Abstract
Natural competence for transformation is a developmental program that allows certain bacteria to take up free extracellular DNA from the environment and integrate this DNA into their genome. Thereby, natural transformation acts as mode of horizontal gene transfer and impacts bacterial evolution. The number of genes induced upon competence induction varies significantly between organisms. However, all of the naturally competent bacteria possess competence genes that encode so-called DNA-uptake machineries. Some components of these multi-protein complexes resemble subunits of type IV pili and type II secretion systems. However, knowledge on the mechanistic aspects of such DNA-uptake complexes is still very limited. Here, we discuss some new findings regarding the DNA-uptake machinery of the naturally transformable human pathogen Vibrio cholerae. The potential of this organism to initiate the competence program was discovered less than a decade ago. However, recent studies have provided new insight into both the regulatory pathways of competence induction and into the DNA uptake dynamics.
Keywords: horizontal gene transfer, V. cholerae, DNA uptake machinery, type IV pilus, natural transformation
Cellular Localization of the DNA-Uptake Machinery within Naturally Competent V. cholerae Cells
Horizontal gene transfer (HGT) is a major driving force of bacterial evolution. The rapid exchange of genetic information mediated by HGT enables bacteria to adapt to new environmental niches, to spread harmful traits such as antibiotic resistance cassettes or pathogenicity islands and to maintain genome integrity.1-5 HGT by means of conjugation and transduction relies on cell-cell contacts and/or mobile genetic elements, whereas natural transformation consists of the uptake of naked DNA from the environment. The ability to acquire exogenous DNA is called natural competence, a physiological state that is of transient nature for most bacterial species.6,7 The development of natural competence is often a highly regulated process, which frequently requires environmental cues such as nutrient availability or species-specific competence pheromones.7-9 Initiation of natural competence results from the expression of so-called competence genes, which in part encode specialized proteins that constitute the so-called DNA-uptake machinery.8,10,11 Contrary to the different regulatory networks controlling the onset of natural competence, the components of the DNA-uptake machinery are often conserved in Gram-positive and Gram-negative bacteria. Moreover, many of these proteins share homology with type IV pili (Tfp) and type II secretion systems (T2SS).8,11 Thus, it is tempting to speculate that DNA uptake by naturally competent bacteria occurs via nearly universal machineries. However, DNA-uptake complexes, especially their composition and cellular localization, have so far been poorly characterized. Moreover, most of the existing information is based on data that were acquired from the naturally competent Gram-positive bacterium Bacillus subtilis12-14 and, more recently, also from Streptococcus pneumoniae.15,16 However, the situation is different and less well studied for Gram-negative bacteria, where external DNA must be first translocated across the outer membrane (and the periplasmic space).
In our recent study, we aimed to gain insight into the process of DNA uptake and to visualize the DNA-uptake machinery in the human pathogen Vibrio cholerae using a cell-biology based approach.17 In this Gram-negative bacterium, natural competence is induced during growth on chitinous surfaces,7,18 which the bacterium encounters in its natural reservoir.19 Substantial information concerning the regulatory circuit of natural competence and transformation of this organism has been gathered in less than a decade (first reviewed by Seitz and Blokesch7 and later also by Sun et al.20). However, the DNA-uptake machinery of V. cholerae has never been investigated and has remained a mystery until recently.17,21,22
As a first step, Seitz and Blokesch identified the minimum competence regulon of V. cholerae based on previously unpublished and published expression data (18,23 and Blokesch, unpublished), locus organization, homology to other naturally competent bacteria and conservation in other naturally transformable Vibrio species.17 A minimal (most likely still incomplete) competence set of 19 genes was identified with the majority of genes encoding proteins with homology to biogenesis or structural components of Tfp.
