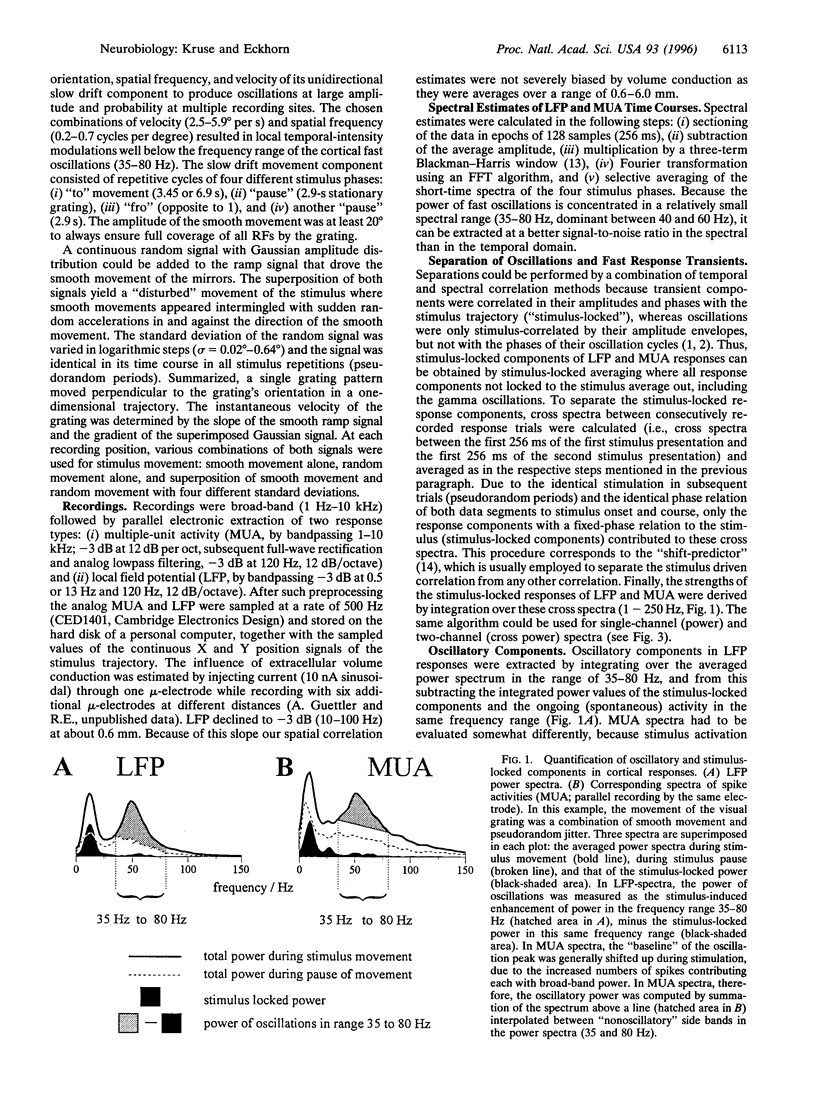
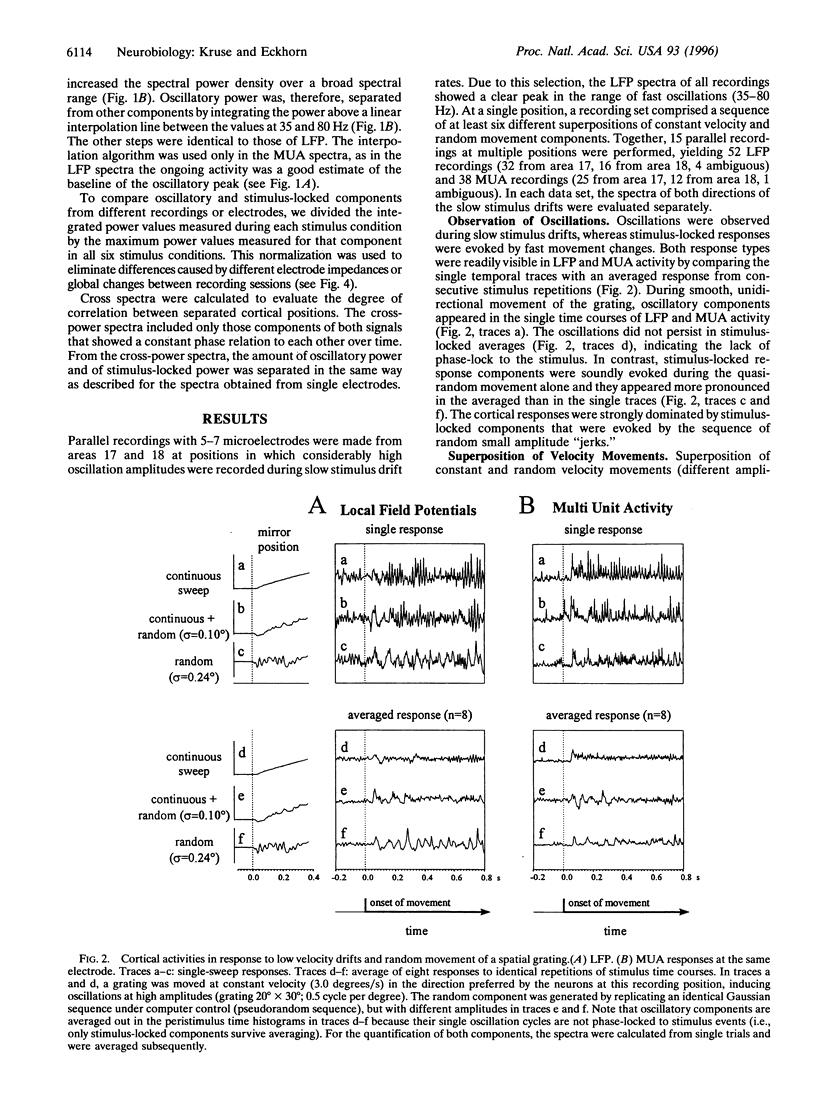
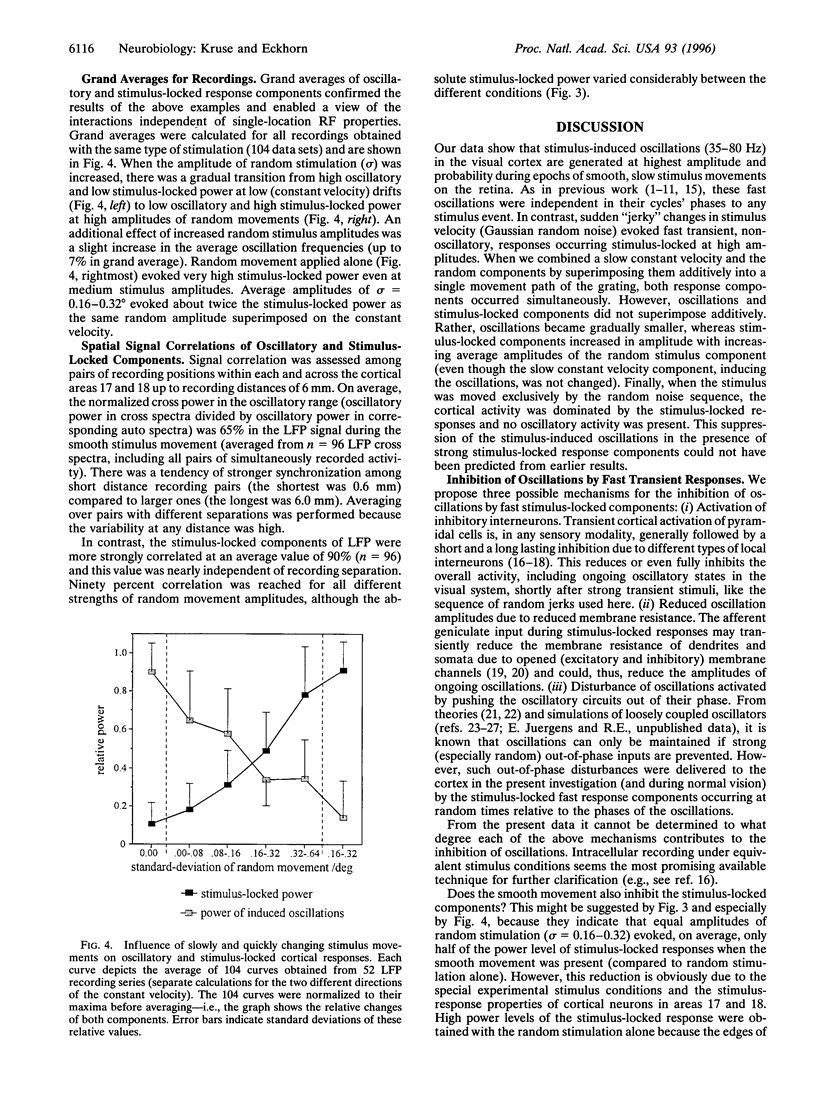
Abstract
Interactions between stimulus-induced oscillations (35-80 Hz) and stimulus-locked nonoscillatory responses were investigated in the visual cortex areas 17 and 18 of anaesthetized cats. A single square-wave luminance grating was used as a visual stimulus during simultaneous recordings from up to seven electrodes. The stimulus movement consisted of a superposition of a smooth movement with a sequence of dynamically changing accelerations. Responses of local groups of neurons at each electrode were studied on the basis of multiple unit activity and local slow field potentials (13-120 Hz). Oscillatory and stimulus-locked components were extracted from multiple unit activity and local slow field potentials and quantified by a combination of temporal and spectral correlation methods. We found fast stimulus-locked components primarily evoked by sudden stimulus accelerations, whereas oscillatory components (35-80 Hz) were induced during slow smooth movements. Oscillations were gradually reduced in amplitude and finally fully suppressed with increasing amplitudes of fast stimulus-locked components. It is argued that suppression of oscillations is necessary to prevent confusion during sequential processing of stationary and fast changing retinal images.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agmon-Snir H., Segev I. Signal delay and input synchronization in passive dendritic structures. J Neurophysiol. 1993 Nov;70(5):2066–2085. doi: 10.1152/jn.1993.70.5.2066. [DOI] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A. A functional microcircuit for cat visual cortex. J Physiol. 1991;440:735–769. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Frien A., Bauer R., Woelbern T., Kehr H. High frequency (60-90 Hz) oscillations in primary visual cortex of awake monkey. Neuroreport. 