Abstract
While there are several published comprehensive stepwise algorithmic methods for diagnosing pigmented skin malignancy, only limited material has been published for the stepwise assessment of non-pigmented lesions. We present a method based on pattern analysis, with a stepwise assessment, first, for ulceration, second, for white clues (defined as white lines, or in the case of a raised lesion any of the keratin clues: dermatoscopic white circles, dermatoscopic white structureless areas or surface keratin), and third, if no ulceration or white clues are present, proceed to vessel pattern analysis.
This is a novel method, and apart from the assessment of white clues in raised lesions, it has not been formally tested. The priority of keratin clues in raised lesions over vessel pattern analysis has, however, been verified.
It is conceded that this method is less specific than methods which have clues of pigmented structures, and accepting these limitations, Prediction without Pigment is a decision algorithm intended to guide the clinician in the decision as to whether to perform a biopsy rather than consistently leading to a specific diagnosis. Reaching a more specific diagnosis at the end of our flowchart can be achieved by weighing of clues both clinical and dermatoscopic, and that ability can be expected to improve with both knowledge and experience, but no diagnostic method, including this one, can be 100% sensitive in diagnosing malignancy, in particular, melanoma. Taking these limitations into account, any non-pigmented lesion, regardless of pattern analysis, which is raised and firm (nodular) and for which a confident, specific benign diagnosis cannot be made, should be excised to exclude the nodular variant of amelanotic melanoma.
Keywords: dermatoscopy, dermoscopy, non-pigmented, skin cancer, algorithm, white circles, keratin, vessels
Introduction
While limited material has been published for stepwise dermatoscopic assessment of non-pigmented skin lesions [1,2], dermatoscopy has mainly been proposed as an aid to the diagnosis of pigmented skin lesions, and it has been shown to increase diagnostic accuracy for all pigmented lesions, melanocytic and non-melanocytic [3–5]. Pattern analysis was the original method of description and diagnosis for pigmented lesions [6] and, despite the development of many “simplified” algorithms, remains the method of experts. In 2007, Kittler proposed a revision of pattern analysis [7], introducing an algorithm based on geometric, objective, well-defined descriptive terms that are applicable regardless of diagnosis. In 2008, Kittler et al proposed a similar system for objective description of blood vessel patterns [8] to replace metaphoric descriptions of dermatoscopic features of non-pigmented skin lesions [1, 2, 9–16].
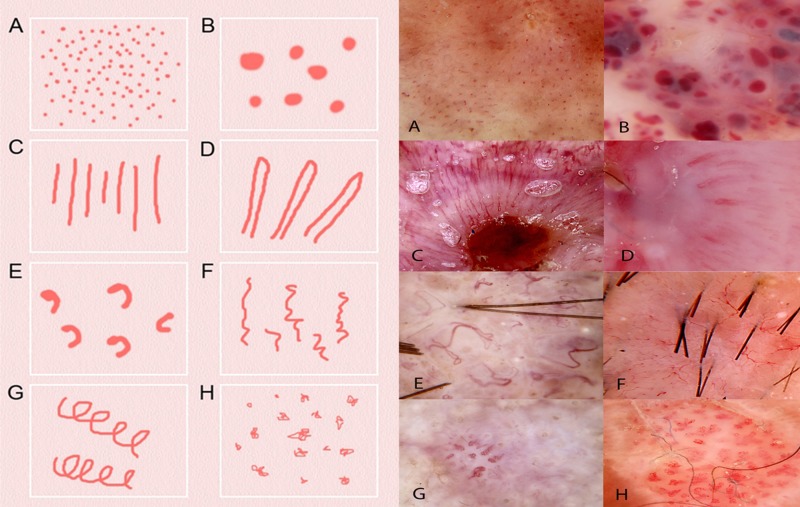
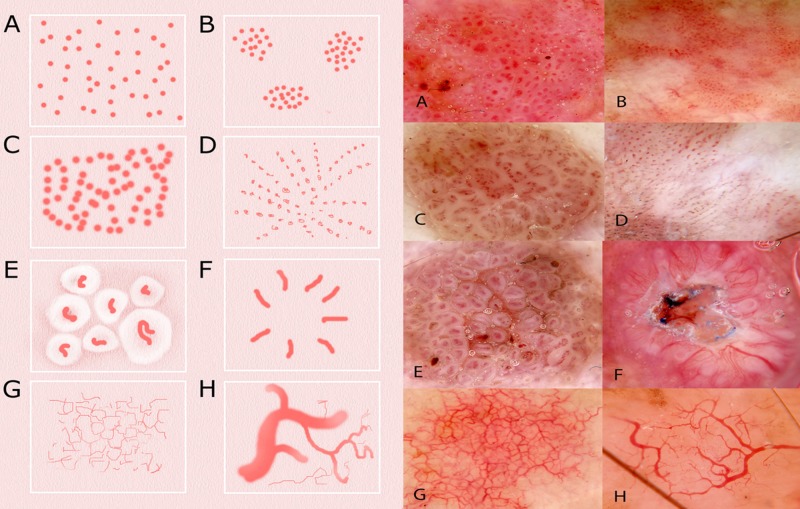
Revised pattern analysis precisely defines eight types of vessel structures and eight vessel arrangements [17]. The types are as follows (closest metaphoric term italicized in brackets where available and different):
Structures (Figure 1): Dots, clods (lacunes), linear straight, linear looped (hairpin), curved (comma), serpentine (linear irregular), helical (corkscrew) and coiled (glomerular).
