Abstract
Purpose
Joubert syndrome is a genetic disorder characterized by hypoplasia of the midline cerebellum and deficiency of crossed connections between neural structures in the brain stem that control eye movements. The goal of the study was to quantify the eye movement abnormalities that occur in Joubert syndrome.
Methods
Eye movements were recorded in response to stationary stimuli and stimuli designed to elicit smooth pursuit, saccades, optokinetic nystagmus (OKN), vestibulo-ocular reflex (VOR), and vergence using video-oculography or Skalar search coils in 8 patients with Joubert syndrome. All patients underwent high-resolution magnetic resonance imaging (MRI).
Results
All patients had the highly characteristic molar tooth sign on brain MRI. Six patients had conjugate pendular (n = 4) or see-saw nystagmus (n = 2); gaze holding was stable in four patients. Smooth-pursuit gains were 0.28 to 1.19, 0.11 to 0.68, and 0.33 to 0.73 at peak stimulus velocities of 10, 20, and 30 deg/s in six patients; smooth pursuit could not be elicited in four patients. Saccade gains in five patients ranged from 0.35 to 0.91 and velocities ranged from 60.9 to 259.5 deg/s. Targeted saccades could not be elicited in five patients. Horizontal OKN gain was uniformly reduced across gratings drifted at velocities of 15, 30, and 45 deg/s. VOR gain was 0.8 or higher and phase appropriate in three of seven subjects; VOR gain was 0.3 or less and phase was indeterminate in four subjects.
Conclusions
The abnormalities in gaze-holding and eye movements are consistent with the distributed abnormalities of midline cerebellum and brain stem regions associated with Joubert syndrome.
Joubert syndrome (JS) is a genetic disorder characterized by developmental delay, hypotonia, ataxia, episodic breathing difficulties in infancy, and eye movement abnormalities.1–6 Brain magnetic resonance imaging (MRI) reveals the characteristic molar tooth sign, a term that refers to the appearance of the cerebral spinal fluid (CSF)-filled interpeduncular fossa, hypoplasia of the cerebellar vermis, and horizontally oriented and thick superior cerebellar peduncles.7–11 Histopathologic studies show loss of Purkinje cells and deep cerebellar nuclei, faulty decussation of the superior cerebellar peduncle, and hypoplasia of the inferior olives and other brain stem nuclei.2–13 Each of these cerebellar and brain stem regions contain neuronal ensembles that influence specific types of oculomotor behavior.14–16 Therefore, characterization of the eye movement abnormalities in JS should provide functional information about the specific oculomotor structures that underlie them. Investigators have reported qualitative abnormalities of gaze-holding and conjugate eye movements in JS.1,2,4,7,17–19 In this study, we used video-oculography or scleral search coil techniques to systematically measure conjugate eye movements in 10 subjects with JS and characterize the oculomotor phenotype(s) in JS and related disorders.
Subjects and Methods
Ten subjects with JS (newborn to 10 years of age) were evaluated in the Genetics and Ophthalmology Clinics at Children's Hospital and Regional Medical Center. Diagnosis of JS was established based on clinical evidence of hypotonia, developmental delay, apnea and/or tachypnea in infancy, ataxia (three of four clinical features), and brain MRI evidence of the molar tooth sign.
All subjects had complete eye examinations including quantitative acuity assessments. Preverbal and nonverbal children had acuities measured with Teller Acuity Cards (TACs). Verbal children had acuities measured with HOTV, Allen, or Snellen optotypes.
MRI scans were performed on a scanner at 1.5 Tesla (Siemens, Iselin, NJ). All scans included T1-weighted sequences and imaging in axial, coronal, and sagittal planes (3-mm thickness). Additional imaging sequences included T2-weighted, T2/FLAIR, and proton density sequences.
The study adhered to the tenets of the Declaration of Helsinki.