To verify the importance of these candidate genes with respect to DNA uptake and natural transformation, each gene was deleted from the parental V. cholerae O1 El Tor strain, and the mutant was assessed for its transformability. Notably, pilD was excluded in this study as the deletion mutant displayed a strong growth defect (both in liquid culture and on solidified agar plates). Furthermore, the gene coding for the single-strand binding protein (ssb) could not be deleted.17 The latter finding is in agreement with recent studies that report the essentiality of ssb in V. cholerae.24-26 All of the other mutants were impaired for transformation even though low numbers of transformants were consistently obtained for strains lacking components of the Tfp portion of the DNA-uptake machinery. Such residual transformability was never observed for those mutants devoid of the competence genes comEA, comEC, and comF as well as the recA mutant, which served as a control in this assay.17 The encoded competence proteins were further categorized (using a recently developed whole-cell duplex PCR DNA-uptake assay27) as required for transport across the outer membrane or inner membrane. All of the Tfp-related proteins and ComEA were required for DNA-uptake across the outer membrane, whereas ComEC, ComF, and RecA were necessary only for inner membrane DNA translocation or recombination.17 Moreover, the results demonstrated that all of the steps involving Tfp-related components or ComEA function upstream of the inner-membrane channel ComEC, whereas the cytoplasmic protein RecA was confirmed as acting downstream of ComEC (similar to DprA; Seitz and Blokesch, data not shown). Finally, as the comEC and comF mutant of V. cholerae consistently displayed the same phenotype with respect to the absence of transformability and the accumulation of transforming DNA within the periplasmic space, it was hypothesized that the two proteins ComEC and ComF work in concert in mediating the translocation of the incoming DNA across the inner membrane (Fig. 1).17
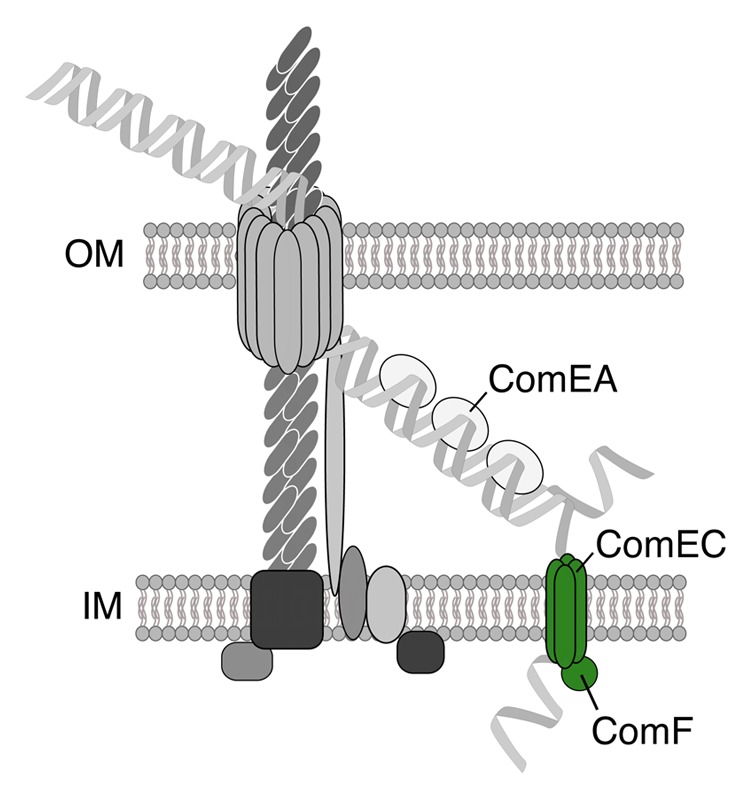
Figure 1. The ComEC and ComF proteins might drive DNA import into the cytoplasm. Based on the similar phenotypes of both the comEC and comF mutants, it was suggested that ComF works in concert with ComEC in the translocation of the DNA across the inner membrane.