Arrangements (Figure 2): Random (non-specific), clustered, serpiginous (string of pearls), linear, centred, radial (starburst), reticular and branched (arborizing).
Figure 1.
Schematic representation (left) and dermatoscopic image of examples (right) of the eight types of vessel structure defined in revised pattern analysis. The closest equivalent metaphoric term, when different, is italicized in brackets and the diagnosis of the lesion is in brackets at the end: (A) dots (melanoma); (B) clods (lacunes)(hemangioma); (C) lines straight (ulcerated BCC); (D) lines looped (hairpin vessels)(SCC); (E) lines curved (comma vessels)(dermal nevus); (F) lines serpentine (linear irregular vessels) (BCC); (G) lines helical (corkscrew vessels)(IEC); (H) lines coiled (glomerular vessels)(IEC). [Copyright: ©2014 Rosendahl et al.]
Abbreviations used: BCC- basal cell carcinoma; SCC—squamous cell carcinoma; IEC—intraepidermal squamous cell carcinoma.
Figure 2.
Schematic representation (left) and dermatoscopic image of examples (right) of the eight types of vessel arrangement defined in revised pattern analysis: (A) random (non-specific)(IEC); (B) clustered (IEC); (C) serpiginous (string of pearls) (CCA); (D) linear (IEC); (E) centered (seborrheic keratosis); (F) radial (starburst) (SCC); (G) reticular (sun-damaged skin); (H) branched (arborizing) (BCC). [Copyright: ©2014 Rosendahl et al.]
Abbreviations used: BCC- basal cell carcinoma; SCC—squamous cell carcinoma; IEC—intraepidermal squamous cell carcinoma; CCA—clear cell acanthoma
Prediction without Pigment: a decision algorithm for non-pigmented skin malignancy
In this context, “non-pigmented” means lesions with no melanin pigmentation (which may be black, brown, gray, or blue, depending on depth in the skin [18]). Such lesions may still have pigment due to keratin (yellow or orange) or hemoglobin (red, purple, blue or black). Both melanin and hemoglobin may produce blue and black pigmentation, and occasionally this can cause confusion. Usually this can be resolved by considering the spectrum of colors present; when associated with brown or gray, black and blue are best interpreted as due to melanin, when associated with only red, they are best interpreted as due to hemoglobin [17].
Only when there are no structures pigmented by melanin should other features be used to reach a decision about the need for biopsy and, second, to attempt to make a specific diagnosis [17]. In contrast to the dermatoscopic diagnosis of pigmented lesions, for non-pigmented lesions dermatoscopic features must be supplemented by clinical findings to achieve acceptable diagnostic accuracy [17]. In fact, with non-pigmented lesions it is often possible to make a confident specific benign diagnosis clinically; examples are cases of viral warts, seborrheic keratoses (SK) and dermal (Unna or Miescher) nevi. Dermatoscopy can then be used simply to confirm this clinical diagnosis. If such a clinical diagnosis is not possible a lesion can be assessed in a stepwise pattern according to the “Prediction without Pigment” diagnostic flowchart (Figure 3).
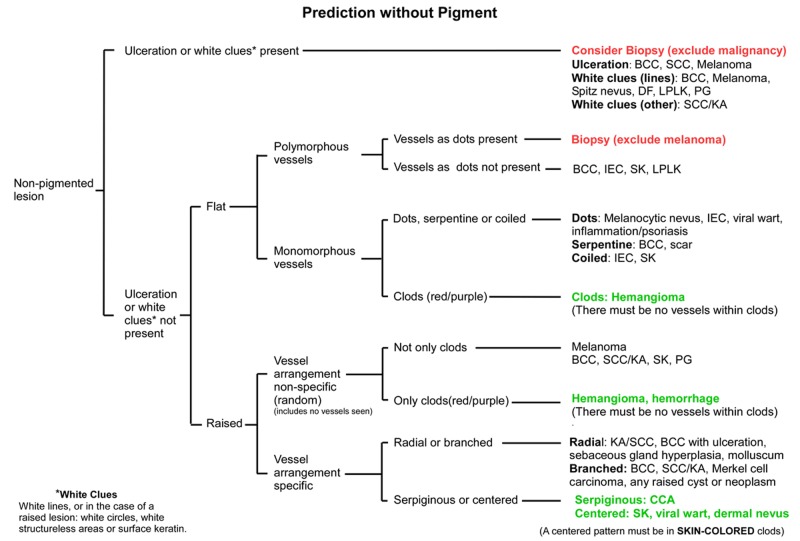
Figure 3.
