mice were injected with 300,000 cells (3 μl) into the right striatum using 26-gauge needle. Animals were monitored daily and imaged 1–2 x weekly until euthanized when exhibiting significant neurological deficit, or greater than 15% reduction from their initial body weight. The number of animals consisted of 8–10 for each treatment group. CDV treatments were by intraperitoneal injection (100 mg/kg), 3x/week until required euthanasia, as indicated by animal subject body condition. The vehicle used was sterile saline.
Bioluminescence monitoring of intracerebral tumor growth
In preparation for bioluminescence imaging (BLI), mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), then administered 150 mg/kg of luciferin (D-luciferin potassium salt, Gold Biotechnology, MO) via intraperitoneal injection. Ten minutes after luciferin injection, mice were examined for tumor bioluminescence using an IVIS Lumina imaging station (Caliper Life Sciences, Alameda, CA). Regions of interest, defined using Living Image software (Caliper Life Sciences, Alameda, CA), were recorded as photons per second per steradian per square cm (31, 32).
Mouse irradiation
Mice were anesthetized via inhalation of 2.5% isoflurane with 1 liter of oxygen per minute for 5 minutes prior to being positioned on an irradiation platform located 16.3 cm from a cesium-137 source (J. L. Shepherd & Associates, San Fernando, CA). Their eyes, respiratory tracts and bodies were protected with lead shielding. Mice received whole brain irradiation (32) at a dose rate of 2.47 Gy/min until 2 Gy radiation for U87MG or 1 Gy radiation for SF7796 had been delivered. After irradiation, animals were monitored until recovery. Radiation treatment was initiated when tumors were in a log-phase growth, as determined by bioluminescence monitoring, with mice irradiated once daily for 5 consecutive days.
Tissue processing, immunohistochemistry and quantification of staining
Within each treatment group of mice, 3 animals were euthanized under deep general anesthesia (sodium pentobarbital, 90 mg/kg intraperitoneal) and brains were harvested for IHC analysis. Detailed procedures are described in the Supplementary Methods.
Statistical analysis
PRISM 5, Version 5.03 (GraphPad Software) was used to conduct all statistical analyses (EC50 values, log-rank Mantel-Cox test for survival analysis, and the student’s t-test for tumor bioluminescence). The EC50 values with corresponding 95% confidence limits were compared using the unpaired Student’s t-test. All data are presented as a mean ± SD. We considered P values of less than 0.05 as statistically significant.
Results
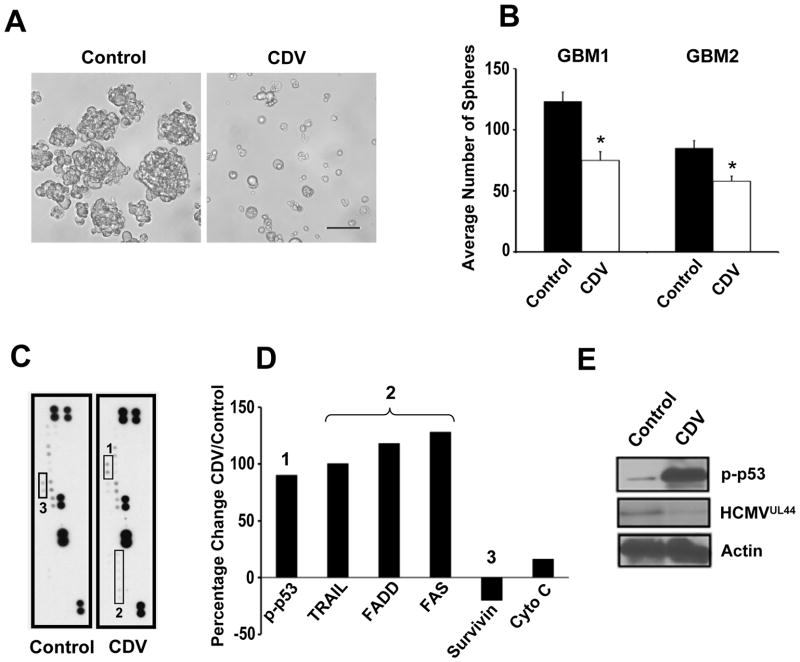
To investigate whether CDV inhibits HCMV-infected GBM tumor cell growth, we treated primary patient-derived GBM cells (passage zero) with CDV (20 μM) for 72 hrs following initial establishment of neurosphere cultures. Cells treated with CDV (Figure 1A–B) displayed phenotypic changes suggestive of cell apoptosis. To investigate the mechanism underlying this response, we used an apoptosis antibody array to profile cell lysates from control and CDV-treated samples, with results indicating at least two apoptotic pathways being activated in the presence of CDV: p53 and Fas/TNFa-Trail (Figure 1C). Results from the antibody array were quantified (Figure 1D) and validated using western blot analysis (Figure 1E). Furthermore, the expression of HCMV genes (e.g., UL44) in this GBM sample was inhibited by CDV treatment (Figure 1E). We similarly examined the CD133+ (stem-like) cellular fraction from another GBM sample, which was also positive for endogenous HCMV. CDV treatment inhibited tumor sphere formation for these CD133+ cells (Fig 1B, white bars) as well as the expression levels of various viral genes (e.g., pp71) and cellular proteins that are important contributors to GBM pathogenesis (e.g., Sox2, SCF). These latter data (Supplementary Figure S1) suggest that the use of antiviral drug CDV inhibits the expansion of primary GBM spheres by multiple mechanisms, including the activation of pro-apoptotic pathways, and the inhibition of self-renewal and mitogenic pathways.
