Abstract
An excessive release of glutamate is considered to be a molecular mechanism associated with several neurological diseases that causes neuronal damage. Therefore, searching for compounds that reduce glutamate neurotoxicity is necessary. In this study, the possibility that the natural flavone acacetin derived from the traditional Chinese medicine Clerodendrum inerme (L.) Gaertn is a neuroprotective agent was investigated. The effect of acacetin on endogenous glutamate release in rat hippocampal nerve terminals (synaptosomes) was also investigated. The results indicated that acacetin inhibited depolarization-evoked glutamate release and cytosolic free Ca2+ concentration ([Ca2+]C) in the hippocampal nerve terminals. However, acacetin did not alter synaptosomal membrane potential. Furthermore, the inhibitory effect of acacetin on evoked glutamate release was prevented by the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channel blocker known as ω-conotoxin MVIIC. In a kainic acid (KA) rat model, an animal model used for excitotoxic neurodegeneration experiments, acacetin (10 or 50 mg/kg) was administrated intraperitoneally to the rats 30 min before the KA (15 mg/kg) intraperitoneal injection, and subsequently induced the attenuation of KA-induced neuronal cell death and microglia activation in the CA3 region of the hippocampus. The present study demonstrates that the natural compound, acacetin, inhibits glutamate release from hippocampal synaptosomes by attenuating voltage-dependent Ca2+ entry and effectively prevents KA-induced in vivo excitotoxicity. Collectively, these data suggest that acacetin has the therapeutic potential for treating neurological diseases associated with excitotoxicity.
Introduction
Glutamate is the principal excitatory neurotransmitter in the central nervous system (CNS) and plays a critical role in numerous functions, such as cognition, movement, learning, and memory [1], [2]. However, in addition to the physiological role of glutamate, excessive glutamate release and activation of the glutamate receptors induce an increase in intracellular Ca2+ levels, which subsequently triggers a cascade of cellular responses, including enhanced oxygen free radical production, disturbed mitochondrial function, and protease activation, which ultimately kill the neurons [3], [4], [5]. This process has been implicated as a pathophysiological factor in multiple neurological disorders, both acute, such as stroke and head trauma, and chronic, such as neurodegenerative disorders [6], [7], [8]. Therefore, inhibiting the central glutamatergic neurotransmission might provide a potential strategy for treating these diseases. Consequently, several glutamatergic modulators are being developed, including N-methyl-D-aspartic acid (NMDA) receptor antagonists, and metabotropic glutamate receptor agonists and antagonists. However, these drugs have been unsuccessful in clinical trials because of numerous side effects, such as ataxia and memory impairment [9], [10]; thus, the search for new drugs that target neurological disorders continues.
Recently, numerous studies have focused on herbal compounds that may prevent specific neurological disorders. For example, berberine (an active component of berberis), curcumin (an active component of turmeric), honokiol (an active component of magnolia officinalis), and tanshinone IIA (an active component of danshen), were able to penetrate the brain and protect it against brain damage in various animal models with neurological disorders [11], [12],[13],[14]. These plant-derived compounds also have been demonstrated to decrease the glutamate release in rat brain tissues [15], [16], [17], [18]. In the current study, we focused on acacetin (5,7-dihydroxy-4-methoxyflavone, Figure 1A), a flavonoid compound isolated from Clerodendrum inerme (L.) Gaertn (CI), which is a local herb that was suggested to be a potential therapeutic application for treating neuropsychiatric disorders [19]. Acacetin exhibits various biological activities including those that are anti-inflammatory, antioxidant, and anticarcinogenic [20], [21], [22]. However, the biological targets and effects of acacetin on the CNS are largely unknown, although the neuroprotective effects of acacetin on the CNS were previously suggested [23].
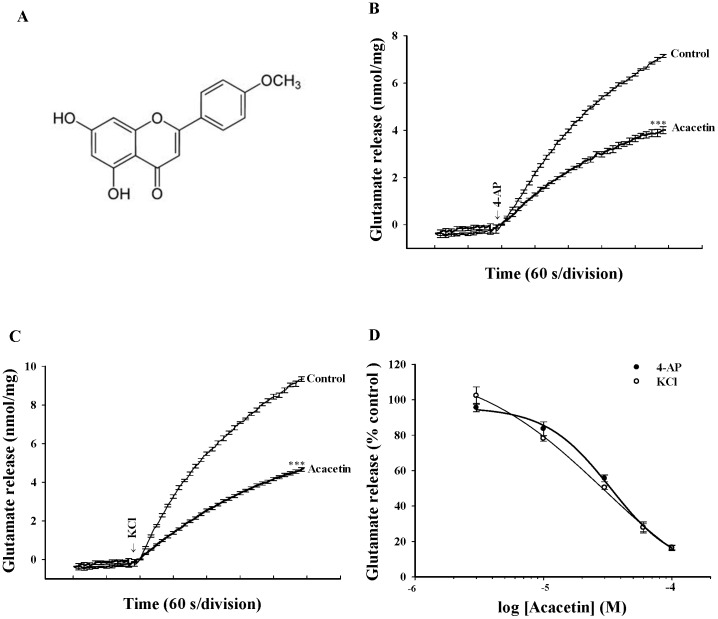
Figure 1. Acacetin inhibits 4-AP and KCl-evoked glutamate release from rat hippocampal nerve terminals in a concentration-dependent manner.
A: Chemical structure of acacetin. B, C: Glutamate release was measured under control conditions or in the presence of 30 µM acacetin added 10 min prior to the addition of 4-AP (1 mM) or KCl (15 mM). D: Dose-response curves of decrease in 4-AP or KCl-evoked glutamate release in the presence of acacetin. Results are mean ± SEM of independent experiments, using synaptosomal preparations from six animals. ***, P<0.001 versus control group.
Considering that the excitotoxicity caused by excessive glutamate is believed to play pivotal roles under numerous neuropathological conditions [24], we investigated (1) whether acacetin affects glutamate release; and (2) whether acacetin executes a protective action against the excitotoxicity induced by kainic acid (KA), which is a glutamate analog. In the first series of experiments, we used isolated nerve terminals (synaptosomes) purified from the rat hippocampus as a model to examine the effects of acacetin on the release of endogenous glutamate, the synaptosomal plasma membrane potential, the Na+ influx, and the activation of the voltage-dependent Ca2+ channels (VDCCs). The isolated presynaptic terminals (synaptosomes) also represent a model for directly investigating the underlying molecular mechanisms of presynaptic phenomena. Specifically, synaptosomes are capable of accumulating, storing, and releasing neurotransmitters, and is devoid of functional glial and nerve cell body elements that might cause the findings to be misinterpreted because of modulatory loci at the non-neuronal, postsynaptic, or network levels [25]. In the second experimental series, we used a rat model that was treated using KA, which induced hippocampal neuronal death and microglial activation, to examine whether acacetin was effective in this model. This model was chosen because KA-induced neurodegeneration and neuronal cell death have been proposed to be linked to the pathological release of glutamate [26], [27], [28]. Therefore, administering KA to rodents is generally assumed to be an adequate model of excitotoxicity.