Flowchart for the Prediction without Pigment algorithm. End-points colored red are highly suspicious for malignancy, while those colored green should be benign. All other endpoints should be assessed by weighing all clues, both clinical and dermatoscopic, as there are malignant options in the differential diagnosis. The diagnoses listed are not exhaustive but are selected to guide the decision process. It may be useful to have this flowchart open when looking at the lesions depicted in Figures 4–10. [Copyright: ©2014 Rosendahl et al.]
Abbreviations used: BCC—basal cell carcinoma; SCC—squamous cell carcinoma; KA—keratoacanthoma; DF—dermatofibroma; LPLK—lichen-planus-like-keratosis (benign lichenoid keratosis); PG—pyogenic granuloma; IEC—intraepidermal carcinoma (Bowen’s disease or SCC in-situ); SK—seborrheic keratosis; CCA—clear cell acanthoma
Ulceration
The first assessment in the Prediction without Pigment algorithm is for the presence of ulceration. The presence of ulceration, either clinically or dermatoscopically, without a clear history of trauma should lead to a biopsy. Ulceration is usually better appreciated clinically than by dermatoscopy. However, serum leaking from ulcerated areas may trap fibers of clothing or loose hair, so adherent fiber constitutes a dermatoscopic clue to ulceration [17]. Ulceration is most commonly seen in basal cell carcinoma (BCC) and it is a published clue to BCC [19], but it can be seen in any malignancy.
White clues: white lines and keratin clues
In the absence of ulceration, the lesion is next assessed for presence of white clues, which are defined as white lines in any lesion, and in the case of a raised lesion, keratin clues: white circles, white structureless areas or surface keratin. The clue of white lines has only been formally evaluated in pigmented lesions [5], but the authors also consider it as an important clue in non-pigmented lesions, and have incorporated it into this algorithm [17]. The keratin clues which apply to raised lesions—white circles, white structureless areas and surface keratin—however, have been evaluated and have been found to be more robust than vessel analysis in differentiating squamous cell carcinoma (SCC) and keratoacanthoma (KA) from other raised non-pigmented lesions [20]. A raised lesion is one that is significantly raised visibly or palpably and includes some papules and all nodules.
White lines
There are two types of white lines that can be present in both pigmented and non-pigmented lesions. First, there are white lines that may be seen with both polarizing and non-polarizing dermatoscopy (including reticular white lines originally called negative, white or inverse network or reticular depigmentation) and then there are polarizing-specific white lines (also known as chrysalis and as shiny or bright white lines [21]), which are only seen with polarizing dermatoscopes (see Figure 4). Both types of white lines are clues to malignancy [17], but they may also be seen in benign lesions, so the clinical context needs to be considered. For example, while white lines seen with both polarizing and non-polarizing dermatoscopes are a clue to malignancy, they also occur in lichen planus as Wickham’s striae.
Figure 4.
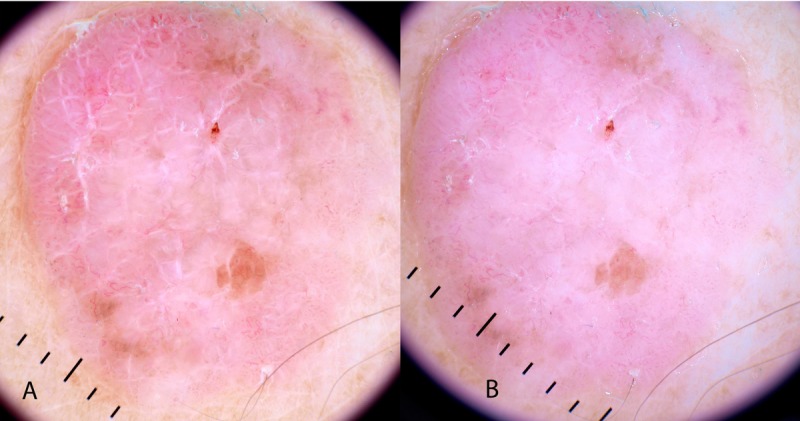
Polarized (A) and non-polarized (B) dermatoscopic image of a non-pigmented lesion reveals the dermatoscopic clue of polarizing-specific white lines. Note the perpendicular orientation of these lines. This is a fibroepithelioma of Pinkus—a variant of BCC. [Copyright: ©2014 Rosendahl et al.]