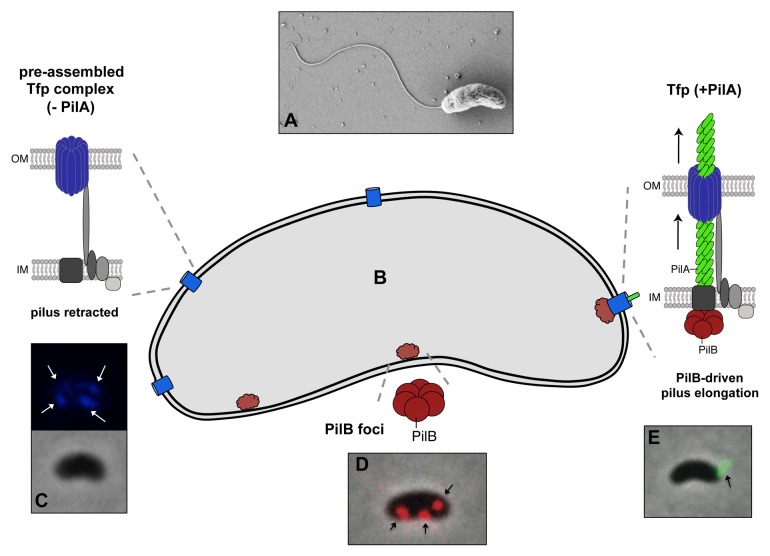
As a second step, Seitz and Blokesch imaged a variety of competence-related proteins (Fig. 2).17 First, the authors used immunofluorescence labeling against an affinity tag that was genetically added to the major pilin subunit PilA. Using this approach, the competence-induced Tfp became evident. Notably, this structure extended well beyond the outer membrane, thereby contradicting the previously hypothesized pseudopilus model for V. cholerae.18 For the majority of piliated cells (95%) only one pilus structure was detectable per cell (Fig. 2). In the absence of each of the Tfp-related components encoded by the minimum competence gene set, the cells appeared non-piliated. However, a pilus structure was still observable in the mutants lacking either a Tfp-unrelated competence gene (comEA, comEC, comF, and recA; all non-transformable) or the potential retraction ATPase (PilT).17 Interestingly, the latter mutant population contained a significantly higher fraction of cells with two or more Tfps.
Figure 2. Model of the localization of the DNA-uptake machinery (including the Tfp) in V. cholerae. Top of the figure: scanning micrograph of V. cholerae (A). Lower part: Schematic representation of a V. cholerae cell (B). Upon induction of natural competence, several PilQ protein foci were observed (in blue; image: mCherry-PilQ translational fusion false-colored in blue [C]). A similar multi-foci pattern also became evident for the ATPase PilB (in red; image: PilB-dsRed translational fusion [D]). Nonetheless, the majority of cells only displayed a single Tfp (in green; image: immunofluorescence image of PilA-Strep [E]). It was therefore hypothesized that a few pre-assembled Tfp complexes containing, among other components, PilQ (blue) but lacking the major pilin subunit PilA exist within the cells and that PilA polymerization is only initiated after the elongation ATPase PilB (in red) co-localizes with one of the pre-assembled complexes.
Next, the authors sought to also localize other competence proteins that were produced as translational fusions (with fluorescent proteins) and replaced the native proteins. All of the competence-induced cells displayed several distinct foci for the secretin PilQ as well as for the putative traffic ATPase PilB (Fig. 2), whereas no localization pattern was obvious for the retraction ATPase PilT.17 However, how can such a discrepancy between a single pilus and several outer membrane secretins (PilQ) and elongation ATPases (PilB) be explained? To address this question, Seitz and Blokesch demonstrated that the Tfp consistently co-localized with one of the PilQ foci. Moreover, the PilB ATPase foci were dynamic within the cell (which was not the case for a PilB variant with a mutated Walker B motif) and sometimes aggregated in close proximity of the PilQ protein complexes. The authors therefore hypothesized that each cell might contain several pre-assembled Tfp complexes that might solely lack the major pilin subunit and that pilus elongation – one at a time – occurs upon stimulation of pilin polymerization through PilB (Fig. 2). Such a model would suggest that a higher percentage of cells contain more than one pilus during PilB overproduction, which was exactly what the authors described.17
Putative Roles of other Competence Tfp-Associated Proteins