Materials and Methods
Chemicals
Acacetin was isolated from Clerodendrum inerme, as described previously [19]. Air dried leaves of Clerodendrum inerme (3.0 kg) were grinded and repeatedly extracted with 95% EtOH (10 L) for three times. The combined EtOH layers were concentrated in vacuo to give a residue (246 g). The residue was triturated with n-hexane, CH2Cl2, n-BuOH and H2O, respectively. CH2Cl2 layer was concentrated in vacuo to provide a residue (16 g) and then subjected to silica gel chromatography to yield fractions 1–9. One compound that was crystallized from fraction 7 using EtOH as a solvent was identified as acacetin based on NMR spectrum comparable to literature reported. The purity of the compound was more than 98% on high-performance liquid chromatography (HPLC). 3′, 3′, 3′-dipropylthiadicarbocyanine iodide [DiSC3(5)] and fura-2-acetoxymethyl ester (Fura-2-AM) were purchased from Invitrogen (Carlsbad, CA, USA). 4-aminopyridine (4-AP), ω-conotoxin MVIIC (ω-CgTX MVIIC), dantrolene, 7-chloro-5-(2-chloropheny)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP37157), kainic acid (KA), and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO). Acacetin, DiSC3(5), SBFI, and Fura-2 were dissolved in 0.1% dimethylsulfoxide (DMSO). 4-AP, ω-CgTX MVIIC, dantrolene, and CGP37157 were dissolved in normal saline.
Animals
Adult male Sprague-Dawley rats (150-200 g) (n = 45) were used in this study. All of the animal experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996), and were approved by the Fu Jen Institutional Animal Care and Utilization Committee (FJIACUC; Permit Number: A9942). All efforts were made to minimize the number of animals used and their sufferings.
Synaptosomal preparation
Percoll-purified synaptosomes were prepared using the hippocampus of rats, as described previously [29]. The rats were sacrificed by decapitation and the brains were remove at 4°C. The hippocampus was rapidly dissected, homogenized in a medium containing 320 mM sucrose, pH 7.4. The homogenate was centrifuged at 3000 g (5000 rpm in a JA 25.5 rotor; Beckman Coulter, Inc., USA) for 10 min at 4°C, and the supernatant was centrifuged again at 14,500 g (11 000 rpm in a JA 25.5 rotor) for 12 min at 4°C. The pellet was gently resuspended in 8 ml of 320 mM sucrose, pH 7.4. Two milliliters of this synaptosomal suspension was placed into 3 ml Percoll discontinuous gradients containing 320 mM sucrose, 1 mM EDTA, 0.25 mM DL-dithiothreitol, and 3, 10 and 23% Percoll, pH 7.4. The gradients were centrifuged at 32,500 g (16 500 rpm in a JA 20.5 rotor) for 7 min at 4°C. Synaptosomes sedimenting between the 10 and the 23% Percoll bands were collected and diluted in a final volume of 30 ml of HEPES buffer medium (HBM) consisting of 140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1 mM MgCl2⋅6H2O, 1.2 mM Na2HPO4, 10 mM glucose, and 10 mM HEPES (pH 7.4). Protein concentration was determined using the Bradford assay. Synaptosomes were centrifuged in the final wash to obtain synaptosomal pellets with 0.5 mg protein. The synaptosomal pellets were stored on ice and used within 4–6 h.
Glutamate release
The glutamate release was assayed by using on-line fluorimetry, as described previously [29]. Synaptosomal pellets (0.5 mg of protein) were resuspended in HBM containing 16 µM bovine serum albumin and incubated in a stirred and thermostated cuvette at 37°C in a Perkin-Elmer LS-55 spectrofluorimeter (PerkinElmer Life and Analytical Sciences, Waltham, MA). NADP+ (2 mM), glutamate dehydrogenase (GDH, 50 U/mL) and CaCl2 (1.2 mM) were added after 3 min. After an additional 10 min of incubation, 4-AP (1 mM) or high external KCl (15 mM) was added to stimulate glutamate release. Glutamate release was monitored by measuring the increase in fluorescence (excitation and emission wavelengths of 340 and 460 nm, respectively) caused by NADPH being produced by the oxidative deamination of released glutamate by GDH. Data were accumulated at 2-s intervals. A standard of exogenous glutamate (5 nmol) was added at the end of each experiment, and the fluorescence response used to calculate released glutamate was expressed as nanomoles of glutamate per milligram of synaptosomal protein (nmol/mg). Values quoted in the text and depicted in bar graphs represent the levels of glutamate cumulatively released after 5 min of depolarization, and are expressed as nmol/mg/5 min. Estimation of the IC50 was based on a one-site model [Inhibition = (Inhibition MAX × [acacetin] / (IC50 + [acacetin])], and calculated using the nonlinear curve-fitting function provided in MicroCal Origin. Cumulative data were analyzed using Lotus 1-2-3.
Plasma membrane potential
The plasma membrane potential was determined using a membrane-potential-sensitive dye, DiSC3(5)[30]. Synaptosomes were resuspended in HBM and incubated in a stirred and thermostated cuvette at 37°C in a Perkin-Elmer LS-55 spectrofluorimeter. After 3 min of incubation, 5 µM DiSC3(5) were added and allowed to equilibrate before the addition of CaCl2 (1 mM) after 4 min of incubation. 4-AP was then added to depolarize the synaptosomes for 10 min, and DiSC3(5) fluorescence was monitored at excitation and emission wavelengths of 646 and 674 nm, respectively. Cumulative data were analyzed using Lotus 1-2-3 and expressed in fluorescence units.
The cytosolic free Ca2+ concentration ([Ca2+]C) in the synaptosomal population
The [Ca2+]C was measured using the Ca2+ indicator fura-2. Synaptosomes (0.5 mg/mL) were resuspended in HBM containing 0.1 mM CaCl2 and loaded with 5 µM Fura-2-AM for 30 min at 37°C. The synaptosomes were washed with HBM by being centrifuged, resuspended in 2 mL of HBM containing BSA, and placed in a Perkin-Elmer LS-55 spectrofluorometer at 37°C with stirring in the presence of 1.2 mM CaCl2. The synaptosomes were incubated for 10 min in the presence of acacetin (30 µM) prior to being depolarized with 4-AP (1 mM). Fura-2-Ca fluorescence was determined at excitation wavelengths of 340 and 380 nm (emission wavelength, 505 nm), and data were accumulated at 2 s intervals. [Ca2+]C (nM) was calculated by using calibration procedures [31] and equations described previously [32]. Cumulative data were analyzed using Lotus 1-2-3.