Figure 1. CDV inhibits neurosphere growth, HCMV gene expression, and induces apoptosis in primary GBM cultures.
(A) Photomicrographs of representative control (left panel) and CDV treated (right panel, 20 μM, 72h) primary patient tumor derived neurosphere cultures. Bar = 50μm. (B) Quantification of neurospheres in CDV vs. vehicle-treated cultures, 96 h following treatment. Results from two distinct primary GBM cultures, each examined using quadruplicate samples per treatment condition, are shown as means ± SD (experiment was repeated once for each cell source). Black bars- control treatment, white bars- CDV treatment. *p=0.01, student t-test. (C) Scanned image of apoptosis arrays hybridized with control (left panel) and CDV-treated (right panel) cell lysates from primary GBM cultures shown in A, 48 hours following treatment. (D) Results from quantitative densitometry analysis of the arrays shown in C for the indicated proteins. (1): p-p53, (2): TRAIL, FADD, FAS, and (3) Survivin. (E) Validation of apoptosis array result by western blot analysis, using a portion of the same lysates as applied to the arrays. Antibodies against p-p53, HCMV UL44 and Actin were used.
To further investigate HCMV as a determinant of GBM cell response to treatment with CDV we next utilized an isogenic model in which U87MG cells, +/− HCMV infection, were examined. The viability of infected cells was more affected by CDV than was uninfected U87MG (Figure 2A, p=0.0059). Importantly, however, viability of uninfected cells was also reduced by CDV treatment (but not HCMV alone – Supplementary Figure S2), suggesting that additional mechanisms, independent of CDV suppression of HCMV gene expression (which is demonstrated in Supplementary Figure S3), underlie the pro-apoptotic effects of CDV. Cidofovir treatment of HCMV-infected U87MG and primary glioma stem cell culture (GSC) 387 resulted in significant inhibition of expression of several viral genes, including UL84, UL55 (gB), UL83 (pp65) and UL82 (pp71) (Supplementary Figure S3). Primary-derived GBM culture SF7796 was found to be endogenously infected with HCMV (Supplementary Figure S4) and used for subsequent testing of CDV anti-tumor effects, as described below. In vitro super-infection of SF7796 with the HCMV TR strain (MOI =0.5) further sensitized these cells to CDV (Figure 2B, p = 0.034). Thus, viability assay results, from both an established glioma cell line (U87MG) and a patient derived GBM primary culture, demonstrate that CDV inhibits GBM cell expansion, regardless of the presence or extent of HCMV infection. In the dose response curve, the EC50 was significantly higher (635.3 μM) in normal MRC-5 fibroblasts treated with CDV (Figure 2A) than in both tested GBM cell lines (371.1 μM for U87MG and 375.2 μM for SF7796). Interestingly, when the fibroblasts were grown at confluence (no mitoses due to contact inhibition), the EC50 was dramatically increased (Supplementary Figure S5A).
Figure 2. Effect of CDV on glioblastoma and normal cells in vitro.
CDV dose-response for U87MG (A) and SF7796 (B) cells infected (or superinfected (+++) in case of SF7796) with HCMV (MOI = 0.5) vs. mock infected controls. MRC-5 fibroblasts (A) were HCMV(−). Each incubation condition for each cell source was in quadruplicate, with cell viability measured using Cell Counting Kit-8 (Dojindo Molecular Technology, Inc.), at 5 days after adding CDV. Note that HCMV infection increases sensitivity of both GBM cell lines to CDV treatment. (C) Incorporation of 14C-CDV into genomic DNA: U87MG cells and MRC-5 fibroblasts (in their log phase and 100% confluent cultures) were exposed to 14C-labeled CDV (100 μM) for 24, 48 and 72 hours. After DNA extraction, dpm of incorporated 14C was measured with ultrasensitive accelerator mass spectrometry (AMS technology). Control culture with no 14C-labeled CDV added showed typical background value of 4.2 ×10−6 dpm/μg DNA. Values on axis Y are expressed as number of CDV molecules per base pair. Black columns – U87MG cells; gray columns – MRC-5 fibroblasts in their log phase of growth; white columns: 100% confluent MRC-5 cultures (contact inhibition – no mitoses). Means ± SD are shown; * p < 0.0001 when compared with control (100% confluent MRC-5) for each time point. (D) DNA damage response after treatment with CDV in vitro: Western blot analysis of extracts from U87MG cells treated with CDV (100 μM for 96 hours), radiation (1.5 Gy), or both CDV and radiation. CDV treatment increases phosphorylation of H2A.X at Ser139. (E) DNA damage response after treatment of MRC-5 fibroblasts (+/− CMV) with CDV (100 μM for 96 hours). CDV treatment induces phosphorylation of H2A.X only in previously CMV-infected cells (HCMV increases cell sensitivity to CDV treatment). Infection with CMV (without CDV) also causes slight induction of H2A.X phosphorylation. Protein pp71 was used as the marker for HCMV infection. (F) shows results from the densitometric analysis of phospho-H2A.X following signal normalization to Actin.