Another important finding of this study was that a gene cluster of five genes (e.g., VC0857 to VC0861) encoding hypothetical proteins or putative type IV pilins contributed significantly to efficient transformation.17 Interestingly, a minor pilin (ComP) was recently shown to directly interact with the species-specific DNA of Neisseria28 thereby answering one of the many open questions in the field of natural transformation, namely “which, if any, protein acts as a receptor for transforming DNA”29 in Neisseria. However, the comP mutants of N. gonorrhoeae, although impaired for natural transformation, are properly piliated and exert a normal Tfp function.30 We recently showed that in contrast to Neisseria and Haemophilus influenzae, V. cholerae does not differentiate between species-specific and species-non-specific DNA at the level of the DNA-uptake process,27 thus excluding a role of the VC0857-0861 operon-encoded proteins in species-specific DNA recognition. Notably, no pili were observed in any of the five mutant strains, consistent with the absence of transforming DNA in the periplasm.17 A putative function of these proteins could therefore be to activate the Tfp assembly machinery, which could potentially work in a similar manner as recently demonstrated for three minor pseudopilins involved in T2SS assembly in Klebsiella oxytoca.31
Another competence gene that was part of this study but not further investigated was VC1612. In previous studies, the VC1612 gene was slightly upregulated when V. cholerae was grown on chitin surfaces and was inducible by the transformation regulatory protein TfoX.18,23 The VC1612 gene product was initially annotated as “fimbrial biogenesis and twitching motility protein, putative.”32 Furthermore, a BLAST analysis against the Neisseria gonorrhoeae FA1090 genome indicated that the VC1612 protein was homologous to JCVI locus NT03NG0804 (“type IV pilus biogenesis-stability protein PilW (pilF)”) (supplementary data in ref. 17). Indeed, as a V. cholerae VC1612 knockout strain behaved similar to other Tfp-related mutants (e.g., lack of both piliation and DNA uptake into the periplasm), it was suggested that this protein might participate in the Tfp portion of the DNA-uptake complex.17 Next, Seitz and Blokesch aimed to predict the subcellular localization of VC1612 using the web-based PSORTb 3.0 algorithm.33 However, as the scores were similar for cytoplasmic and outer-membrane localization, the program proposed that the protein might have multiple localization sites. Given that a signal peptide was also not predicted by the SignalP server,34 the authors suggested in their model that the localization of VC1612 is cytoplasmic (Fig. 3).17 Interestingly, the VC1612 protein shows 34% identity (and 51% similarity) to PilF of Pseudomonas aeruginosa strain PAO1 (NCBI reference sequence: NP_252494.1). For P. aeruginosa, it was initially suggested that PilF might be localized to the inner face of the cytoplasmic membrane,35 although a more recent study by Koo et al. challenged this notion and demonstrated that PilF is an outer membrane lipoprotein involved in the insertion and polymerization of the secretin PilQ36 (in a chaperone-like manner37). Thus, given that future studies will also demonstrate outer membrane localization for the VC1612 protein of V. cholerae, it would be tempting to speculate that the protein also acts as pilotin, which could foster PilQ secretin assembly (Fig. 3). We therefore propose to annotate the VC1612 gene of V. cholerae as pilF.
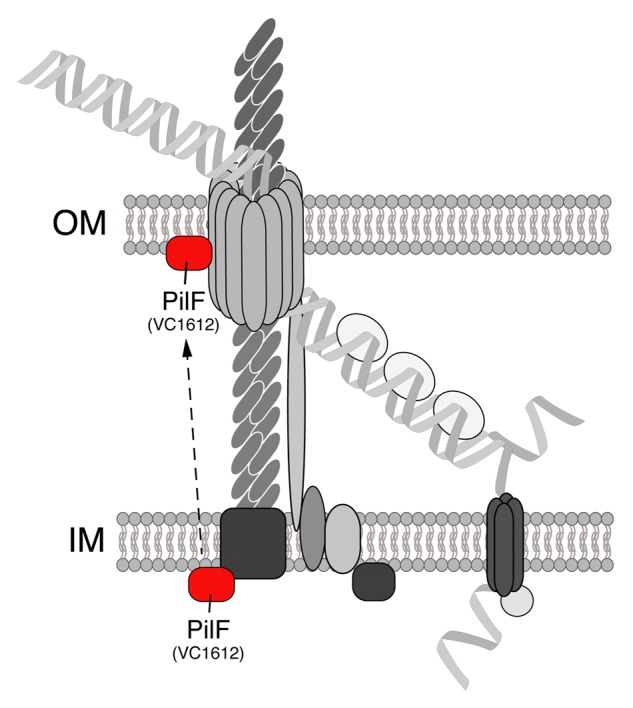
Figure 3. The PilF protein of V. cholerae might act as pilotin. In our previous study, the localization of the VC1612 protein was not unambiguously predictable and not experimentally addressed.17 However, BLAST comparisons with PilF of P. aeruginosa indicate that the VC1612 protein of V. cholerae (suggested annotation: PilF) might act as a pilotin, thereby assisting the PilQ secretin in its assembly.