Cytosolic free Na+ concentration ([Na+]C)
Na+ measurements were performed in essentially the same manner as those performed in the [Ca2+]C determinations, except the synaptosomes were preincubated with 5 µM SBFI-AM instead of fura-2 [33]). SBFI fluorescence was monitored by applying the same method used in the fura-2 experiments. The results were expressed as ratios of fluorescence (emission wavelength, 505 nm) at excitation wavelengths of 340 and 380 nm (340/380 nm).
Histological analysis of neuronal death by neutral red and Fluoro-Jade B staining
Rats (n = 24) were randomly divided into four experimental groups: (1) control group; (2) KA-treated group; (3) KA and acacetin 10 mg/kg-treated group; and (4) KA and acacetin 50 mg/kg-treated group. Acacetin was injected intraperitoneally (i.p.) 30 min before KA (15 mg/kg; i.p.) injection. Rats were sacrificed 3 days after KA (15 mg/kg) injection by using an overdose of anesthetics (chloral hydrate, 650 mg/kg, i.p.). The animals were then perfused transcardially with saline (room temperature) followed by cold 4% paraformaldehyde in 0.1 M PBS. The brains were removed immediately and post-fixed in the same fixative overnight at 4°C, and then cryoprotected in 30% sucrose for 24–48 h. To perform neutral red staining, the brains were sectioned coronally into 30-µm-thick sections in a cryostat. The sections were then mounted on gelatin-coated slides, air dried and then stained with neutral red solution. Fluoro-Jade B (Chemicon, Millipore Ltd, Billerica, MA) staining was performed as described previously [34]. In summary, the sections (20 µm) were mounted on gelatin-coated slides and dried at room temperature followed by a solution containing 1% sodium hydroxide in 80% ethanol for 5 min. After the slides were immersed in 70% ethanol for 2 min and in distilled water for 2 min, the sections were oxidized in 0.06% potassium permanganate for 15 min, washed with water, and then immersed in 0.001% Fluoro-Jade B solution for 30 min in the dark. The slides were then washed in distilled water, air dried, cleared, and coverslipped. According to previous studies [35], [36], the hippocampus CA3 is the most vulnerable area to excitotoxic lesions caused by kainic acid. Therefore, the CA3 region was visualized under 100X magnification using an upright fluorescence microscope (Zeiss Axioskop 40, Goettingen, Germany) and digitized photomicrographs used for analysis were captured using a digital camera (Nikon D80, Tokyo, Japan) between bregma −2.30 mm and −3.60 mm according to the rat brain atlas of Paxino and Watson [37]. To compare neuronal death among the experimental groups, the number of Fluoro-Jade B-positive cells was measured in a 255×255 µm area of the hippocampal CA3 in 6 to 8 randomly chosen sections from each animal and averaged for each animals using a computer-assisted image analysis system (Image J; NIH Image, National Institutes of Health, Bethesda, MD, USA) by an examiner blind to experimental conditions. Results were expressed as mean ± SEM of labeled cells per 0.1 mm2.
Immunohistochemistry
Fixed brains were cut into 40-µm-thick coronal sections in a cryostat and then free-floating staining was performed using an immunohistochemical ABC method. In summary, after rinsing the sections 3 times with PBS, the sections were blocked with 2% normal goat serum containing 0.3% Triton X-100 for 1 h at room temperature. The sections were then incubated overnight at 4°C with a mouse monoclonal anti-OX-42 antibody (1∶500; Santa Cruz Biotechnology Inc). The sections were then incubated with a goat biotinylated anti-mouse secondary antibody (1∶200; Vector Laboratories, Burlingame, CA) for 2 h, and subsequently incubated with ExtrAvidin peroxidase (1∶1000, Sigma-Aldrich) for 1 h at room temperature. After rinsing the sections in 0.1 M PBS for 20 min, the sections were reacted with 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution in PBS containing 0.0025% hydrogen peroxide for 6 min. The sections were then mounted on gelatin-coated glass slides, air-dried, dehydrated, cleared with xylene, and coverslipped with Entellan mounting medium (Merck, Darmstadt, Germany).
Statistical analysis
Data were expressed as mean ± SEM. The data reported were analyzed by using the unpaired Student's t test or by using one-way ANOVA accompanied by post-hoc LSD comparison tests for multiple comparisons. The analysis was completed using SPSS software (17.0; SPSS Inc., Chicago, IL). P<0.05 was considered to represent a significant difference.
Results
Acacetin inhibits evoked glutamate release in rat hippocampal synaptosomes
In the first set of experiments, isolated nerve terminals were depolarized using the potassium channel blocker 4-AP or high external [K+] to investigate the effect of acacetin on glutamate release. In synaptosomes incubated in the presence of 1 mM CaCl2, 4-AP (1 mM) evoked a glutamate release of 7.2±0.1 nmol/mg/5 min. The application of acacetin (30 µM) decreased the amount of 4-AP-evoked glutamate release to 4.0±0.1 nmol/mg/5 min (P<0.001) without altering the basal release of glutamate (Figure 1B). Similarly, the release of glutamate evoked by KCl (15 mM) was also inhibited in the presence of acacetin (30 µM; P<0.001; Figure 1C). The acacetin-mediated inhibition of 4-AP- or KCl-evoked glutamate release was concentration dependent, and produced an IC50 value of approximately 31 µM and 29 µM, respectively, which was derived from a dose-response curve (Figure 1D).