Eye Movement Testing
Eye movement recordings were performed during binocular viewing in eight children with binocular infrared video-oculography (VOG; Sensorimotoric Instruments [SMI], Berlin) and in two infants with search coils (Skalar, Delft, The Netherlands) in a magnetic field (CNC Engineering, Seattle, WA). Temporal resolution for VOG and search coils is 60 and 1000 Hz, respectively. The spatial resolution for VOG is 0.2°. Subjects sat independently or in a parent's lap, with the head manually restrained. They viewed a back-projected visual stimulus on a screen subtending approximately 60° at a fixation distance of 60 to 80 cm. Gaze holding was measured in primary gaze in the dark and while fixating a point target at eccentricities of 15° up, down, right, and left. To elicit saccades, we pseudorandomly stepped the target between 5° and 20° horizontally or vertically. Smooth pursuit was elicited by moving a point target sinusoidally ±10° along the horizontal meridian at peak velocities of 10, 20, and 30 deg/s. Optokinetic nystagmus (OKN) was elicited by drifting square wave gratings with a spatial frequency of 0.1 cyc/deg and >80% contrast horizontally or vertically on a screen at constant velocities of 15, 30, and 45 deg/s. Vestibulo-ocular reflex (VOR) was elicited by rotating the child ±10° in complete darkness about an earth vertical axis at frequencies of 0.16, 0.32, and 0.50 Hz deg/s.
Data Analysis
The VOG analysis program calculates eye position in space by tracking the center of the pupil and identifies the presence of an eye movement on the basis of preselected criterion. For saccades, we allowed a maximum latency of 300 ms, from the target step, a minimum peak velocity of 50 deg/s, and a velocity of 10 deg/s to detect saccade onset. For analysis we selected epochs of pursuit eye movement with a Vmax of 50 deg/s. Each cycle was analyzed off-line for gain and phase after removing saccades and movement artifact. For OKN, the following criteria were selected: minimum slow phase amplitude, 1°; minimum velocity of fast phase, 50 deg/s; and minimum and maximum duration of slow phase, 50 and 1000 ms, respectively. For coil recording, the analog data stream was digitized (CED 1401) and analyzed off-line using commercial (Spike 2, MatLab; The MathWorks, Natick, MA) and locally written analysis software. Determination of gain was problematic for patients with pendular nystagmus. For saccades, final eye position represented the average position across the postsaccadic horizontal oscillations. For smooth pursuit and VOR, eye velocity represented a sinusoidal fit of the combined eye movement and superimposed nystagmus by least squares regression. For OKN, the slow phase velocity represented the additive or subtractive sum of the OKN component and the superimposed nystagmus.
A report of the preliminary results of this work was published in abstract form (Weiss AH, et al. IOVS 2004;45:ARVO E-Abstract 2324).
Results
Clinical Data
Table 1 shows the relevant ocular and systemic findings for 10 subjects with JS evaluated between infancy and 10 years of age. Subjects 1, 2, 3, and 4 were evaluated before 5 months of age because of pendular nystagmus and lack of visual orienting behavior. These infants showed no response to TACs, but acuities were measurable at an older age. All subjects with pendular or see-saw nystagmus had mild to moderate reductions of acuity relative to the age-matched control subjects. Subject 3, with severely reduced acuity, had a chorioretinal coloboma without involvement of the macula and optic nerve. Subjects 4 and 6, with asymmetric acuity loss, had strabismic amblyopia in the nonpreferred eye. Refractive errors ranged from +4.00 to −15.00 D. Full-field electroretinograms (ERGs) were performed in 2 of 10 subjects. Subject 10 showed severely reduced photopic and scotopic responses; subject 5 had normal ERG responses. Subjects 1 and 10 had mutations in the AHI1 gene, whereas subject 9 had a homozygous NPHP1 deletion.
Table 1.