Since CDV is a nucleoside analog, we hypothesized that it might induce apoptosis by becoming incorporated into cellular DNA, leading to DNA injury and subsequent activation of programmed cell death. Further, we hypothesized that the combination of CDV with ionizing radiation (RT), which is the standard of care for GBM, might act in concert to promote further tumor cell apoptosis. To test these hypotheses, we examined proliferating U87MG incorporation of 14C-labeled CDV into newly synthesized DNA, both in the presence and absence of 1.5 Gy irradiation. Cells were harvested at 2, 6, and 24 hours post-treatment, and DNA was extracted and analyzed using ultrasensitive accelerator mass spectrometry (AMS) (Accium BioSciences, Seattle, WA). After 2-hour incubation, 14C-labeled CDV incorporates into genomic DNA at a frequency of 10−9 nucleotide per DNA base pair in both “CDV only” and “CDV + RT” -treated cultures. Analysis of additional time points revealed that the extent of CDV incorporation was time-dependant, but was not significantly affected by RT treatment (Supplementary Figure S5B).
To test the effects of CDV on normal cells, MRC-5 fibroblasts were exposed to the same doses of 14C-labeled CDV as U87MG (without irradiation). The results showed that the GBM cell line incorporates the drug into DNA at a much higher rate (Figure 2C) than MRC-5. In addition, when cellular divisions were halted by contact inhibition, the incorporation was minimal. This would indicate that CDV treatment primarily affect rapidly dividing cells.
To test the hypothesis that CDV causes cellular DNA damage, and that the combination of CDV plus RT promotes more robust DNA damage than either agent alone, we performed Western blot analysis with antibodies specific to proteins involved in DNA repair, using cell lysates from U87MG cells exposed to CDV, irradiation, or the two in combination. Analysis of cellular phospho-histone H2A.X, a sensitive indicator of double-stranded DNA breaks (33), showed that both CDV and RT induce H2A.X phosphorylation. This effect was dramatically enhanced when CDV was combined with RT (27.6- or 21-fold increase when compared to treatment with only radiation or CDV, respectively) (Figure 2D). Interestingly, when normal fibroblasts were treated with CDV, no induction of H2A.X phosphorylation was observed (Figure 2E and 2F). However, when fibroblasts were previously infected with HCMV, exposure to CDV caused significant upregulation of activated H2A.X (sensitizing effect). HCMV infection alone (without CDV) caused only slight induction of H2A.X (Figure 2E and 2F), in concordance with previous studies which demonstrated that HCMV can induce chromosomal and DNA damage (34).
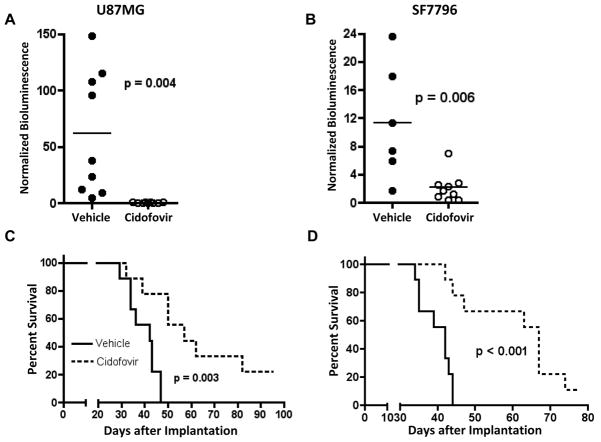
In order to determine if the antitumor effects of CDV observed in cultured GBM cells would translate to in vivo conditions, we used a mouse intracranial GBM xenograft model. Our initial in vivo analysis was aimed at addressing the anti-tumor activity of CDV, as administered systemically, via intraperitoneal injection, to athymic mice in which human GBM xenografts had been established by intracranial injection of tumor cells infected with HCMV, and/or that express HCMV gene products (Supplementary Figure S4). As anticipated based on published work showing that virus-associated subcutaneous xenograft tumors responded to CDV treatment (13, 17), mice administered with 100 mg/kg CDV, 3 times weekly, either delayed or prevented intracranial tumor development, compared to mice treated with vehicle only (Figures 3A, 3B). Importantly, CDV treatment significantly extended animal subject survival for each of the two xenograft models we tested (Figures 3C, 3D).