Acacetin reduces the depolarization-induced increase in [Ca2+]C but does not alter the synaptosomal membrane potential
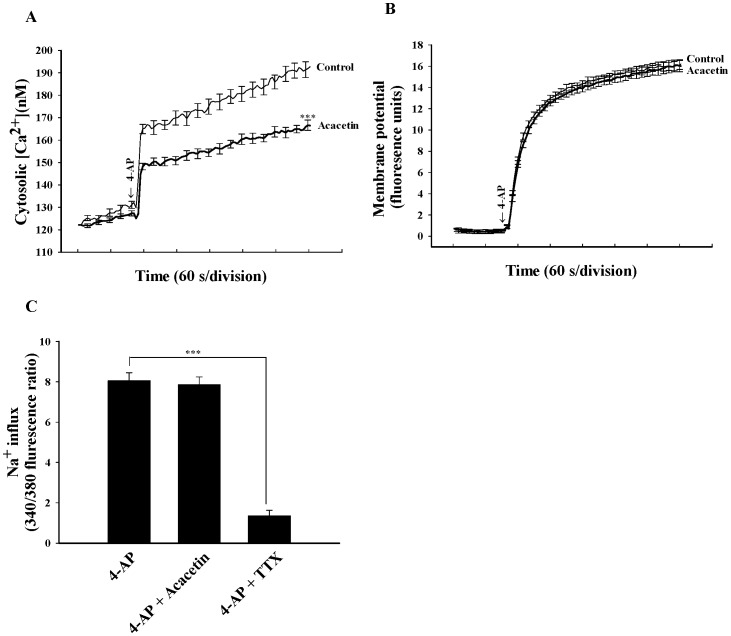
Transmitter release can be modulated by regulating the plasma membrane potential, and consequently altering the calcium influx. To investigate the potential mechanisms underlying the acacetin-mediated inhibition of glutamate release, the effect of acacetin on intrasynaptosomal Ca2+ levels was determined by using the Ca2+ indicator Fura-2. In Figure 2A, 4-AP (1 mM) caused a rise in cytosolic Ca2+ concentration ([Ca2+]C) from 127.1±1.2 nM to a plateau level of 196.8±5.5 nM (P<0.001). Applying acacetin (30 µM) did not affect basal Ca2+ levels, but caused an approximately 18% decrease in the 4-AP-evoked rise in [Ca2+]c (161.5±4.6 nM; P<0.001; Figure 2A). The inhibition of the [Ca2+]C elevation by acacetin might be attributed either to a direct reduction in the amount of Ca2+ entering through VDCCs, or to secondary effects caused by, for example, the modulation of potassium channels and the consequently altered plasma membrane potential. To discern between these two possibilities, the effect of acacetin on the synaptosomal plasma membrane potential under resting conditions and on depolarization was examined using membrane potential-sensitive dye DiSC3(5). Figure 2B demonstrates that 4-AP (1 mM) caused an increase in DiSC3(5) fluorescence of 16.3±0.8 fluorescence units/5 min. Preincubation of the synaptosomes using acacetin (30 µM) for 10 min before adding 4-AP did not alter the resting membrane potential, and produced no substantial change in the 4-AP-mediated increase in DiSC3(5) fluorescence (16.1±0.6 units/5 min; P = 0.812). In addition, the Na+-sensitive probe SBFI was used to measure cytosolic Na+ levels. Figure 2C indicates that 4-AP (1 mM) caused a clear rise in Na+ influx, but acacetin (30 µM) failed to affect this increase (P = 0.792). The failure of acacetin to produce an effect on this increase was not caused by an insufficient level of sensitivity of the SBFI probe to alterations in Na+ channel activity because in parallel experiments, the Na+ channel blocker tetrodotoxin (TTX; 2 µM) caused an 82% inhibition of 4-AP-evoked Na+ influx (Figure 2C; P<0.001). These results indicate that the observed effect of acacetin on [Ca2+]C is most likely caused by a direct modulation of VDCCs activity.
Figure 2. Acacetin reduces intrasynaptosomal Ca2+ levels but does not alter the synaptosomal membrane potential and Na+ influx.
Cytosolic free Ca2+ concentration (nM) (A) or synaptosomal membrane potential (B) was measured in the absence (control) and in the presence of 30 µM acacetin, added 10 min before depolarization with 1 mM 4-AP. C: Na+ influx was induced by 1 mM 4-AP in the absence (control) or presence of 30 µM acacetin or 2 µM TTX, added 10 min before depolarization. Results are mean ± SEM of independent experiments, using synaptosomal preparations from five animals. ***, P<0.001 versus control group.
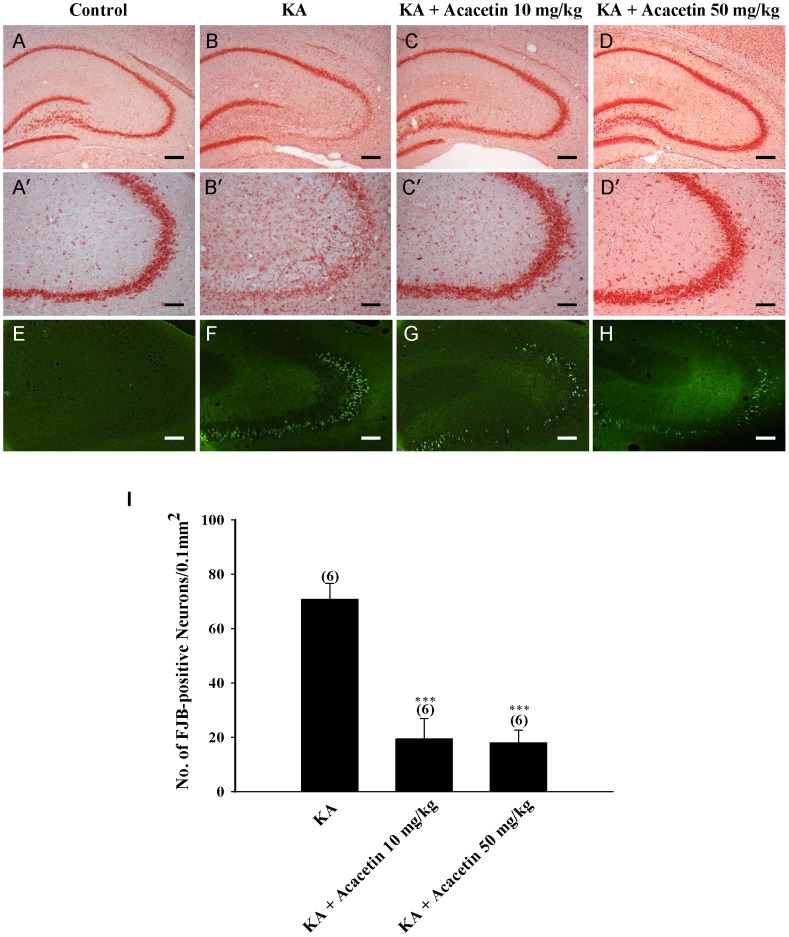
Figure 4. Acacetin attenuates KA-induced neuronal cell death in the CA3 region of the hippocampus.
Acacetin (10 or 50 mg/kg) was administrated intraperitoneally 30 min before KA injection, and extents of neuronal losses in the hippocampus were evaluated at 3 days after KA injection by staining with neutral red (A-D) and Fluoro-Jade B (E-H). Representative photomicrographs illustrating neuronal cell death in the hippocampal CA3 region of control, KA, KA + acacetin 10 mg/kg, and KA + acacetin 50 mg/kg. (I) Quantification of Fluoro-Jade B-positive neurons in the CA3 region of the hippocampus. Data are expressed as mean ± SEM of six independent experiments. ***P<0.001, as compared with the KA-treated group. Scale bar for A-D, E-H = 250 µm, A′-D′ = 100 µm.
