Abstract
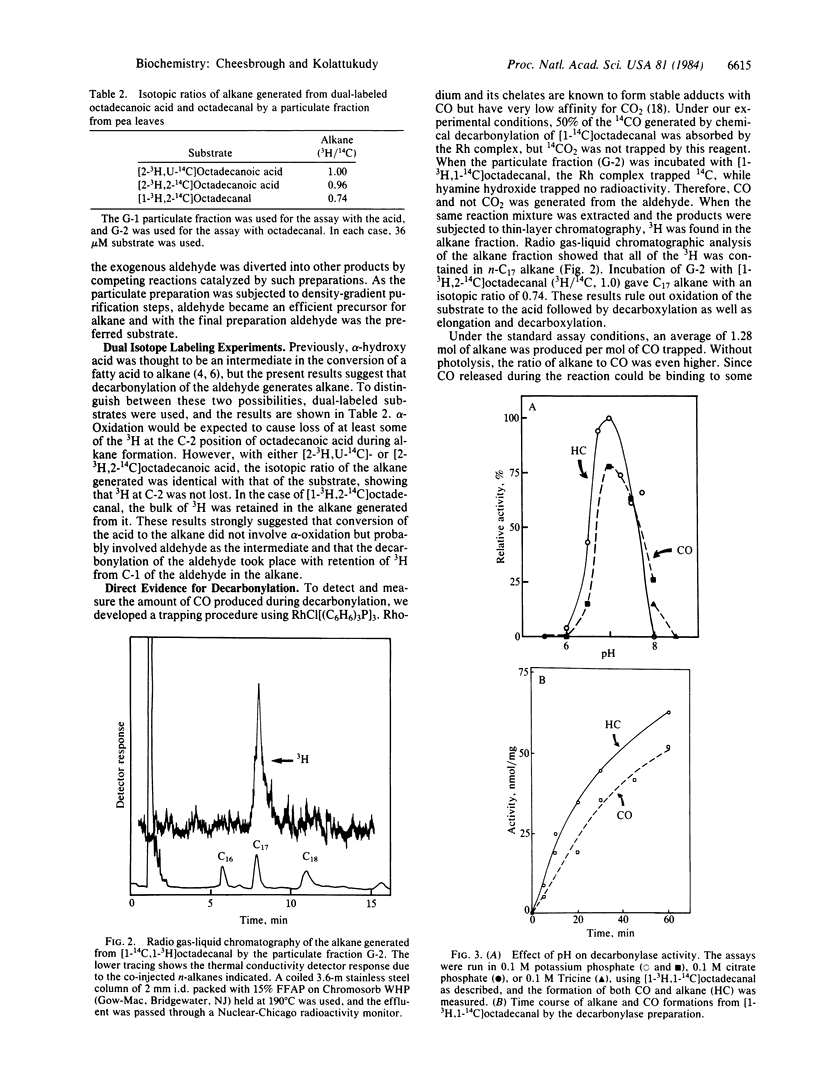
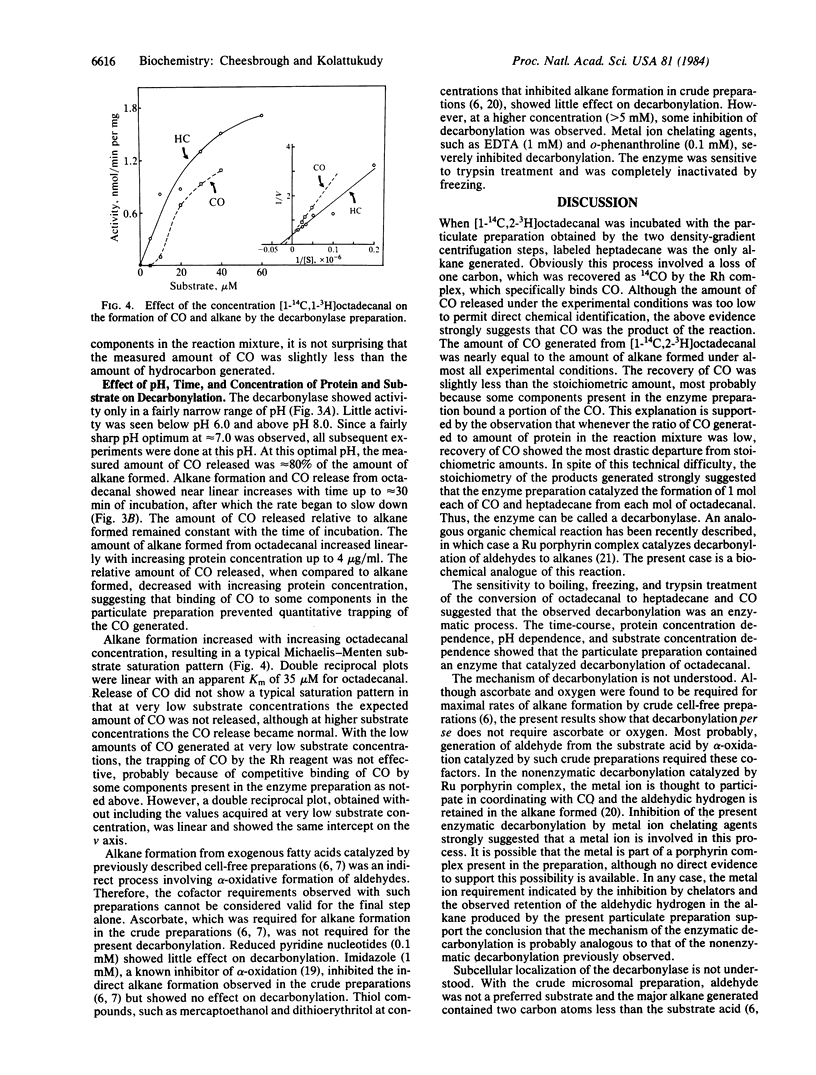
Mechanism of enzymatic conversion of a fatty acid to the corresponding alkane by the loss of the carboxyl carbon was investigated with particulate preparations from Pisum sativum. A heavy particulate preparation (sp. gr., 1.30 g/cm3) isolated by two density-gradient centrifugation steps catalyzed conversion of octadecanal to heptadecane and CO. Experiments with [1-3H,1-14C]octadecanal showed the stoichiometry of the reaction and retention of the aldehydic hydrogen in the alkane during this enzymatic decarbonylation. This decarbonylase showed an optimal pH of 7.0 and a Km of 35 microM for the aldehyde. This enzyme was severely inhibited by metal ion chelators and showed no requirement for any cofactors. Microsomal preparations and the particulate fractions from the first density-gradient step catalyzed acyl-CoA reduction to the corresponding aldehyde. Electron microscopic examination showed the presence of fragments of cell wall/cuticle but no vesicles in the decarbonylase preparation. It is concluded that the aldehydes produced by the acyl-CoA reductase located in the endomembranes of the epidermal cells are converted to alkanes by the decarbonylase located in the cell wall/cuticle region.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal V. P., Kolattukudy P. E. Purification and characterization of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase from potato tuber disks (Solanum tuberosum L.). Arch Biochem Biophys. 1978 Dec;191(2):452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
- Ashani Y., Catravas G. N. Highly reactive impurities in Triton X-100 and Brij 35: partial characterization and removal. Anal Biochem. 1980 Nov 15;109(1):55–62. doi: 10.1016/0003-2697(80)90009-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. E., Hajra A. K. A method for the chemical synthesis of 14C-labeled fatty acyl coenzyme A's of high specific activity. Anal Biochem. 1980 Aug;106(2):344–350. doi: 10.1016/0003-2697(80)90531-x. [DOI] [PubMed] [Google Scholar]