1993 Mar;4(3):243–246. doi: 10.1097/00001756-199303000-00004. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Obermueller A. Single neurons are differently involved in stimulus-specific oscillations in cat visual cortex. Exp Brain Res. 1993;95(1):177–182. doi: 10.1007/BF00229667. [DOI] [PubMed] [Google Scholar]
- Engel A. K., Kreiter A. K., König P., Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. K., König P., Kreiter A. K., Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991 May 24;252(5009):1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Engel A. K., König P., Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frien A., Eckhorn R., Bauer R., Woelbern T., Kehr H. Stimulus-specific fast oscillations at zero phase between visual areas V1 and V2 of awake monkey. Neuroreport. 1994 Nov 21;5(17):2273–2277. doi: 10.1097/00001756-199411000-00017. [DOI] [PubMed] [Google Scholar]
- Gerstner W., Ritz R., van Hemmen J. L. A biologically motivated and analytically soluble model of collective oscillations in the cortex. I. Theory of weak locking. Biol Cybern. 1993;68(4):363–374. doi: 10.1007/BF00201861. [DOI] [PubMed] [Google Scholar]
- Ghose G. M., Freeman R. D. Oscillatory discharge in the visual system: does it have a functional role? J Neurophysiol. 1992 Nov;68(5):1558–1574. doi: 10.1152/jn.1992.68.5.1558. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989 Jul;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. M., Engel A. K., König P., Singer W. Synchronization of oscillatory neuronal responses in cat striate cortex: temporal properties. Vis Neurosci. 1992 Apr;8(4):337–347. doi: 10.1017/s0952523800005071. [DOI] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Kreiter A. K., Singer W. Oscillatory Neuronal Responses in the Visual Cortex of the Awake Macaque Monkey. Eur J Neurosci. 