The Conserved Operon Structure of pilMNOPQ
While comparing the competence/Tfp-related genes of different organisms, it became obvious that there were many variations with respect to the organization of the genes (e.g., whether they clustered together in operons or not). For example, whereas the major pilin-encoding gene pilA and the gene coding for the elongation ATPase PilB exist in a single operon in V. cholerae (supplementary table of ref. 17), this is not the case for many other Tfp-containing organisms (for an example, see the genetic map of the Tfp genes of Myxococcus xanthus38). Interestingly the pilMNOPQ operon (Fig. 4) is conserved in many proteobacteria, including for example P. aeruginosa strain PAO1, Pseudomonas stutzeri A1501, Escherichia coli K12, Legionella pneumophila strain Paris, Legionella longbeachae strain NSW150, and M. xanthus (according to the GenoList database39; see also review40).

Figure 4. The conserved pilMNOPQ operon encoding components of the Tfp-part of the DNA-uptake machinery. For details, see text.
What is so special about these five genes that warrants such a conserved operon structure? Indeed, many studies have recently addressed potential protein-protein interactions between these five proteins,38,41-46 and all of these studies suggested that the encoded proteins form a complex that connects the inner and outer membrane (including the outer membrane secretin PilQ). Interestingly, Friedrich et al. recently demonstrated that this protein complex is assembled in a sequential outside-in pathway,38 which therefore occurs opposite to the gene succession (PilQ-P-O-N-M). It is tempting to speculate that the genetic organization aims to avoid the toxic effects of the gene products that are encoded toward the end of the operon. Thus, the conserved gene sequence would ensure that a given stoichiometry is preserved and that the assembly is only initiated after all of the proteins are synthesized (e.g., full-length mRNA is available in the cell). Notably, the enhanced expression of pilQ and pilP in trans results in severe toxicity in both V. cholerae and E. coli.17
In summary, we conclude that we are only beginning to understand the basic components of the DNA-uptake machinery of V. cholerae and its functionality.17,22 Moreover, the composition and mechanistic aspects of type IV pili and the DNA-uptake machineries are generally not well understood, even though many hypothetical models of these multiprotein complexes exist. Notably, many of those models have not changed dramatically over the last decade (compare, for example, refs. 47 and 48) but by now are much better supported by experimental data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We like to thank Patrick Seitz for providing of the fluorescence images and for discussing on the content of the manuscript and Graham Knott (BioEM facility of EPFL) for his help with the electron microscopy. This work was supported by the Swiss National Science Foundation (grants 31003A_127029 and 31003A_143356) and by the European Research Council (309064-VIR4ENV).
Glossary
Abbreviations:
- HGT
horizontal gene transfer
- Tfp
type IV pilus
- tDNA
transforming DNA
Citation: Metzger LC, Blokesch M. Composition of the DNA-uptake complex of Vibrio cholerae. Mobile Genetic Elements 2014; 4:e28142; 10.4161/mge.28142
Footnotes
Previously published online: www.landesbioscience.com/journals/mge/article/28142
References
- 1.Popa O, Dagan T. Trends and barriers to lateral gene transfer in prokaryotes. Curr Opin Microbiol. 2011;14:615–23. doi: 10.1016/j.mib.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 2.Wiedenbeck J, Cohan FM. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol Rev. 2011;35:957–76. doi: 10.1111/j.1574-6976.2011.00292.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–21. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 4.Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbø CL, Case RJ, Doolittle WF. Lateral gene transfer and the origins of prokaryotic groups. Annu Rev Genet. 2003;37:283–328. doi: 10.1146/annurev.genet.37.050503.084247. [DOI] [PubMed] [Google Scholar]