Involvement of presynaptic N- and P/Q-type Ca2+ channels in the acacetin-mediated inhibition of glutamate release
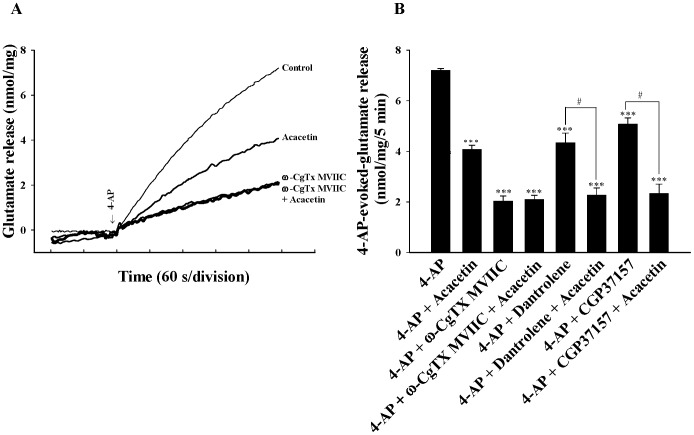
In the adult rat cerebrocortical nerve terminals, the release of glutamate evoked by depolarization is supported by Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels [38], [39], [40]. To determine whether the decrease in Ca2+ channel activity was involved in the effect of acacetin on 4-AP-evoked glutamate release, we examined the effect of acacetin in the presence of ω-conotoxin MVIIC (ω-CgTX MVIIC), a wide spectrum blocker of Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels. In Figure 3A, 4-AP (1 mM)-evoked glutamate release (7.2±0.3 nmol/mg/5 min) was substantially reduced in the presence of ω-CgTX MVIIC (2 µM) (2.0±0.2 nmol/mg/5 min; P<0.001). Although the 4-AP-evoked glutamate release was considerably reduced in the presence of acacetin (30 µM), this effect was prevented by the presence of ω-CgTX MVIIC. The release measured in the presence of both ω-CgTX MVIIC and acacetin was similar to that obtained in the presence of ω-CgTX MVIIC alone [F(2,13) = 413.856, P = 0.792] (Figure 3A and 3B). In addition to the Ca2+ influx through VDCCs, the release of glutamate evoked by depolarization was reported to be caused by a Ca2+ release from intracellular stores such as endoplasmic reticulum (ER) and mitochondria [41]. Therefore, a potential role of intracellular Ca2+ release in the acacetin-mediated inhibition of glutamate release was tested in the presence of dantrolene, an inhibitor of intracellular Ca2+ release from endoplasmic reticulum, and CGP37157, a membrane-permeant blocker of mitochondrial Na+/Ca2+ exchange. Figure 3B indicates that 4-AP (1 mM)-evoked glutamate release was reduced by dantrolene (100 µM) (4.3±0.4 nmol/mg/5 min; P<0.001), indicating that the Ca2+ release from ER ryanodine receptors contributes substantially to the levels of 4-AP-evoked glutamate release. In the presence of dantrolene, however, acacetin (30 µM) still effectively inhibited 4-AP-evoked glutamate release [F(2,13) = 95.346, P<0.05]. Compared to the effects of dantrolene, CGP37157 (100 µM) similarly decreased the levels of 4-AP-evoked glutamate release, but it produced no effect on the acacetin-mediated inhibition of 4-AP-evoked glutamate release [F(2,13) = 104.843, P<0.05] (Figure 3B).
Figure 3. Acacetin-mediated inhibition of glutamate release is prevented by blocking the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channels.
A: Glutamate release was evoked by 1 mM 4-AP in the absence (control) or presence of 30 µM acacetin, 2 µM ω-CgTX MVIIC, 2 µM ω-CgTX MVIIC and 30 µM acacetin. B: Quantitative comparison of the extent of glutamate release by 1 mM 4-AP in the absence or presence of 30 µM acacetin, and absence and presence of 2 µM ω-CgTX MVIIC, 100 µM dantrolene, or 100 µM CGP37157. Results are mean ± SEM of independent experiments, using synaptosomal preparations from five to six animals. ***, P<0.001 versus control group; #, P<0.05 versus dantrolene-, or CGP37157-treated group.
Acacetin pretreatment reduces KA-induced neuronal cell death and microglial activation in the CA3 region of the hippocampus
An excessive release of glutamate was implicated in the pathogenesis of acute and chronic brain disorders [24]. The experiments performed in this study demonstrated that acacetin inhibits glutamate release from hippocampal nerve terminals, thus supporting the hypothesis that acacetin produces a neuroprotective effect against exocytotoxic insults. To confirm this hypothesis, we examined the effect of acacetin on neuronal death induced by kainic acid (KA), an excitotoxic substance. The neuronal death that occurred after KA administration (15 mg/kg, i.p., 72 h) was verified using neutral red and Fluoro-Jade B staining. As displayed in Figure 4A and B, neutral red staining indicated a significant neuronal loss in the hippocampal CA3 and CA4 of KA-injected rats compared with that of the DMSO-treated rats (control). Acacetin administration (10 or 50 mg/kg, i.p.) performed 30 min before KA administration substantially reduced KA-induced neuronal death in CA3 and CA4 (Figure 4C and D). A similar protective effect of acacetin against neuronal death was observed by using Fluoro-Jade B staining. As illustrated in Figure 4E, no staining was observed in the DMSO-injected rats (control). KA treatment caused a substantial increase in the number of Fluoro-Jade B-positive neurons in the CA3 region of the hippocampus (P<0.001; Figure 4G and I). In rats pretreated with acacetin (10 or 50 mg/kg), the number of KA-induced degenerative neurons in CA3 was substantially reduced [F(2,15) = 24.158, P<0.05](Figure 4G-I).
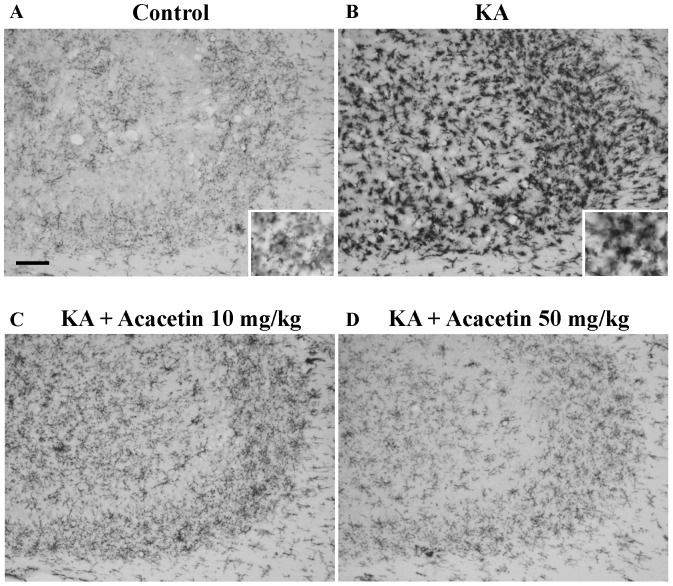
KA-induced hippocampal neuronal death is accompanied by the increased activation of the microglia [42], [43]. To examine whether acacetin affected inflammatory processes in KA-injected brains, the activation of microglia after administering KA (72 h) was analyzed by detecting the expression of OX42, a surface marker used for microglia. In the DMSO-treated rats (control), microglial cells in the CA3 region exhibited a resting morphology with small cell bodies and thin processes (Figure 5A). Conversely the number of microglial cells in KA-injected rats increased remarkably in the CA3 region. These cells displayed enlarged cell bodies with considerably shorter and thicker processes (indicating the activation state; Figure 5B). KA-induced microglial activation was substantially suppressed in the rats pretreated with acacetin (10 or 50 mg/kg); most of the microglial cells were in a ramified or resting state (n = 6; Figure 5C and 5D).