Clinical and Genetic Findings
| Subject | Age* (y)/Sex | Additional Findings† | Mutation Testing | Visual Acuity‡ RE/LE | Alignment (Primary Gaze) | Nyatagmus | Fundus |
|---|---|---|---|---|---|---|---|
| 1 | 0.2/F | High myopia (−10.50 SE) | AMI1 W420X/IVS8 (−2 A>G) | 20/160 | Var XT, RHT | Pendular | Normal |
| 20/200 | |||||||
| 2 | 0.7/M | None | None | 20/300 | Ortho | Pendular | Normal |
| 20/300 | |||||||
| 3 | 0.8/F | Chorioretinal coloboma | No NPHP1 deletion | 20/1400 | 30E(T) | Pendular | Normal |
| 20/1400 | |||||||
| 4 | 1.8/F | Oculomotor paresis RE | No NPHP1 deletion | 20/600 | Var 20RXT | Pendular | Normal |
| <20/2700 | |||||||
| 5 | 2.5/F | High myopia (−15.00 SE) | None | 20/50 | 0–30 ET | See-saw | Pigmentary irregularities |
| 20/60 | |||||||
| 6 | 4.3/F | Duane's LE | None | 20/25 | Ortho | None | Normal |
| 20/60 | |||||||
| 7 | 4.9/M | Occipital encephalocele polydactyly, hydrocephalus | None | 20/130 | Ortho | None | Normal |
| 20/130 | |||||||
| 8 | 9.5/M | Duane's RE | No AHI1 mutations, No NPHP1 deletion | 20/20 | Ortho | None | Normal |
| 20/20 | |||||||
| 9 | 10.6/M | Renal failure | Homozygous NPHP1 deletion | 20/25 | Ortho | Cyclotorsional | Normal |
| 20/30 | |||||||
| 10 | 16.9/F | Diabetes type 1 | AHI1 K246X/L832X | 20/100 | 20 XT, RHT | See-saw | Pigmentary retinopathy |
| 20/30 |
Ortho, orthotropia; XT, exotropia; ET, esotropia; RHT, right hypertropia.
Age at which eye movement recording was performed.
Ocular and systemic findings in addition to developmental delay, hypotonia, and ataxia.
Acuity when eye movement recording was performed.
Imaging
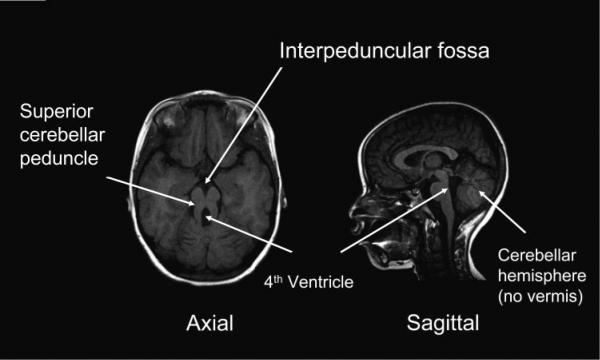
Standard clinical MRIs were reviewed by a pediatric neuroradiologist (DS) and a developmental pediatrician (DD). Imaging uniformly revealed a small or absent cerebellar vermis, horizontally orientated long, thick superior cerebellar peduncles, and an abnormally deep interpeduncular fossa (Fig. 1). Although individual nuclei could not be identified, the region containing the deep cerebellar nuclei was abnormal in all subjects except subject 9 (Table 2). The pons was hypoplastic in 3 of 10 subjects. Subject 3 had generalized pontine hypoplasia, and subjects 4 and 7 had a small pons in the rostral- caudal and axial dimensions, respectively (Table 2). The midbrain tectum, middle and inferior cerebellar peduncles, and medulla appeared normal by imaging criteria. The nodulus and uvula were uniformly small or absent. Cortical structures were normal in all subjects except 3 in whom periventricular heterotopias were present. Subject 7 had an occipital encephalocele and hydrocephalus.
Figure 1.
T1-weighted MRI in T1-weighted image in Joubert Syndrome. Axial images show the prominent interpeduncular fossa and vertically oriented superior cerebellar peduncles. Sagittal images show the enlarged fourth ventricle with high take-off of the roof and absence of the cerebellar vermis.
Table 2.