Figure 3. CDV inhibits the growth of CMV-infected U87MG and SF7796 in vivo.
Tumor bioluminescence (A and B) and animal subject survival (C and D), for athymic mice receiving intracranial human GBM tumor cell implantation with U87MG (A and C) or SF7796 (B and D). For each bioluminescence graph, results are shown at day 36 post tumor cell implantation, a time at which there were only 6 surviving mice in the untreated control group for SF7796. Treatments were initiated at day 19 post tumor cell implantation for mice with intracranial SF7796, and on day 11 post U87MG cell implantation. Indicated p-values, all of which are < 0.05, are based onapplication of student’s t-test (A and B) and log rank test (C and D). There was detectable bioluminescence in all CDV-treated animals implanted with U87MG (A) with values ranging from 0.11 to 1.31 at the imaging time-point. Because these values are low, in relation to the scale used for the y-axis, the values appear to be zero.
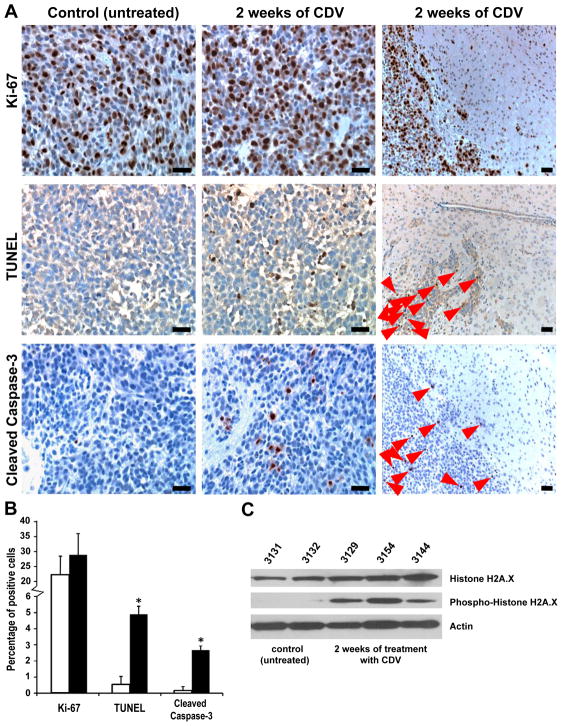
No significant differences in cell proliferation were identified in the control vs. CDV –treated SF7796 primary xenograft GBM tumors after two weeks of treatment: Ki-67 proliferation index for mice receiving CDV was 28.9 ± 7.1%, vs. untreated control tumors showing an average of 22.4 ± 6.0 % cells positive for Ki-67 (Figure 4A and 4B, p=0.13). In contrast, immunohistochemical analysis of tumors from mice receiving treatment with CDV showed extensive apoptotic response of tumor cells, as indicated by DNA fragmentation revealed by TUNEL staining (Figure 4A): 4.9 ± 0.5% for CDV-treated tumors vs. 0.54 ± 0.06% in controls (Figure 4B, p<0.0001). Detection of apoptotic cells in treated tumors was also confirmed by staining for cleaved Caspase 3 (Figure 4A) (2.68 ± 0.25% vs. 0.17 ± 0.03% in untreated mice: p<0.0001, Figure 4B). Interestingly, the positive cells for TUNEL and cleaved Caspase-3 were detected only within the tumor areas of CDV-treated mice (Figure 4A, third column). All non-tumor areas of treated and control animals were negative for both markers. CDV treatment also inhibited expression of the late HCMV antigen in these endogenously infected primary GBM cells (Supplementary Figure S6). Furthermore, phospho-histone H2A.X was detected in the tumor lysates from mice treated with CDV, but not in tumors from untreated control mice (Figure 4C). Taken together, these data indicate that CDV induces apoptosis in proliferating GBM cells by promoting DNA damage and, as a result, CDV treatment decreases tumor growth and extends animal subject survival. To address the importance of anti-HCMV effects of CDV in terms of tumoricidal activity, we repeated the experiment with U87MG cells, but this time without HCMV infection of U87MG prior to implantation. Consistent with our in vitro results, substantial anti-tumor effect from CDV treatment was evident (Figure 5A, 5B), suggesting that CDV anti-tumor activity is not dependent on, and is not necessarily affected by presence of virus.
Figure 4. Effect of CDV on human SF7796 xenografts in vivo.