- 5.Stokes HW, Gillings MR. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol Rev. 2011;35:790–819. doi: 10.1111/j.1574-6976.2011.00273.x. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seitz P, Blokesch M. Cues and regulatory pathways involved in natural competence and transformation in pathogenic and environmental Gram-negative bacteria. FEMS Microbiol Rev. 2013;37:336–63. doi: 10.1111/j.1574-6976.2012.00353.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2:241–9. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- 9.Chen I, Christie PJ, Dubnau D. The ins and outs of DNA transfer in bacteria. Science. 2005;310:1456–60. doi: 10.1126/science.1114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allemand JF, Maier B. Bacterial translocation motors investigated by single molecule techniques. FEMS Microbiol Rev. 2009;33:593–610. doi: 10.1111/j.1574-6976.2009.00166.x. [DOI] [PubMed] [Google Scholar]
- 11.Burton B, Dubnau D. Membrane-associated DNA transport machines. Cold Spring Harb Perspect Biol. 2010;2:a000406. doi: 10.1101/cshperspect.a000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidane D, Graumann PL. Intracellular protein and DNA dynamics in competent Bacillus subtilis cells. Cell. 2005;122:73–84. doi: 10.1016/j.cell.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Kramer N, Hahn J, Dubnau D. Multiple interactions among the competence proteins of Bacillus subtilis. Mol Microbiol. 2007;65:454–64. doi: 10.1111/j.1365-2958.2007.05799.x. [DOI] [PubMed] [Google Scholar]
- 15.Laurenceau R, Péhau-Arnaudet G, Baconnais S, Gault J, Malosse C, Dujeancourt A, Campo N, Chamot-Rooke J, Le Cam E, Claverys JP, et al. A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS Pathog. 2013;9:e1003473. doi: 10.1371/journal.ppat.1003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergé MJ, Kamgoué A, Martin B, Polard P, Campo N, Claverys JP. Midcell recruitment of the DNA uptake and virulence nuclease, EndA, for pneumococcal transformation. PLoS Pathog. 2013;9:e1003596. doi: 10.1371/journal.ppat.1003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seitz P, Blokesch M. DNA-uptake machinery of naturally competent Vibrio cholerae. Proc Natl Acad Sci U S A. 2013;110:17987–92. doi: 10.1073/pnas.1315647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meibom KL, Blokesch M, Dolganov NA, Wu C-Y, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–7. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- 19.Lipp EK, Huq A, Colwell RR. Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev. 2002;15:757–70. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Bernardy EE, Hammer BK, Miyashiro T. Competence and natural transformation in vibrios. Mol Microbiol. 2013;89:583–95. doi: 10.1111/mmi.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molloy S. Bacterial physiology: Vibrio uptake apparatus. Nat Rev Microbiol. 2013;11:820–1. doi: 10.1038/nrmicro3165. [DOI] [PubMed] [Google Scholar]
- 22.Seitz P, Pezeshgi Modarres H, Borgeaud S, Bulushev RD, Steinbock LJ, Radenovic A, Dal Peraro M, Blokesch M, Com EA. ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Vibrio cholerae Cells. PLoS Genet. 2014;10:e1004066. doi: 10.1371/journal.pgen.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci U S A. 2004;101:2524–9. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci U S A. 2008;105:8736–41. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamp HD, Patimalla-Dipali B, Lazinski DW, Wallace-Gadsden F, Camilli A. Gene Fitness Landscapes of Vibrio cholerae at Important Stages of Its Life Cycle. PLoS Pathog. 2013;9:e1003800. doi: 10.1371/journal.ppat.1003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao MC, Pritchard JR, Zhang YJ, Rubin EJ, Livny J, Davis BM, Waldor MK. High-resolution definition of the Vibrio cholerae essential gene set with hidden Markov model-based analyses of transposon-insertion sequencing data. Nucleic Acids Res. 2013;41:9033–48. doi: 10.1093/nar/gkt654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suckow G, Seitz P, Blokesch M. Quorum sensing contributes to natural transformation of Vibrio cholerae in a species-specific manner. J Bacteriol. 2011;193:4914–24. doi: 10.1128/JB.05396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, Pallett M, Brady J, Baldwin GS, Lea SM, Matthews SJ, et al. Specific DNA recognition mediated by a type IV pilin. Proc Natl Acad Sci U S A. 2013;110:3065–70. doi: 10.1073/pnas.1218832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton HL, Dillard JP. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol. 2006;59:376–85. doi: 10.1111/j.1365-2958.2005.04964.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolfgang M, van Putten JP, Hayes SF, Koomey M. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol Microbiol. 1999;31:1345–57. doi: 10.1046/j.1365-2958.1999.01269.x. [DOI] [PubMed] [Google Scholar]