Polarizing-specific white lines are a published clue to the benign conditions of scar tissue, Spitz nevus, dermatofibroma (DF), lichen planus like keratosis (LPLK) [22] and pyogenic granuloma (termed “white rail lines” in the reference cited) [23], as well as the malignancies melanoma and BCC [22]. With respect to melanomas, they were more prominent in invasive (41%) than in-situ (17%) melanomas [22].
Keratin clues
If a non-pigmented lesion is not ulcerated and there are no white lines as a clue to malignancy, then there is one step to assess prior to the need to perform vessel pattern analysis. If the lesion is raised, then the three keratin clues of clinical or dermatoscopic surface keratin, dermatoscopic white structureless areas or dermatoscopic white circles are valuable clues to malignancy, specifically SCC and KA. White circles have the highest specificity (87%) for SCC/KA with a sensitivity of 44% for this diagnosis [20]. Surface keratin is best appreciated clinically, as one of the purposes of dermatoscopy is to render keratin invisible to better appreciate structures beneath the stratum corneum, but the other two keratin clues, white circles and white structureless areas, are dermatoscopic clues (Figures 5 and 6).
Figure 5.
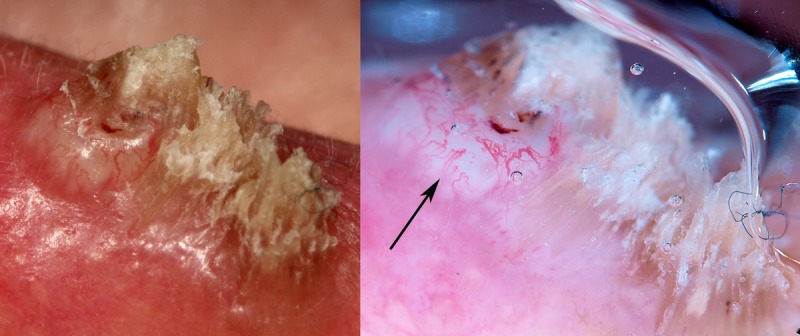
Surface keratin and a white structureless area are seen clearly on the close-up clinical image of a non-pigmented raised lesion on the ear of a 60-year-old man (left). They are also apparent in the dermatoscopic image (right) with the white structureless area indicated by an arrow. It is an SCC. [Copyright: ©2014 Rosendahl et al.]
Figure 6.
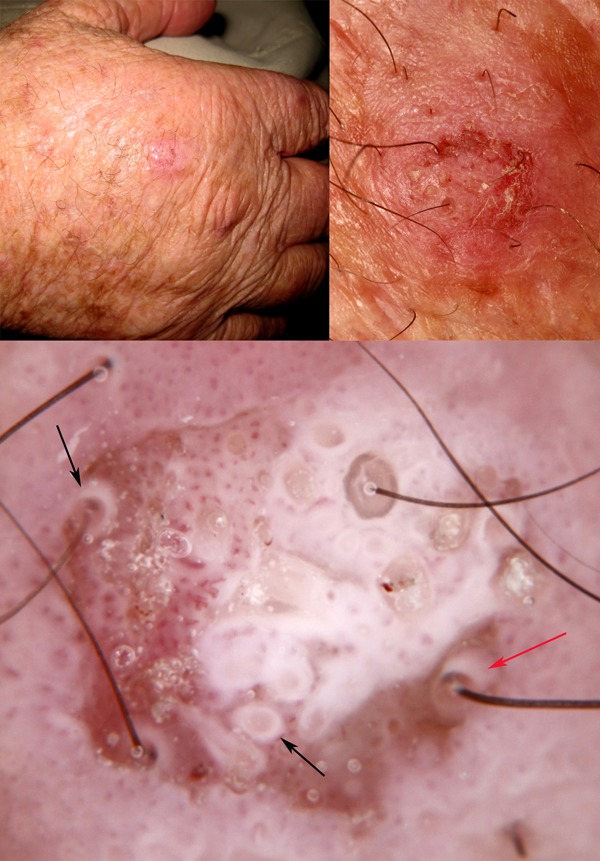
Clinical and close-up images of a lesion on the hand of a 72-year-old-man which cannot be confidently diagnosed as benign clinically (upper images). Dermatoscopically (lower image) there are intensely white circles (black arrows) and one of these in particular is projected obliquely (red arrow), showing a thin white cylinder from the center of which emerges a hair shaft. White circles are produced by highly keratinized malignant squamous cells invading hair follicles. [Copyright: ©2014 Rosendahl et al.]