- Buckner J. S., Kolattukudy P. E. Specific inhibition of alkane synthesis with accumulation of very long chain compounds by dithioerythritol, dithiothreitol, and mercaptoethanol in Pisum sativum. Arch Biochem Biophys. 1973 May;156(1):34–45. doi: 10.1016/0003-9861(73)90338-x. [DOI] [PubMed] [Google Scholar]
- Cassagne C., Darriet D., Bourre J. M. Evidence of alkane synthesis by the sciatic nerve of the rabbit. FEBS Lett. 1977 Oct 1;82(1):51–54. doi: 10.1016/0014-5793(77)80883-1. [DOI] [PubMed] [Google Scholar]
- Khan A. A., Kolattukudy P. E. Decarboxylation of long chain fatty acids to alkanes by cell free preparations of pea leaves (Pisum sativum). Biochem Biophys Res Commun. 1974 Dec 23;61(4):1379–1386. doi: 10.1016/s0006-291x(74)80436-5. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthetic relationships among very long chain hydrocarbons, ketones, and secondary alcohols and the noninvolvement of alkenyl glyceryl ethers in their biosynthesis. Arch Biochem Biophys. 1970 Nov;141(1):381–383. doi: 10.1016/0003-9861(70)90149-9. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Walton T. J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry. 1972 May 9;11(10):1897–1907. doi: 10.1021/bi00760a026. [DOI] [PubMed] [Google Scholar]
- MARTIN R. O., STUMPF P. K. Fat metabolism in higher plants. XII. alpha-Oxidation of long chain fatty acids. J Biol Chem. 1959 Oct;234:2548–2554. [PubMed] [Google Scholar]
- Vederas J. C., Graf W., David L., Tamm C. Biosynthesis of cytochalasans. Part 4. The mode of incorporation of common naturally-occurring carboxylic acids into cytochalasin D1. Helv Chim Acta. 1975 Nov 5;58(7):1886–1898. doi: 10.1002/hlca.19750580704. [DOI] [PubMed] [Google Scholar]