MRI Findings and Eye Movements
| Subject | Vermis | Deep Cerebellar Nuclei | Flocculus | Pons | Smooth Pursuit | Saccade | HOKN | VOR |
|---|---|---|---|---|---|---|---|---|
| 1 | Hypoplastic (severe) | Region abnormal | Present bilaterally | Normal | + | + | + | + |
| 2 | Hypoplastic (severe) | Region abnormal | Present bilaterally | Normal | + | + | + | ND |
| 3* | Absent | Region abnormal | Small or absent bilaterally | Hypoplastic | + | + | + | + |
| 4 | Hypoplastic (severe) | Region abnormal | Small or absent bilaterally | Hypoplastic (axial) | + | + | ++ | ND |
| 5 | Hypoplastic (severe) | Region abnormal | Small or absent bilaterally | Normal | + | ++ | + | + |
| 6 | Hypoplastic (moderate) | Region abnormal | Right present; left small or absent | Normal | ++ | ++ | ++ | + |
| 7† | Absent | Region abnormal | Images inadequate | Hypoplastic (rostral-caudal) | + | + | + | ND |
| 8‡ | Hypoplastic (moderate) | Region abnormal | Small or absent bilaterally | Normal | ++++ | ++++ | + | ++++ |
| 9 | Hypoplastic (mild) | Normal | Images inadequate | Normal | ++ | ++ | +++ | ++++ |
| 10 | Absent | Region abnormal | Small or absent bilaterally | Normal | ++ | ++ | ++ | ++++ |
normal gain;
mild reduction in gain;
moderate reduction in gain;
severe reduction in gain; ND, no data.
Subject also had periventricular heterotopias.
Subject also had occipital encephalocele.
Subject had Duane syndrome.
Gaze Holding
Nystagmus was present in 7 of 10 subjects. Of these, subjects 1, 2, 3, and 4 had horizontal, pendular nystagmus with a small vertical component that appeared before 6 months of age. Initially, there was a conjugate drift between the extreme right and left orbital positions of variable periodicity. With increasing age in individual subjects, we observed clinically that the amplitude of the drift progressively decreased as the frequency increased. Slow-phase velocities of the pendular nystagmus in both primary and eccentric gaze positions ranged from 0.8 to 10.0 deg/s horizontally and 1.0 to 14.7 deg/s vertically. Subject 4, with unilateral ocular motor palsy, fixated with the paretic eye rather than the eye with full oculomotor range. Two subjects had see-saw nystagmus with amplitudes of vertical dysconjugacy that averaged 15° and 60° and a horizontal conjugate component that was less than 3°. Slow-phase velocities for the vertical component ranged from 4.9 to 9.9 deg/s. Subject 9 had intermittent cyclotorsional nystagmus (amplitude < 5°).
Smooth Pursuit
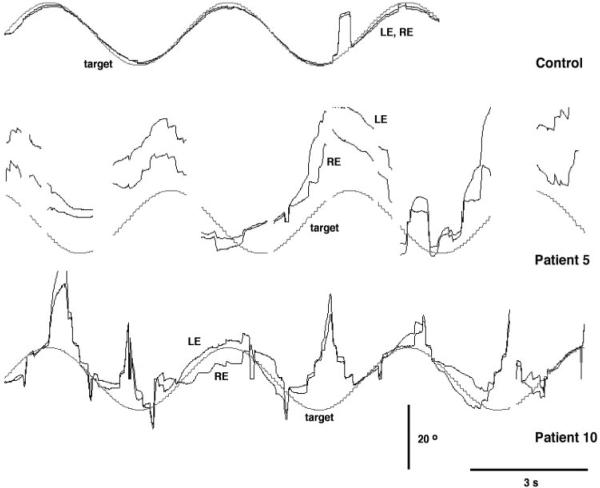
Representative horizontal smooth pursuit data are shown in Figure 2 for a 10-year-old control subject and two subjects with JS. The control recording shows smooth eye movements in which the gain approaches 1.0 (top trace). Note the one saccadic intrusion. Subject 5 predominantly tracked the stimulus with a sequence of hypometric saccades but seldom acquired the target (middle trace). Similarly, subject 10 inconsistently tracked the stimulus, although the elicited tracking response roughly matched the periodicity of the stimulus. However, there were abrupt transitions where the eye moved toward or away from the target (bottom trace).
Figure 2.
Representative smooth eye movements from an age-matched control (top trace), patient 5 (middle trace) and patient 10 (bottom trace). Upward traces indicate rightward movement, and downward traces indicate leftward movement.