There are other clues provided by keratin not included in the flowchart because we regard them as of inadequate specificity for malignancy but which are useful when applied at the right-hand side of the algorithmic flowchart to help reach a specific diagnosis. White clods and dots (also known as milia-like cysts) occur commonly in seborrheic keratosis but also in SCC and melanoma. Polarizing-specific white clods (referred to as white shiny areas and white shiny clods in the reference cited) are described as a published clue to BCC and melanoma [24]. Areas of structureless pink and white are published as a clue to BCC [25]. Four-dot-clods are frequently seen on actinic keratosis and intraepidermal squamous cell carcinoma (IEC)—called rosettes in the reference cited [16]—but can be seen on any sun-damaged skin including on melanoma.
Vessel pattern analysis
If a non-pigmented lesion cannot be confidently diagnosed clinically, is not ulcerated and there are no white clues, the final step in the algorithm is vessel pattern analysis [8,17]. One might also choose to perform vessel pattern analysis when the decision has already been made to perform a biopsy due to ulceration or white clues for the purpose of reaching a more specific diagnosis. First, the lesion is assessed to determine whether it is flat or raised, and the stepwise decision process proceeds differently in each case.
If a lesion is flat then it is assessed as to whether the vessel pattern is polymorphous or monomorphous [8]. A pattern of vessels is termed polymorphous if there is more than one type of vessel without one predominating by far. A single dot, clod or line does not make a pattern polymorphous. A polymorphous pattern can be produced by either two distinct vessel patterns on different parts of a lesion or by more than one vessel type present in a speckled arrangement in significant numbers over the lesion. A pattern of vessels is termed monomorphous if one type of vessel predominates by far.
A polymorphous pattern of vessels including a pattern of dot vessels requires biopsy on suspicion of melanoma [8] (Figure 7) although the differential diagnosis also includes benign lesions. A polymorphous pattern of vessels without a pattern of dot vessels also requires consideration of biopsy because the differential diagnosis includes BCC and IEC, as well as benign conditions including SK and LPLK [17].
Figure 7.
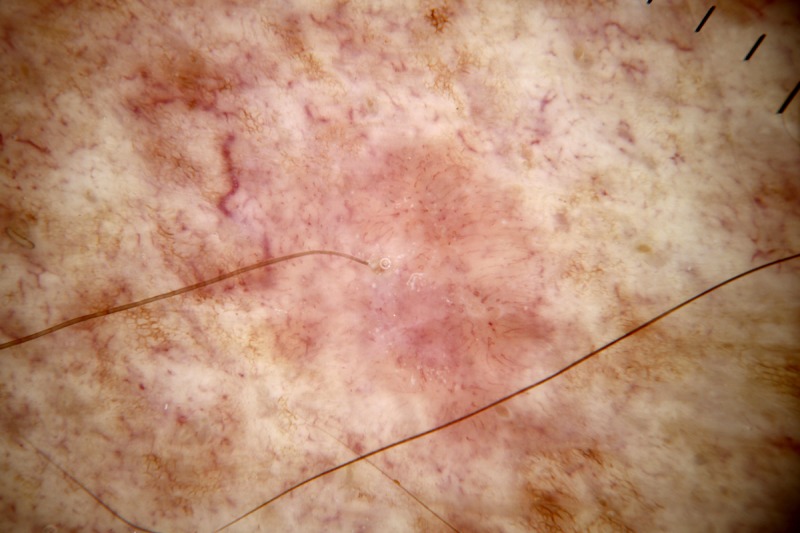
Dermatoscopic image of a non-pigmented lesion. There is no ulceration and there are no “white clues” (as defined in the text). There are linear vessels covering the majority of the lesion with a pattern of dot vessels at the upper extremity. A polymorphous pattern of vessels, including a pattern of dot vessels, raises suspicion for melanoma. This was an invasive amelanotic melanoma (Breslow thickness 0.3 mm). [Copyright: ©2014 Rosendahl et al.]
Any monomorphous vessel pattern in a flat lesion other than clods-only should be considered for biopsy. Flat lesions with a monomorphous pattern of dot vessels include IEC (where tightly coiled vessels may sometimes appear as dots at standard dermatoscopic magnification), as well as the benign conditions of melanocytic nevus, viral wart, psoriasis and inflammatory conditions [17]. Flat lesions with monomorphous serpentine vessels include BCC (Figure 1F), as well as desmoplastic trichoepithelioma and scar tissue. Flat lesions with monomorphous, coiled vessels include IEC (referred to as glomerular vessels in the reference cited) [11] (Figure 1H), as well as the benign lesion SK, although SK will more commonly have polymorphous vessels and in most cases will have been diagnosed with confidence clinically [17].
If a flat lesion has a monomorphous pattern of vessels and the vessel pattern is (red/purple) clods-only, this is a benign pattern consistent with the diagnosis of hemangioma [17] (Figure 1B). For this benign diagnosis to be made there must be no vessels within any of the clods and there must not be a history inconsistent with a benign diagnosis; the lesion must not be reported to be changing.