1992;4(4):369–375. doi: 10.1111/j.1460-9568.1992.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Legatt A. D., Arezzo J., Vaughan H. G., Jr Averaged multiple unit activity as an estimate of phasic changes in local neuronal activity: effects of volume-conducted potentials. J Neurosci Methods. 1980 Apr;2(2):203–217. doi: 10.1016/0165-0270(80)90061-8. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Properties of the evoked potential generators: current source-density analysis of visually evoked potentials in the cat cortex. Int J Neurosci. 1987 Mar;33(1-2):33–59. doi: 10.3109/00207458708985928. [DOI] [PubMed] [Google Scholar]
- NACIMIENTO A. C., LUX H. D., CREUTZFELDT O. D. POSTSYNAPTISCHE POTENTIALE VON NERVENZELLEN DES MOTORISCHEN CORTEX NACH ELEKTRISCHER REIZUNG SPEZIFISCHER UND UNSPEZIFISCHER THALAMUSKERNE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Oct 5;281:152–169. [PubMed] [Google Scholar]
- PURPURA D. P., SHOFER R. J., MUSGRAVE F. S. CORTICAL INTRACELLULAR POTENTIALS DURING AUGMENTING AND RECRUITING RESPONSES. II. PATTERNS OF SYNAPTIC ACTIVITIES IN PYRAMIDAL AND NONPYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Mar;27:133–151. doi: 10.1152/jn.1964.27.2.133. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H. G., Wagner P. A model for neuronal oscillations in the visual cortex. 2. Phase description of the feature dependent synchronization. Biol Cybern. 1990;64(1):83–85. doi: 10.1007/BF00203634. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Golomb D, Kleinfeld D. Cooperative dynamics in visual processing. Phys Rev A. 1991 Jun 15;43(12):6990–7011. doi: 10.1103/physreva.43.6990. [DOI] [PubMed] [Google Scholar]
- Zeki S., Shipp S. The functional logic of cortical connections. Nature. 1988 Sep 22;335(6188):311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]