Figure 5. Acacetin suppresses KA-induced microglial activation in the CA3 region of the hippocampus.
Acacetin (10 or 50 mg/kg, i.p.) was administrated 30 min before KA injection, and the hippocampal sections were stained with anti-OX-42 antibody at 3 days after KA injection. Representative photomicrographs illustrating OX-42 immunoreactivity in the hippocampal CA3 region of control, KA, KA + acacetin 10 mg/kg, and KA + acacetin 50 mg/kg. Insets in figure show the morphological changes after KA administration under higher magnification. Representative picture from six independent experiments were presented. Scale bar = 100 µm for A-D.
Discussion
Excessive glutamate release is an underlying cause of neuronal damage in a variety of CNS diseases including cerebral ischemia, epilepsy and neurodegenerative disorders [7], [8], [24]. As a result, reducing glutamate release may have critical consequences and may be a potential mechanism of neuroprotective drugs. Natural products derived from medicinal herbs have recently received a considerable amount of attention because of the beneficial effects of these products on the CNS, specifically, the neuroprotective effects. Experiments performed on animals revealed that acacetin, a naturally occurring flavonoid, plays a neuroprotective role [23]. However, the detailed mechanism of this role remains unresolved. Therefore, the purpose of this study was to enhance the understanding of the mechanism responsible for the neuroprotective effect of acacetin produced in response to excitotoxic insults. By considering both in vitro glutamate release and in vivo KA-induced excitotoxicity, we discovered that acacetin preferentially inhibits glutamate release evoked from rat hippocampal nerve terminals, and attenuates KA-induced neuronal cell death and microglia activation in the CA3 region of the hippocampus. This is the first study to assess the possible influence of acacetin on glutamate release at the presynaptic level, and on glutamate-induced excitotoxicity.
The release of glutamate from a presynaptic site is a possible target for the drug modulation of excitability and synaptic transmission in central neurons [44]. Therefore, one purpose of this study was to investigate the relationship between acacetin and the presynaptic modulation of glutamate release, and to determine the underlying molecular mechanisms. By preparing nerve terminals from rat hippocampi, we discovered that acacetin inhibited depolarization-evoked glutamate release. However, acacetin did not affect the basal release of glutamate from the nerve terminals, suggesting that acacetin might reduce the release of glutamate when it is triggered by neuronal activation.
In nerve terminals, the inhibition of Na+ channels or activation of K+ channels stabilizes membrane excitability and, consequently, causes a reduction in the levels of Ca2+ entry and neurotransmitter release [45], [46], [47]. The observed inhibitory effect of acacetin on evoked glutamate release could occur through a reduction of nerve terminal excitability, but this is unlikely because of the following 2 reasons: First, acacetin inhibited the release of glutamate evoked by 4-AP and KCl. Although 4-AP-evoked glutamate release involves the activation of Na+ and Ca2+ channels, 15 mM external KCl-evoked glutamate release involves only Ca2+ channels [48], [49], and this indicates that Na+ channels are not involved in the effect of acacetin on glutamate release. This suggestion was supported by the observation that acacetin did not affect the 4-AP-evoked Na+ influx; Second, no substantial acacetin effect on synaptosomal plasma membrane potential was observed, which indicated a lack of effect on the K+ conductance. These results suggest that the decreased glutamate release caused by acacetin is not the result of a reduction in synaptosomal excitability caused by ion channel (e.g., the Na+ or K+ channels) modulation. This finding disagrees with previous electrophysiological studies, which have shown that acacetin inhibits K+ currents in human atrial myocytes and HEK 293 cells [50], [51]. The reason for this discrepancy between the current and previous in vitro studies is unclear, but may be related to the different experimental models applied.
Therefore, if the effect is not caused by the modulation of synaptosomal excitability, then acacetin possibly inhibits evoked glutamate release by decreasing the levels of Ca2+ entry through the Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels that are coupled to glutamate exocytosis in the nerve terminals [38], [39]. This hypothesis is plausible because we demonstrated that acacetin decreased the 4-AP-evoked increase in [Ca2+]C. In addition, the inhibitory effect of acacetin on glutamate release was prevented by ω-CgTX MVIIC, a wide spectrum blocker of the Cav2.2 (N-type) and Cav2.1 (P/Q-type) Ca2+ channels. However, neither dantrolene, an inhibitor of intracellular Ca2+ release from the endoplasmic reticulum ryanodine receptors, nor CGP37157, a mitochondrial Na+/Ca2+ exchange blocker, affected the inhibitory effect of acacetin on glutamate release. Based on these results, we suggest that acacetin inhibits evoked glutamate release by reducing presynaptic Ca2+ influx rather than by indirectly affecting neuronal excitability.
We hypothesized that acacetin would have a neuro-protective effect because we discovered that acacetin depressed glutamate release from nerve terminals, and that the excessive release of glutamate is a critical element in the neuropathology of acute and chronic brain disorders. This hypothesis was confirmed in this study by applying the KA model to adult rats. KA is an analogue of glutamate, and the systemic administration of KA to animals causes neuronal cell death or neurodegeneration in specific brain regions, such as the hippocampus, piriform cortex, thalamus, and amygdala [52]. Furthermore, previous studies have suggested that KA-induced neuronal death is linked to the pathological release of glutamate [26], [27], [28]. Therefore, administering KA to rodents is generally assumed to be an adequate method of excitotoxicity. In this study, administering KA (15 mg/kg, i.p.) caused a considerable neuronal death in the CA3/CA4 hippocampus area. This result is consistent with the results obtained when other groups applied the same dose of KA [35], [53]. In the hippocampus, CA3 pyramidal cells are most severely damaged after KA administration [35], [36], [54]. Such a phenomenon may be attributed to the highest density of KA receptors in the CA3 region [55]. In this study, KA-induced hippocampal neuronal death was also attenuated by acacetin pretreatment (10 or 50 mg/kg), suggesting that acacetin acts as a neuroprotective agent. This suggestion is in line with the results of a previous study that demonstrated that acacetin protects dopaminergic neurons against neurotoxicity in experimental models [23]. In addition, KA is an agonist for a subtype ionotropic glutamate receptors, KA and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propioni acid) receptors. Overstimulation of these receptors is believed to contribute to KA-induced neurodegeneration [26], [27]. Thus, the present study does not exclude the possibility that the inhibition of KA-induced neuronal cell death by acacetin is linked to antagonism at the KA/AMPA receptor.