Horizontal smooth pursuit was abnormal in all subjects (Fig. 3). Four of the 10 subjects failed to demonstrate any smooth pursuit across target velocities from 10 to 30 deg/s. Smooth pursuit was elicited in the remaining six subjects but average gains (eye velocity/target velocity) were low. Gains ranged from 0.28 to 1.19 (2–9 cycles) for cycles with peak stimulus velocities of 10 deg/s, 0.11 to 0.68 (4–9 cycles) for cycles with peak stimulus velocities of 20 deg/s, and 0.33 to 0.73 (4–14 cycles) for cycles with peak stimulus velocities of 30 deg/s. At the higher velocities, tracking became increasingly saccadic, and eye position never matched the target position except in primary gaze. In our laboratory, smooth pursuit gains are low in normal children less than 1 year of age, and >0.8 in older children.
Figure 3.
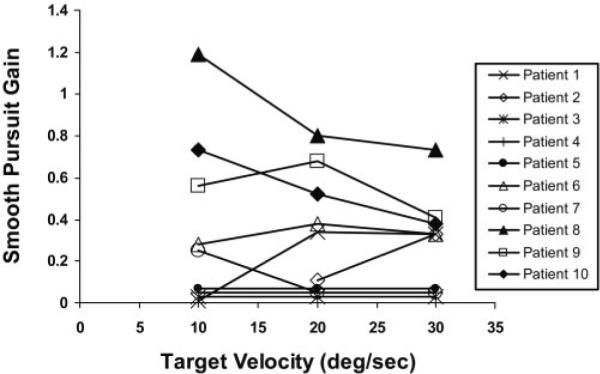
Smooth-pursuit gains for all subjects across stimulus velocities. The ratio of eye position and target position (Gain) during horizontal sinusoidal motion of a target moving at peak velocities of 10, 20, and 30 deg/s are plotted. Conventions for the direction of target and eye movement traces are the same as in Figure 2.
Saccades
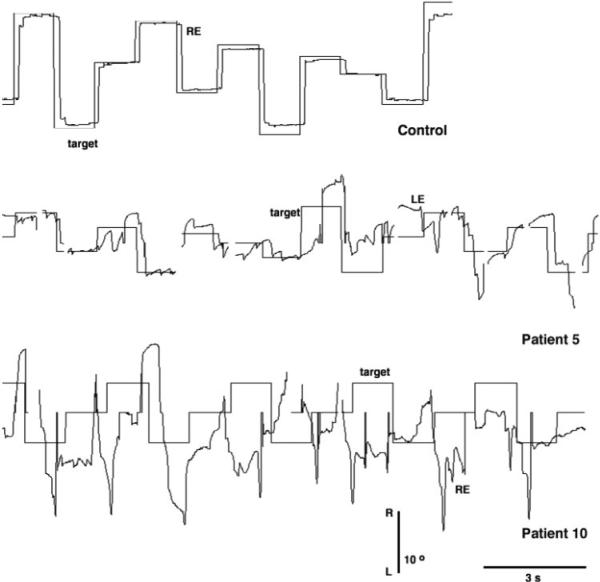
Figure 4 shows representative horizontal saccades for a 10-year-old control and for two subjects with JS. The control accurately acquired the target with a gain near 1.0. Subject 5 (middle trace) acquired the target with a sequence of hypometric saccades in some trials, but fell short of or overshot the target in other trials. Subject 10, the oldest subject, generated randomly directed saccades in response to a target stepped horizontally 5° to 20°. All saccades seemed to be nontargeted and unrelated to the stimulus. Multiple saccades were preceded by a slow eye movement, ending with a glissade and were followed by postsaccadic drift. Intrusion of a saccade with vertical component (not shown) was probably related to the underlying see-saw nystagmus.
Figure 4.
Representative horizontal saccades for control (top trace) and two patients with JS (middle and bottom traces). Conventions for the direction of target and eye movement traces are the same as in Figure 2.