If a lesion is raised assessment according to monomorphous versus polymorphous vessel pattern is not helpful for a decision algorithm. Instead, raised lesions are first assessed as to whether the vessel arrangement is non-specific or alternatively one of four different specific vessel arrangements (radial, branched, serpiginous or centered) [17]. There are eight different vessel arrangements (Figure 2), and of these the last seven are specific, but three of these arrangements (clustered, linear and reticular) are not expected in a raised lesion.
If none of the four designated specific vessel arrangements are present the arrangement is described as “non-specific.” If the arrangement is non-specific and not clods-only, then a biopsy is indicated as melanoma, BCC and SCC/KA are in the differential diagnosis as are benign lesions including SK and pyogenic granuloma (PG) [17] (Figure 8). If the pattern is non-specific but clods-only the pattern is regarded as benign (hemangioma) [17]. As with the clods-only pattern in flat lesions, the clods must be red or purple and there must not be any vessels within any of the clods (see Figure 9) [17]. If the vessel arrangement is specific, either radial, branched, serpiginous or centered, then the lesions are assessed according to each of these specific arrangements. Lesions with a radial pattern of vessels include SCC/KA (referred to as a starburst vessel pattern in the reference cited [16] (Figure 2F—SCC) and BCC with ulceration, as well as sebaceous gland hyperplasia (additional clue: several white or yellow clods in central location) and molluscum contagiosum (additional clue: single skin-colored or orange clod in central location). Lesions with a branched vessel pattern include BCC, SCC/KA, Merkel cell carcinoma and any raised cyst or neoplasm, benign or malignant [17] (Figure 2H—BCC). The serpiginous vessel pattern is specific to the benign lesion, clear cell acanthoma (CCA) [2] (Figure 2C). A centered pattern is also regarded as a benign pattern and is seen with papillomatous dermal nevi, seborrheic keratosis (Figure 2E) and viral wart, but to qualify for this pattern the vessels must be in the center of skin-colored (not red or purple) clods (see Figure 10) and the lesion must be truly non-pigmented [17].
Figure 8.
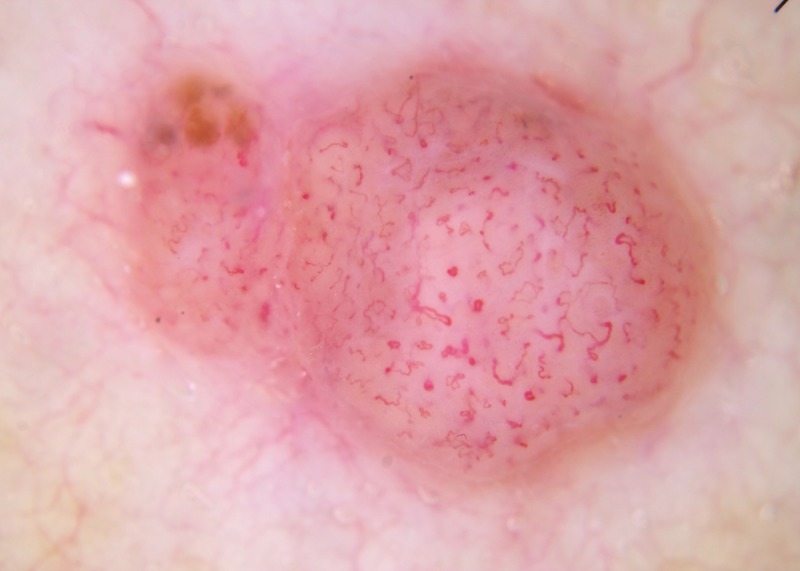
Dermatoscopic image of a focally pigmented lesion. Because focal pigment is present, this should be assessed as a pigmented lesion, and as it has chaos (asymmetry of structure or color) and the clue of an eccentric structureless area, it should be excised [5]. If the focal pigment is ignored and it is assessed as a non-pigmented lesion; it is raised, the vessel pattern is non-specific and is not clods-only, so it should be excised. This was an invasive melanoma (Breslow thickness 2.5 mm). [Copyright: ©2014 Rosendahl et al.]
Figure 9.