(A) Ex vivo immunohistochemistry staining for Ki-67, TUNEL, and cleaved Caspase-3 in xenografts treated with CDV. Results shown for Ki-67 are at 2 weeks, although similar results were obtained at other time points. Representative photomicrographs (control and week 2) of TUNEL and cleaved Caspase-3 immunohistochemical staining similarly show increased numbers of positive cells in the tumors of mice treated with CDV (magnification x 20). The third column of photographs shows lower magnification (x 10) of the margin between invasive tumor and surrounding non-tumor tissues. Positive cells (red arrows) were detected only in tumor areas of CDV-treated mice. Size bars = 200 μm. (B) Statistical analysis of staining for Ki-67, TUNEL, and cleaved Caspase-3 between treated (black columns) and control animals (white columns) is shown as means ± SD. Asterisks depict statistically significant differences with p < 0.001. (C) Western blot analysis of extracts from SF7796 xenografts treated with CDV. CDV treatment results in increased phosphorylation of the histone H2A.X at Ser139.
Figure 5. Growth inhibition of U87MG-derived tumors lacking HCMV.

Tumor bioluminescence (A) and animal subject survival (B), for athymic mice receiving intracranial injection of U87MG cells. Bioluminescence results (means ± SD) are at day 29-post tumor cell implantation. CDV treatments were as described in Figure 4, and were initiated day 11 post U87MG cell implantation. Indicated p-values are based on statistical analysis as described in Figure 3.
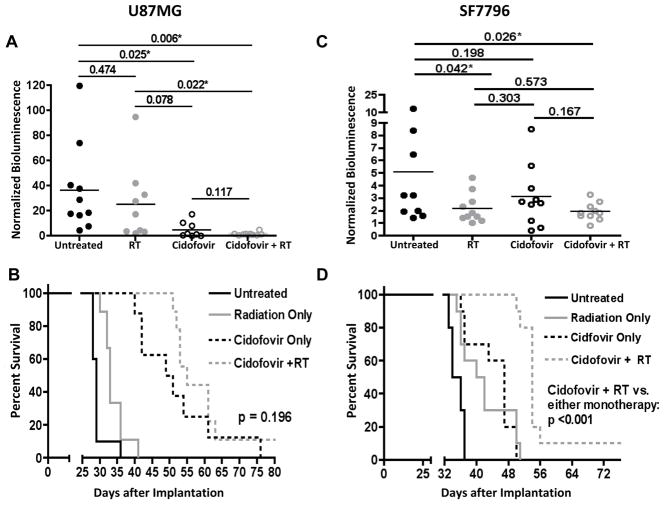
Our next in vivo experiment was conducted to evaluate the anti-tumor effect of CDV when administered in combination with RT. For U87MG-derived tumors without HCMV infection, comparison of group bioluminescence values revealed substantial anti-tumor effect from RT or CDV treatment alone. Although combination therapy further suppressed tumor growth (Figure 6A), and increased animal survival, as indicated by comparison of group mean values, and in relation to corresponding monotherapies the effects of combination therapy were not significantly different relative to monotherapy effects (Figure 6B).
Figure 6. CDV potentiates the anti-tumor effect of ionizing radiation in mice intracerebrally implanted with human GBM cells.
Tumor bioluminescence (A, C) and animal survival (B, D) of athymic mice treated with CDV only, radiation only, or with concurrent radiation and CDV therapy. Bioluminescence results (means ± SD) are at days 26 (U87MG) - A and 29 (SF7796) - C post tumor cell implantation. Student’s t-test values are indicated for all 2-way comparison (asterisks denote values <0.05). CDV treatments were as described in Materials and Methods, and were initiated day 11-post U87MG (B) and at day 19 post SF7796 cell (D) implantation. Radiotherapy was 2 Gy/treatment/day x 5 consecutive days, beginning at days 11 and 19 post U87MG and SF7796 implantation, respectively.
A repeat of the combination therapy experiment with SF7796-derived tumors (primary-derived GBM, endogenously positive for HCMV expression – Supplementary Figures S4 and S6) showed that combined treatment significantly extended survival compared to either monotherapy (Figure 6C and 6D). Therefore, based on the results of two distinct intracranial xenograft models, we conclude that combination CDV + RT treatment has the most substantial anti-tumor effect.
Discussion
Here we show for the first time that CDV, an FDA approved drug for treatment of HCMV infection, possesses potent anti-tumor properties against GBM. We found that CDV inhibits HCMV viral gene expression in HCMV-infected primary GBM tumors derived from patient biopsy specimens, but that viral gene expression was not required for CDV anti-tumor activity. Our data indicate that CDV becomes incorporated into the DNA of proliferating tumor cells, which likely initiates DNA double-stranded breaks and/or stalling of the DNA replication fork, leading to tumor cell apoptosis. When CDV treatment was combined with ionizing radiation we observed a dramatic increase in phosphorylation of histone H2AX, a sensitive indicator of double stranded DNA breaks, thereby showing that the DNA damaging effects of RT are exacerbated by CDV.