- 31.Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation. EMBO J. 2012;31:1041–53. doi: 10.1038/emboj.2011.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–83. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–15. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 35.Kim K, Oh J, Han D, Kim EE, Lee B, Kim Y. Crystal structure of PilF: functional implication in the type 4 pilus biogenesis in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 2006;340:1028–38. doi: 10.1016/j.bbrc.2005.12.108. [DOI] [PubMed] [Google Scholar]
- 36.Koo J, Tammam S, Ku SY, Sampaleanu LM, Burrows LL, Howell PL. PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa Type IV pilus secretin. J Bacteriol. 2008;190:6961–9. doi: 10.1128/JB.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo J, Tang T, Harvey H, Tammam S, Sampaleanu L, Burrows LL, Howell PL. Functional mapping of PilF and PilQ in the Pseudomonas aeruginosa type IV pilus system. Biochemistry. 2013;52:2914–23. doi: 10.1021/bi3015345. [DOI] [PubMed] [Google Scholar]
- 38.Friedrich C, Bulyha I, Søgaard-Andersen L. Outside-In Assembly Pathway of the Type IV Pilus System in Myxococcus xanthus. J Bacteriol. 2014;196:378–90. doi: 10.1128/JB.01094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechat P, Hummel L, Rousseau S, Moszer I. GenoList: an integrated environment for comparative analysis of microbial genomes. Nucleic Acids Res. 2008;36:D469–74. doi: 10.1093/nar/gkm1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelicic V. Type IV pili: e pluribus unum? Mol Microbiol. 2008;68:827–37. doi: 10.1111/j.1365-2958.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- 41.Balasingham SV, Collins RF, Assalkhou R, Homberset H, Frye SA, Derrick JP, Tønjum T. Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. J Bacteriol. 2007;189:5716–27. doi: 10.1128/JB.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Georgiadou M, Castagnini M, Karimova G, Ladant D, Pelicic V. Large-scale study of the interactions between proteins involved in type IV pilus biology in Neisseria meningitidis: characterization of a subcomplex involved in pilus assembly. Mol Microbiol. 2012;84:857–73. doi: 10.1111/j.1365-2958.2012.08062.x. [DOI] [PubMed] [Google Scholar]
- 43.Berry JL, Phelan MM, Collins RF, Adomavicius T, Tønjum T, Frye SA, Bird L, Owens R, Ford RC, Lian LY, et al. Structure and assembly of a trans-periplasmic channel for type IV pili in Neisseria meningitidis. PLoS Pathog. 2012;8:e1002923. doi: 10.1371/journal.ppat.1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Wallace RA, Black WP, Li YZ, Yang Z. Type IV pilus proteins form an integrated structure extending from the cytoplasm to the outer membrane. PLoS One. 2013;8:e70144. doi: 10.1371/journal.pone.0070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tammam S, Sampaleanu LM, Koo J, Manoharan K, Daubaras M, Burrows LL, Howell PL. PilMNOPQ from the Pseudomonas aeruginosa type IV pilus system form a transenvelope protein interaction network that interacts with PilA. J Bacteriol. 2013;195:2126–35. doi: 10.1128/JB.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tammam S, Sampaleanu LM, Koo J, Sundaram P, Ayers M, Chong PA, Forman-Kay JD, Burrows LL, Howell PL. Characterization of the PilN, PilO and PilP type IVa pilus subcomplex. Mol Microbiol. 2011;82:1496–514. doi: 10.1111/j.1365-2958.2011.07903.x. [DOI] [PubMed] [Google Scholar]
- 47.Fussenegger M, Rudel T, Barten R, Ryll R, Meyer TF. Transformation competence and type-4 pilus biogenesis in Neisseria gonorrhoeae--a review. Gene. 1997;192:125–34. doi: 10.1016/S0378-1119(97)00038-3. [DOI] [PubMed] [Google Scholar]
- 48.Burrows LL. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol. 2012;66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]