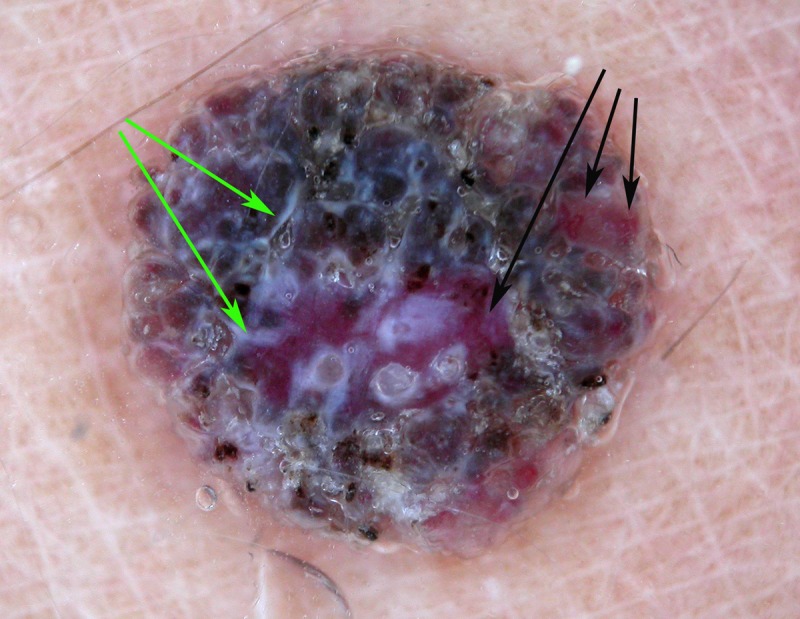
Dermatoscopic image of a raised non-pigmented lesion. It does have the clue to malignancy of white lines (green arrows), which should lead to biopsy. If this was not noted and vessel pattern analysis was done it has a non-specific vessel arrangement. If this were interpreted as a structureless lesion (rather than clods-only), biopsy would therefore be indicated. If, however, it was interpreted as having a “clods-only” pattern, it can be seen on close inspection that there are vessels within some of the clods (black arrows). This by definition disqualifies this lesion from a benign “clods-only” categorization, which is important because this was a nodular melanoma (Breslow thickness 1.2 mm). [Copyright: ©2014 Rosendahl et al.]
Figure 10.
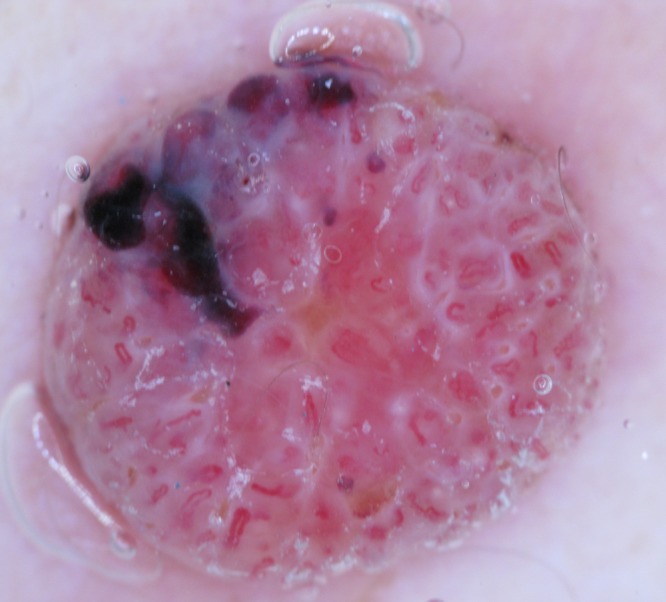
Dermatoscopic image of a raised non-pigmented lesion, which does have vessels centered on non-pigmented clods but the clods are not skin-colored being markedly erythematous and in the upper part of the image there are purple clods. This lesion therefore, by definition, cannot be described as having a “centered” pattern. It was a nodular melanoma (Breslow thickness 2.5 mm). [Copyright: ©2014 Rosendahl et al.]