The current paradigm for the mechanism of action of CDV in treating various types of viral-associated diseases suggests that this nucleoside analog becomes preferentially incorporated into viral DNA (35). Interpretation of preferential antiviral effect is based on results indicating that the viral DNA polymerase, rather than human DNA polymerase, is selectively inhibited. In the case of the antiviral drug ganciclovir, a thymidine nucleoside analog, there is clearly a greater specificity for inhibition of HCMV DNA synthesis, rather than cellular DNA synthesis, because the activity of ganciclovir depends on viral thymidine kinsase for its conversion to the active metabolite that is incorporated into viral DNA as a chain terminator (36). In contrast, CDV does not depend upon a viral gene product to become phosphorylated, but rather is phosphorylated by the human cellular cytidine kinase enzyme. Thus, there is no virus-specific activity required for conversion of CDV to an active state. An important issue is the safety of CDV treatment to normal cells of a patient. Our data strongly suggest that CDV primarily affect rapidly dividing cells, sparing cells that replicate their DNA at a lower rate. The incorporation of CDV molecules into newly synthesized DNA is therefore dependant on the mitotic rate (23). No apoptotic cells were detected outside the tumor tissue of treated mice and normal fibroblasts grown in contact inhibition did not incorporate toxic levels of CDV.
The current literature suggests that, as opposed to other cytosine analog antineoplastic agents such as gemcitodine, CDV does not cause direct DNA chain termination upon incorporation into cellular DNA, but likely promotes stalling of the DNA replication fork (37). DNA fork stalling could lead to “futile cycling” of the replication fork or of DNA double strand break repair. In either case, if the DNA replication process is not sustained, affected cells may undergo programmed cell death.
By the addition of further DNA insult, such as ionizing radiation, we hypothesized that cellular apoptotic response would increase. In vitro data revealed a dramatic increase (over 21-fold) of a phosphorylated histone H2AX indicating DNA injury/instability after exposure to both CDV and ionizing radiation, and that this effect resulted in reduced tumor growth in vivo, which, in turn, results in extending animal subject survival.
Further investigation is needed to address the extent to which the antitumor effect of CDV is due to a direct anti-HCMV effect vs. an effect on cellular DNA synthesis, repair and apoptotic signaling. HCMV infection seems to sensitize cells (both tumor and normal) to CDV treatment. The underlying mechanism is not fully understood, however, this and other studies show that during infection, host cells activate DNA damage signaling pathways (phosphorylation of H2A.X) (38–40). Using HCMV-infected GBM cells (U87MG and SF7796), we determined enhanced in vitro tumor cell CDV sensitivity with the presence of virus. The impact of HCMV gene expression may play a significant role in the pathogenesis of GBMs (3, 6, 7, 41) and medulloblastomas in vivo (42). Carro et al, for example have demonstrated that activation of the STAT3 signaling pathway is a critical event in the progression of proneural GBM to the highly aggressive mesenchymal subtype of GBM (43). We as well as others, have shown that the expression of HCMV IE1 and US28 gene products promote STAT3 signaling (3, 4, 6). Additional studies using endogenously infected GBM tissues, measuring the effects of CDV and related compounds on HCMV-induced growth promoting signaling are under way in our laboratory.
Regardless of the issue concerning the roles and effects of HCMV in GBM oncogenesis, our results show that CDV is an effective drug for interfering with DNA synthesis, both in the presence and absence of HCMV. Its incorporation into cellular DNA activates DNA damage response pathways due to increased DNA breaks, that prompts elevated tumor cell apoptotic response. Given the mode of cell killing by CDV, its use in combination treatments with radiation represents a promising and practical strategy to improve treatment outcomes for GBM patients. Enhanced radiation sensitivity in HPV-positive head and neck cancer has been recently shown (44). Studies with analogs of CDV having more favorable pharmacokinetics are underway.
Supplementary Material
Translational Relevance.
Cidofovir (CDV) is a FDA-approved nucleoside analog used to treat severe human cytomegalovirus (HCMV) infection. Until now, no clear antineoplastic effects of CDV have been reported outside of the setting of viral infection, and no prior evidence has shown a role for CDV as an antineoplastic agent for glioblastoma (GBM). Our pre-clinical evaluation of CDV revealed its potent antitumor activity against GBM, independently of the status of HCMV infection. CDV is incorporated into tumor cell DNA, thereby promoting double-stranded DNA breaks and triggering downstream apoptotic signaling. In-vivo data with human GBM intracranial xenografts showed that, when treatment with CDV was combined with radiation therapy, it significantly increased the survival of tumor-bearing mice. We believe that this finding may represent a new therapeutic avenue for GBM treatment.
Acknowledgments
Financial Support: NIH grants R01NS070289-02 (C.S.C., P.H.); 1R21NS067395-01 (L.S.); ACS grant RSG-09-197-01 (C.S.C., P.H.); ABC2 Foundation and Flaming Foundation; SPORE grant CA097257 (C.D.J. and T.O.)
Grant Support
These studies were supported by the National Institutes of Health, Grants R01NS070289-02 to C.S.C..; 1R21NS067395-01 to L.S., by ACS grant RSG-09-197-01 to C.S.C.; and by additional funds from ABC2 Foundation and the Flaming Foundation. C.D.J. and T.O. were supported by the SPORE grant CA097257. L.M. is a recipient of a fellowship from American Brain Tumor Association in honor of Joel A. Gingras Jr.