Saccade abnormalities were observed in each of the 10 subjects (Fig. 5). Five subjects showed no targeted saccades in response to the stepped stimulus, and eye position seldom matched target position. Velocities of nontargeted saccades were low (<150 deg/s). The remaining five subjects generated targeted horizontal saccades. Gains to target steps of 5° to 20° ranged from 0.35 ± 0.19 to 0.91 ± 0.63. Post saccadic eye position occasionally held steady at eccentric target position but often drifted toward primary gaze position. Combined analysis of targeted and nontargeted saccades (12–53 saccades per subject) showed velocities that ranged from 60.9 ± 19.1 to 259.47 ± 159.0 deg/s. Latencies of targeted saccades ranged from 250 to 350 ms. For comparison, saccades are uniformly hypometric in normal children less than 1 year of age; gains are 0.8 and higher in older children.
Figure 5.
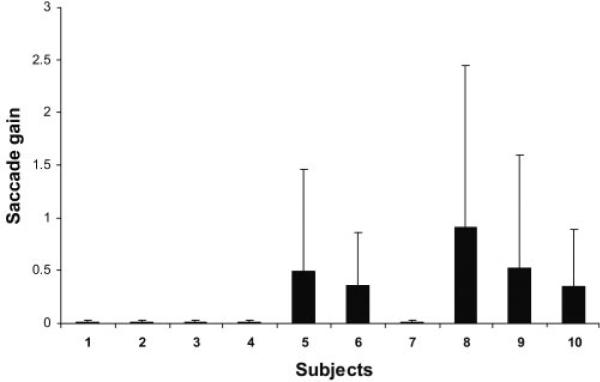
Horizontal saccade gains for all subjects. Plots of saccade gains ± SEM for 10 subjects. Gains near 0 represent subjects who failed to generate targeted saccades.
Optokinetic Nystagmus
Horizontal OKN (HOKN) gains were reduced across all stimulus velocities in 9 of 10 subjects (Fig. 6). For five subjects, gains were less than 0.1 across all stimulus velocities. In the remaining five subjects, gains ranged from 0.25 to 0.65 at a stimulus velocity of 15 deg/s and decreased at stimulus velocities of 30 and 45 deg/s. For comparison, OKN gains for normal young children are on average >0.7, with higher gains at lower stimulus velocities. Three of the five subjects with significant HOKN responses demonstrated the “locked-up” phenomenon in which both eyes remained in extreme orbital position for several seconds. When the subjects were observed clinically in head-free conditions, restoration of gaze to primary position was often initiated by a head thrust directed toward this extreme gaze position. When the VOR is active, thrusting the head ipsiversive to gaze induces ocular counterrotation, thereby centering the eye in the orbit. In subjects with congenital nystagmus, the nystagmus while viewing a moving full-field stimulus was variable but often indistinguishable from the subject's underlying horizontal nystagmus.
Figure 6.
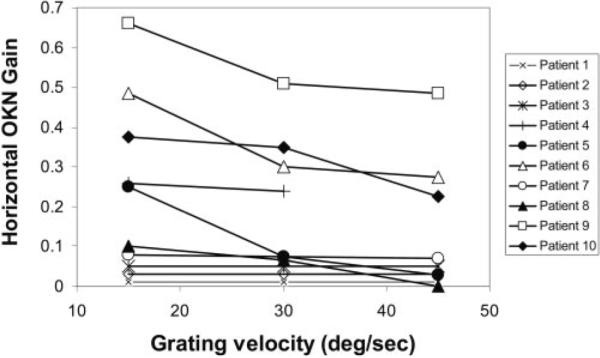
HOKN gains in all subjects. The ratio of slow phase eye velocity and target velocity to gratings drifted horizontally at 15, 30, and 45 deg/s are plotted. Gains represent averages of OKN responses to gratings drifted to the right and to the left.
Vestibulo-ocular Reflex
Seven of the 10 subjects tolerated testing of the horizontal VOR (Fig. 7). Of these, two subjects failed to demonstrate VOR at any chair rotation frequency, and two subjects had VOR with a gain of less than 0.4. The remaining three subjects had gains of 0.8 or higher in response to angular rotation between 0.16 and 0.50 Hz (normal range 0.8 –1.0).20 The corresponding phase of the VOR ranged from 155.7° to 203.8° (2–4 cycles) at a rotation frequency of 0.16 Hz, 194.0° to 199.0° (9 cycles) at rotation a frequency of 0.32 Hz, and 157.5° to 223.0° (9–13 cycles) at a rotation frequency of 0.50 Hz (normal range, 177°–186°).20 For comparison VOR gains in normal children less than 1 year of age are near 1.0 and are adult-like in older children.