Numerous studies have indicated that KA-induced neuronal death is associated with microglia activation in the hippocampus [42], [43]. The prevention of microglia activation protects the hippocampus from neuro-degeneration caused by KA [56]. In our study, we also observed that the amount of activated microglia immunostained by OX-42 antibodies in the hippocampus was substantially higher in KA-treated rats. Acacetin pretreatment reduced the amount of activated microglia. Therefore, acacetin can be supposed to have an anti-inflammatory function, and that this action underlies, at least in part, its protective action against the excitotoxicity induced by KA. This is consistent with previous studies, which showed that acacetin protects neurons against neurotoxin- and ischemia-induced toxicity by inhibiting microglial activation and pro-inflammatory cytokine production [23],[57]. However, the manner in which acacetin affected KA-induced microglial activation is not clear. Neuroinflammation is included in the pathogenesis of numerous acute and chronic neurological disorders [58], [59]. Inflammatory processes, including microglial activation and the consequent production of various neurotoxic factors (including free radicals and pro-inflammatory cytokines), are believed to assist in causing KA-induced neuronal death [60]. Therefore, future research should determine whether acacetin affects the production of these factors in KA animal model.
Studies have reported that acacetin, at 10-25 mg/kg, protects neurons against insults induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and ischemia [23], [57]. However, the neural protective effects of acacetin are also reported at a lower concentration range (i.e., 50–200 nM) [23]. In the present study, 30 µM of acacetin inhibits evoked glutamate release in vitro, and acacetin at 10–50 mg/kg (approximately 50–250 µM) attenuates in vivo KA-induced neuronal death and microglia activation in the hippocampus. Although the dose of acacetin used in our present study to produce the effect was higher, the action of acacetin was specific. The observation supporting this statement revealed the following: (1) acacetin decreased the depolarization-induced increase in [Ca2+]C, whereas it did not alter 4-AP-mediated depolarization and Na+ influx; (2) acacetin-mediated inhibition of glutamate release was abolished by the N-, P- and Q-type Ca2+ channel blocker, but not by the ryanodine receptor blocker, or the mitochondrial Na+/Ca2+ exchanger blocker.
In conclusion, this study is the first to demonstrate that acacetin inhibits glutamate release from hippocampal nerve terminals in rats. This may contribute to the substantial neuroprotective effect of acacetin against KA-induced in vivo excitotoxicity. Although, the relevance of our finding to in vivo clinical situations remains to be determined, this investigation enhances the understanding of acacetin action in the brain and demonstrates the therapeutic potential of this natural compound in treating neurological disorders in which excitotoxic neuronal cell death and inflammation processes are involved.
Acknowledgments
We thank Sin-Yi Tsai for excellent experimental assistance.
Funding Statement
This work was supported by a grant from the National Science Council (NSC 100-2320-B-030-006-MY3), Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fonnum F (1984) Glutamate: a neurotransmitter in mammalian brain. J Neurochem 42: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Greenamyre JT, Porter RH (1994) Anatomy and physiology of glutamate in the CNS. Neurology 44: S7–S13. [PubMed] [Google Scholar]
- 3. Choi DW (1992) Excitotoxic cell death. J Neurobiol 23: 1261–1276. [DOI] [PubMed] [Google Scholar]
- 4. Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689–695. [DOI] [PubMed] [Google Scholar]
- 5. Schinder AF, Olson EC, Spitzer NC, Montal M (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 16: 6125–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi DW, Rothman SM (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13: 171–182. [DOI] [PubMed] [Google Scholar]
- 7. Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44: S14–S23. [PubMed] [Google Scholar]
- 8. Obrenovitch TP, Urenjak J (1997) Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol 51: 39–87. [DOI] [PubMed] [Google Scholar]
- 9. Danysz W, Parsons CG (1998) Glycine and N-methyl-D-asparate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50: 597–664. [PubMed] [Google Scholar]
- 10. Ikonomidou C, Turski L (2002) Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 1: 383–386. [DOI] [PubMed] [Google Scholar]
- 11. Cui HS, Huang LS, Sok DE, Shin J, Kwon BM, et al. (2007) Protective action of honokiol, administered orally, against oxidative stress in brain of mice challenged with NMDA. Phytomedicine 14: 696–700. [DOI] [PubMed] [Google Scholar]
- 12. Ye M, Fu S, Pi R, He F (2009) Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J Pharm Pharmacol 61: 831–837. [DOI] [PubMed] [Google Scholar]
- 13. Wang L, Zhang X, Liu L, Cui L, Yang R, et al. (2010) Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-κB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res 1321: 143–151. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Li Q, Wang X, Yu S, Li L, et al. (2013) Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLOS ONE 8: e59843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sy HN, Wu SL, Wang WF, Wang SJ (2008) Mechanisms underlying the honokiol inhibition of evoked glutamate release from glutamatergic nerve terminals of the rat cerebral cortex. Synapse 62: 890–901. [DOI] [PubMed] [Google Scholar]
- 16. Lin TY, Lu CW, Wang CC, Wang YC, Wang SJ (2011) Curcumin inhibits glutamate release in nerve terminals from rat prefrontal cortex: possible relevance to its antidepressant mechanism. Prog Neuropsychopharmacol Biol Psychiatry 35: 1785–1793. [DOI] [PubMed] [Google Scholar]
- 17. Lin TY, Lu CW, Huang SK, Wang SJ (2013) Tanshinone IIA, a constituent of Danshen, inhibits the release of glutamate in rat cerebrocortical nerve terminals. J Ethnopharmacol 147: 488–496. [DOI] [PubMed] [Google Scholar]
- 18. Lin TY, Lin YW, Lu CW, Huang SK, Wang SJ (2013) Berberine inhibits the release of glutamate in nerve terminals from rat cerebral cortex. PLOS ONE 8: e67215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen HL, Lee HJ, Huang WJ, Chou JF, Fan PC, et al. (2012) Clerodendrum inerme leaf extract alleviates animal behaviors, hyperlocomotion, and prepulse inhibition disruptions, mimicking tourette syndrome and schizophrenia. Evid Based Complement Alternat Med 2012: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pan MH, Lai CS, Wang YJ, Ho CT (2006) Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem Pharmacol 72: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 21. Shim HY, Park JH, Paik HD, Nah SY, Kim DS, et al. (2007) Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK/2-c-Jun activation. Mol Cell 24: 95–104. [PubMed] [Google Scholar]