We thank Dr. Thomas Shenk for the pp71 antibody, Dr. Mark Prichard for providing the sequences for HCMV RT-PCR, Dr. Lee Fortunato for providing the TR virus, and Dr. Jeremy Rich for the GSC 387 line.
Footnotes
Conflict of interest: no conflicts to disclose
Author Contribution
Conception and design: L.S., C.S.C., C.D.J., P.H.; development of methodology: P.H., T.O., L.S., Y.Y., E.S., L.M., E.F.; acquisition of data: P.H., T.O., L.S.; analysis and interpretation of data: P.H., L.S., C.D.J.; Writing of the manuscript: L.S., P.H., C.S.C., C.D.J.; study supervision: C.S.C., P.H.
References
- 1.Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67:279–83. doi: 10.1001/archneurol.2010.5. [DOI] [PubMed] [Google Scholar]
- 2.Dziurzynski K, Chang SM, Heimberger AB, Kalejta RF, McGregor Dallas SR, Smit M, et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro Oncol. 2012;14:246–55. doi: 10.1093/neuonc/nor227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soroceanu L, Matlaf L, Bezrookove V, Harkins L, Martinez R, Greene M, et al. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71:6643–53. doi: 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity. Cancer Res. 2008;68:724–30. doi: 10.1158/0008-5472.CAN-07-2291. [DOI] [PubMed] [Google Scholar]
- 5.Rahbar A, Stragliotto G, Orrego A, Peredo I, Taher C, Willems J, et al. Low levels of Human Cytomegalovirus Infection in Glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae. 2012;3:3. doi: 10.1186/2042-4280-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;3:ra58. doi: 10.1126/scisignal.2001180. [DOI] [PubMed] [Google Scholar]
- 7.Straat K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, et al. Activation of telomerase by human cytomegalovirus. J Natl Cancer Inst. 2009;101:488–97. doi: 10.1093/jnci/djp031. [DOI] [PubMed] [Google Scholar]
- 8.Stragliotto G, Rahbar A, Solberg NW, Lilja A, Taher C, Orrego A, et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: A randomized, double-blind, hypothesis-generating study. Int J Cancer. 2013;133:1204–13. doi: 10.1002/ijc.28111. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq E. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antiviral Res. 2007;75:1–13. doi: 10.1016/j.antiviral.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Snoeck R, Sakuma T, De Clercq E, Rosenberg I, Holy A. (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 1988;32:1839–44. doi: 10.1128/aac.32.12.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann T, Stingele K, Hartmann M, Haas J, von Einsiedel R, Wildemann B. Successful treatment of aids related PML with HAART and cidofovir. Eur J Med Res. 2001;6:190–62. [PubMed] [Google Scholar]
- 12.Bronson JJ, Ho HT, De Boeck H, Woods K, Ghazzouli I, Martin JC, et al. Biochemical pharmacology of acyclic nucleotide analogues. Ann N Y Acad Sci. 1990;616:398–407. doi: 10.1111/j.1749-6632.1990.tb17859.x. [DOI] [PubMed] [Google Scholar]
- 13.Liekens S, Gijsbers S, Vanstreels E, Daelemans D, De Clercq E, Hatse S. The nucleotide analog cidofovir suppresses basic fibroblast growth factor (FGF2) expression and signaling and induces apoptosis in FGF2-overexpressing endothelial cells. Mol Pharmacol. 2007;71:695–703. doi: 10.1124/mol.106.026559. [DOI] [PubMed] [Google Scholar]
- 14.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–17. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82:A84–A98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrei G, Snoeck R, Piette J, Delvenne P, De Clercq E. Inhibiting effects of cidofovir (HPMPC) on the growth of the human cervical carcinoma (SiHa) xenografts in athymic nude mice. Oncol Res. 1998;10:533–9. [PubMed] [Google Scholar]
- 17.Liekens S, Andrei G, Vandeputte M, De Clercq E, Neyts J. Potent inhibition of hemangioma formation in rats by the acyclic nucleoside phosphonate analogue cidofovir. Cancer Res. 1998;58:2562–7. [PubMed] [Google Scholar]
- 18.Liekens S, Verbeken E, De Clercq E, Neyts J. Potent inhibition of hemangiosarcoma development in mice by cidofovir. Int J Cancer. 2001;92:161–7. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1183>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Neyts J, Sadler R, De Clercq E, Raab-Traub N, Pagano JS. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998;58:384–8. [PubMed] [Google Scholar]
- 20.Murono S, Raab-Traub N, Pagano JS. Prevention and inhibition of nasopharyngeal carcinoma growth by antiviral phosphonated nucleoside analogs. Cancer Res. 2001;61:7875–7. [PubMed] [Google Scholar]
- 21.Liekens S, Neyts J, De Clercq E, Verbeken E, Ribatti D, Presta M. Inhibition of fibroblast growth factor-2-induced vascular tumor formation by the acyclic nucleoside phosphonate cidofovir. Cancer Res. 2001;61:5057–64. [PubMed] [Google Scholar]