Conclusion
An algorithmic flowchart for non-pigmented skin lesions is less specific than can be achieved for pigmented lesions. Incorporating the clinical clues of ulceration and surface keratin with the dermatoscopic white line, keratin and vessel clues does, however, facilitate a stepwise process which can exclude some lesions from biopsy with confidence, while also predicting malignancy. Prediction without Pigment, analogous to the method for assessing pigmented skin lesions by revised pattern analysis, Chaos and Clues, provides a framework on which to build the learning that comes from accumulated experience. With non-pigmented lesions that cannot confidently be diagnosed clinically, the limitations of dermatoscopy must be acknowledged, and if there is any doubt following assessment a biopsy should be performed.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Zalaudek I, Argenziano G, Di Stefani A, et al. Dermoscopy in general dermatology. Dermatology. 2006;212(1):7–18. doi: 10.1159/000089015. [DOI] [PubMed] [Google Scholar]
- 2.Zalaudek I, Kreusch J, Giacomel J, et al. How to diagnose non-pigmented skin tumors: a review of vascular structures seen with dermoscopy: part II. Nonmelanocytic skin tumors. J Am Acad Dermatol. 2010;63(3):377–386. doi: 10.1016/j.jaad.2009.11.697. quiz 387–388. [DOI] [PubMed] [Google Scholar]
- 3.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–65. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 4.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669–76. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 5.Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64(6):1068–73. doi: 10.1016/j.jaad.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17(4):571–83. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 7.Kittler H. Dermatoscopy: Introduction of a new algorithmic method based on pattern analysis for diagnosis of pigmented skin lesions. Dermatopathology: Practical & Conceptual. 2007;13(1):3. [Google Scholar]
- 8.Kittler H, Riedl E, Rosendahl C, Cameron A. Dermatoscopy of unpigmented lesions of the skin: a new classification of vessel morphology based on pattern analysis. Dermatopathology: Practical & Conceptual. 2008;14(4):4. [Google Scholar]
- 9.Zalaudek I, Argenziano G, Leinweber B, et al. Dermoscopy of Bowen’s disease. Br J Dermatol. 2004;150(6):1112–6. doi: 10.1111/j.1365-2133.2004.05924.x. [DOI] [PubMed] [Google Scholar]
- 10.Bugatti L, Filosa G, De Angelis R. The specific dermoscopical criteria of Bowen’s disease. J Eur Acad Dermatol Venereol. 2007;21(5):700–1. doi: 10.1111/j.1468-3083.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Chamberlain AJ, Bailey M, et al. Dermatoscopy aids in the diagnosis of the solitary red scaly patch or plaque-features distinguishing superficial basal cell carcinoma, intraepidermal carcinoma, and psoriasis. J Am Acad Dermatol. 2008;59(2):268–74. doi: 10.1016/j.jaad.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Felder S, Rabinovitz H, Oliviero M, Kopf A. Dermoscopic differentiation of a superficial basal cell carcinoma and squamous cell carcinoma in situ. Dermatol Surg. 2006;32(3):423–5. doi: 10.1111/j.1524-4725.2006.32085.x. [DOI] [PubMed] [Google Scholar]
- 13.Zalaudek I, Giacomel J, Argenziano G, et al. Dermoscopy of facial nonpigmented actinic keratosis. Br J Dermatol. 2006;155(5):951–6. doi: 10.1111/j.1365-2133.2006.07426.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuellar F, Vilalta A, Puig S, et al. New dermoscopic pattern in actinic keratosis and related conditions. Arch Dermatol. 2009;145(6):732. doi: 10.1001/archdermatol.2009.86. [DOI] [PubMed] [Google Scholar]
- 15.Kreusch J, Koch F. Incident light microscopic characterization of vascular patterns in skin tumors. Hautarzt. 1996;47(4):264–72. doi: 10.1007/s001050050412. [DOI] [PubMed] [Google Scholar]
- 16.Zalaudek I, Giacomel J, Schmid K, et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: a progression model. J Am Acad Dermatol. 2011;66(4):589–97. doi: 10.1016/j.jaad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Kittler H, Rosendahl C, Cameron A, Tschandl P. Dermatoscopy. Austria: Facultas.wuv; 2011. [Google Scholar]
- 18.Weismann K, Lorentzen HF. Dermoscopic color perspective. Arch Dermatol. 2006;142(9):1250. doi: 10.1001/archderm.142.9.1250. [DOI] [PubMed] [Google Scholar]
- 19.Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62(1):67–75. doi: 10.1016/j.jaad.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Rosendahl C, Cameron A, Argenziano G, et al. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch Dermatol. 2012;148(12):1386–92. doi: 10.1001/archdermatol.2012.2974. [DOI] [PubMed] [Google Scholar]
- 21.Marghoob AA, Cowel L, Kopf AW, Scope A. Observation of chrysalis structures with polarized dermoscopy. Arch Dermatol. 2009;145(5):618. doi: 10.1001/archdermatol.2009.28. [DOI] [PubMed] [Google Scholar]
- 22.Balagula Y, Braun RP, Rabinovitz HS, et al. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and non-melanocytic lesions. J Am Acad Dermatol. 2012;67(2):194.e1–8. doi: 10.1016/j.jaad.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Zaballos P, Llambrich A, Cuéllar F, Puig S, Malvehy J. Dermoscopic findings in pyogenic granuloma. Br J Dermatol. 2006;154(6):1108–11. doi: 10.1111/j.1365-2133.2006.07193.x. [DOI] [PubMed] [Google Scholar]
- 24.Liebman TN, Rabinovitz HS, Balagula Y, Jaimes-Lopez N, Marghoob AA. White shiny structures in melanoma and BCC. Arch Dermatol. 2012;148(1):146. doi: 10.1001/archdermatol.2011.618. [DOI] [PubMed] [Google Scholar]
- 25.Puig S, Cecilia N, Malvehy J. Dermoscopic criteria and basal cell carcinoma. G Ital Dermatol Venereol. 2012;147(2):135–40. [PubMed] [Google Scholar]