- 22. Shen KH, Hung SH, Yin LT, Huang CS, Chao CH, et al. (2010) Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol Cell Biochem 333: 279–291. [DOI] [PubMed] [Google Scholar]
- 23. Kim HG, Ju MS, Ha SK, Lee H, Lee H, et al. (2012) Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Bio Phar Bull 35: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 24. Lipton SA, Rosenberg PA (1994) Excitatory amino acid as a final common pathway for neurologic disorders. N Engl J Med 330: 613–622. [DOI] [PubMed] [Google Scholar]
- 25. Nicholls DG (1989) Release of glutamate, aspartate, and gamma-aminobutyric acid from isolated nerve terminals. J Neurochem 52: 331–341. [DOI] [PubMed] [Google Scholar]
- 26. Ferkany JW, Zaczek R, Coyle JT (1982) Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature 298: 757–759. [DOI] [PubMed] [Google Scholar]
- 27. Ferkany JW, Coyle JT (1983) Kainic acid selectively stimulates the release of endogenous excitatory acid amino acid. J Pharmacol Exp Ther 225: 399–406. [PubMed] [Google Scholar]
- 28. Chittajallu R, Vignes MDev KK, Barnes JM, Collingridge GL, Henley JM (1996) Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379: 78–91. [DOI] [PubMed] [Google Scholar]
- 29. Nicholls DG, Sihra TS (1986) Synaptosomes possess an exocytotic pool of glutamate. Nature 321: 772–773. [DOI] [PubMed] [Google Scholar]
- 30. Akerman KE, Scott IG, Heikkila JE, Heinonen E (1987) Ionic dependence of membrane potential and glutamate receptor-linked responses in synaptoneurosomes as measured with a cyanine dye, DiSC2(5). J Neurochem 48: 552–559. [DOI] [PubMed] [Google Scholar]
- 31. Sihra TS, Bogonez E, Nicholls DG (1992) Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J Biol Chem 267: 1983–1989. [PubMed] [Google Scholar]
- 32. Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450. [PubMed] [Google Scholar]
- 33. Minta A, Tsien RY (1989) Fluorescent indicators for sodium. J Biol Chem 264: 19449–19457. [PubMed] [Google Scholar]
- 34. Schmued LC, Hopkins KJ (2000) Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874: 123–130. [DOI] [PubMed] [Google Scholar]
- 35. Friedman LK, Pellegrini-Giampietro DE, Sperber EF, Bennett MV, Moshe SL, et al. (1994) Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J Neurosci 14: 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park HJ, Kin HJ, Park HJ, Ra J, Zheng LT, et al. (2008) Protective effect of topiramate on kainic acid-induced cell death in mice hippocampus. Epilepsia 49: 163–167. [DOI] [PubMed] [Google Scholar]
- 37.Paxino G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edition. San Diego, Academic Press.
- 38. Turner TJ, Adams ME, Dunlap K (1993) Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Nat Acad Sci USA 90: 9518–9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vazquez E, Sanchez-Prieto J (1997) Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur J Neurosci 9: 2009–2018. [DOI] [PubMed] [Google Scholar]
- 40. Millan C, Sanchez-Prieto J (2002) Differential coupling of N and P/Q-type calcium channels to glutamate exocytosis in the rat cerebral cortex. Neurosci Lett 330: 29–32. [DOI] [PubMed] [Google Scholar]
- 41. Berridge MJ (1998) Neuronal calcium signaling. Neuron 21: 13–26. [DOI] [PubMed] [Google Scholar]
- 42. Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E (2008) Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci 28: 9133–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim DH, Yoon BH, Jung WY, Kim JM, Park SJ, et al. (2010) Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology 59: 20–30. [DOI] [PubMed] [Google Scholar]
- 44. Wu L, Saggau P (1997) Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 20: 204–212. [DOI] [PubMed] [Google Scholar]
- 45. Rehm H, Tempe BL (1991) Voltage-gated K+ channels of mammalian brain. FASEB J 5: 164–170. [DOI] [PubMed] [Google Scholar]
- 46. Li M, West JW, Numann R, Murphy BJ, Scheuer T, et al. (1993) Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science 261: 1439–1442. [DOI] [PubMed] [Google Scholar]
- 47. Pongs O, Leicher T, Berger M, Roeper J, Bahring R, et al. (1999) Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann N Y Acad Sci 868: 344–355. [DOI] [PubMed] [Google Scholar]
- 48. Barrie AP, Nicholls DG, Sanchez-Prieto J, Sihra TS (1991) An ion channel locus for the protein kinase C potentiation of transmitter glutamate release from guinea pig cerebrocortical synaptosomes. J Neurochem 57: 1398–1404. [DOI] [PubMed] [Google Scholar]
- 49. Nicholls DG (1998) Presynaptic modulation of glutamate release. Prog Brain Res 116: 15–22. [DOI] [PubMed] [Google Scholar]
- 50. Li GR, Wang HB, Qin GW, Jin MW, Tang Q, et al. (2008) Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation 117: 2449–2457. [DOI] [PubMed] [Google Scholar]
- 51. Wu HJ, Sun HY, Wu W, Zhang YH, Qin GW, et al. (2013) Properties and molecular determinants of the natural flavone acacetin for blocking hKv4.3 channels. PLOS ONE 8: e57864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben-Ari Y (1985) Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14: 375–403. [DOI] [PubMed] [Google Scholar]
- 53. Spigolon G, Veronesi C, Bonny C, Vercelli A (2010) c-Jun n-terminal kinase signaling pathway in excitotoxic cell death following kainic acid-induced status epilepticus. Eur J Neurosci 31: 1261–72. [DOI] [PubMed] [Google Scholar]
- 54. Pollard H, Héron A, Moreau J, Ben-Ari Y, Khrestchatisky M (1993) Alterations of the GluR-B AMPA receptor subunit flip/flop expression in kainate-induced epilepsy and ischemia. Neuroscience 57: 545–554. [DOI] [PubMed] [Google Scholar]
- 55. Bahn S, Volk B, Widsen W (1994) Kainate receptor gene eexpression in the developing rat brain. J Neurosci 14: 5525–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, et al. (2005) Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res 79: 522–534. [DOI] [PubMed] [Google Scholar]
- 57. Ha SK, Moon E, Lee P, Ryu JH, Oh MS, et al. (2012) Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in vitro and in vivo. Neurochem Res 37: 1560–1567. [DOI] [PubMed] [Google Scholar]
- 58. Choi SH, Aid S, Bosetti F (2009) The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmaco Sci 30: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Klegeris A, McGeer EG, McGeer PL (2007) Therapeutic approaches to inflammation in neurodegenerative disease. Curr Opin Neurol 20: 351–357. [DOI] [PubMed] [Google Scholar]