- 22.Liekens S. Regulation of cancer progression by inhibition of angiogenesis and induction of apoptosis. Verh K Acad Geneeskd Belg. 2008;70:175–91. [PubMed] [Google Scholar]
- 23.Spanos WC, El-Deiry M, Lee JH. Cidofovir incorporation into human keratinocytes with episomal HPV 16 results in nonselective cytotoxicity. Ann Otol Rhinol Laryngol. 2005;114:840–6. doi: 10.1177/000348940511401106. [DOI] [PubMed] [Google Scholar]
- 24.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–37. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 25.Magee WC, Aldern KA, Hostetler KY, Evans DH. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob Agents Chemother. 2008;52:586–97. doi: 10.1128/AAC.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdulkarim B, Sabri S, Deutsch E, Chagraoui H, Maggiorella L, Thierry J, et al. Antiviral agent Cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene. 2002;21:2334–46. doi: 10.1038/sj.onc.1205006. [DOI] [PubMed] [Google Scholar]
- 27.Abdulkarim B, Sabri S, Zelenika D, Deutsch E, Frascogna V, Klijanienko J, et al. Antiviral agent cidofovir decreases Epstein-Barr virus (EBV) oncoproteins and enhances the radiosensitivity in EBV-related malignancies. Oncogene. 2003;22:2260–71. doi: 10.1038/sj.onc.1206402. [DOI] [PubMed] [Google Scholar]
- 28.Sirianni N, Wang J, Ferris RL. Antiviral activity of Cidofovir on a naturally human papillomavirus-16 infected squamous cell carcinoma of the head and neck (SCCHN) cell line improves radiation sensitivity. Oral Oncol. 2005;41:423–8. doi: 10.1016/j.oraloncology.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Soroceanu L, Kharbanda S, Chen R, Soriano RH, Aldape K, Misra A, et al. Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A. 2007;104:3466–71. doi: 10.1073/pnas.0611271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinca EB, Sarkaria JN, Schroeder MA, Carlson BL, Voicu R, Gupta N, et al. Bioluminescence monitoring of intracranial glioblastoma xenograft: response to primary and salvage temozolomide therapy. J Neurosurg. 2007;107:610–6. doi: 10.3171/JNS-07/09/0610. [DOI] [PubMed] [Google Scholar]
- 31.Ozawa T, James CD. Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. J Vis Exp. 2010;41:1986. doi: 10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozawa T, Faddegon BA, Hu LJ, Bollen AW, Lamborn KR, Deen DF. Response of intracerebral human glioblastoma xenografts to multifraction radiation exposures. Int J Radiat Oncol Biol Phys. 2006;66:263–70. doi: 10.1016/j.ijrobp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–7. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 34.Fortunato EA, Dell’Aquila ML, Spector DH. Specific chromosome 1 breaks induced by human cytomegalovirus. Proc Natl Acad Sci USA. 2000;97:853–8. doi: 10.1073/pnas.97.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Clercq E. Therapeutic potential of Cidofovir (HPMPC, Vistide) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verh K Acad Geneeskd Belg. 1996;58:19–47. discussion 47–9. [PubMed] [Google Scholar]
- 36.Matthews T, Boehme R. Antiviral activity and mechanism of action of ganciclovir. Rev Infect Dis. 1988;10 (Suppl 3):S490–4. doi: 10.1093/clinids/10.supplement_3.s490. [DOI] [PubMed] [Google Scholar]
- 37.WC, Hostetler KY, Evans DH. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob Agents Chemother. 2005;49:3153–62. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilley CE, Chaurushiya MS, Weitzman MD. Chromatin at the intersection of viral infection and DNA damage. Biochim Biophys Acta. 2010;1799:319–27. doi: 10.1016/j.bbagrm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siew VK, Duh CY, Wang SK. Human cytomegalovirus UL76 induces chromosome aberrations. J Biomed Sci. 2009;16:107. doi: 10.1186/1423-0127-16-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EX, Pickering MT, Debatis M, Castillo J, Lagadinos A, Wang S, et al. An E2F1-mediated DNA damage response contributes to the replication of human cytomegalovirus. PLoS Pathog. 2011;7:e1001342. doi: 10.1371/journal.ppat.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matlaf LA, Harkins LE, Bezrookove V, Cobbs CS, Soroceanu L. Cytomegalovirus pp71 Protein Is Expressed in Human Glioblastoma and Promotes Pro-Angiogenic Signaling by Activation of Stem Cell Factor. PLoS One. 2013;8:e68176. doi: 10.1371/journal.pone.0068176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baryawno N, Rahbar A, Wolmer-Solberg N, Taher C, Odeberg J, Darabi A, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest. 2011;121:4043–55. doi: 10.1172/JCI57147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–25. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.