Figure 7.
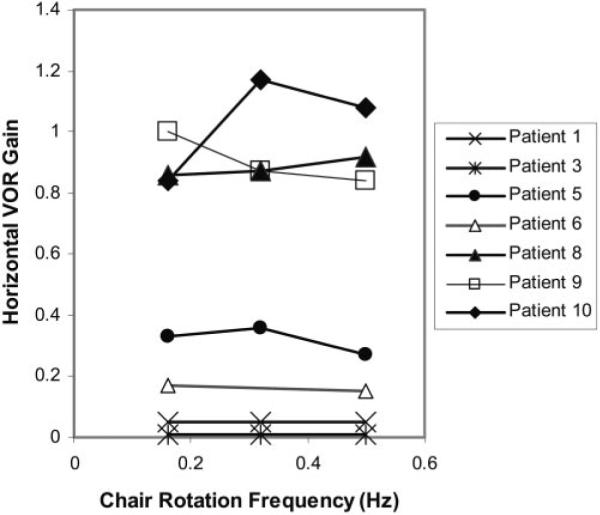
Horizontal VOR gains. The ratio of slow-phase eye velocity and horizontal chair rotation to peak velocities of 10, 20, and 30 deg/s are plotted. Gains represent averages of horizontal VOR responses to chair rotation to the right and to the left.
Table 2 summarizes the anatomic abnormalities revealed by MRI and the oculomotor findings for each subject. Overall, MRI evidence of vermis hypoplasia, abnormalities of the deep cerebellar nuclei, and a hypoplastic or absent flocculus was associated with reduced gains for smooth pursuit, saccades, OKN, and VOR. Since individual deep cerebellar nuclei were not visualized by MRI, and the flocculus was described as present or absent, anatomic characterizations of these structures were limited. Therefore, the specific neural structures within each of these regions involved in the mediation of conjugate eye movements were not visualized by MRI. In general, MRI evidence of a normal versus hypoplastic pons was predictive of the severity of the eye movement abnormality. Each of the four patients with a normal pons had normal to moderately reduced gains for smooth pursuit, saccades, and HOKN. In comparison, three of six patients with a hypoplastic pons showed severe reductions in these conjugate eye movements. Furthermore, within a given patient, the severity of reduction in gain tended to be similar across most types of conjugate eye movements. The only exception to this trend was patient 8 who had Duane syndrome.
DISCUSSION
JS was uniformly associated with a spectrum of ocular motor deficits consistent with malformation of midline structures and crossed neural pathways of the cerebellum and brain stem. Brain MRI provided gross anatomic evidence for hypoplasia of the cerebellar vermis, nodulus, flocculus, deep cerebellar nuclei, and pons. However, quantitative eye movements provided a functional measure of specific nuclei or regions within each of these structures because they influence eye movements in precise ways. All subjects in this study had abnormalities of saccades, smooth pursuit, OKN, and VOR or were unable to consistently generate a specific eye movement in response to the appropriate stimulus. The presence of eye movement abnormalities implies that discrete neural structures were responsible for the movement that were not delineated by standard MRI. Within the cerebellum and brain stem, neural structures are interconnected by neural pathways that cross the midline. Quantitative eye movements also provided indirect evidence for decussation abnormalities of neural pathways that were not visualized by MRI. Therefore, eye movement studies complement the MRI findings by providing indirect functional evidence of neuroanatomic abnormalities within the brain stem and cerebellum that occur in JS.
Gaze-holding instabilities were an early manifestation of JS in six subjects. The appearance of a conjugate pendular nystagmus before 6 months of age is consistent with infantile nystagmus. However, the systemic findings and the abnormalities in conjugate eye movements distinguish the nystagmus associated with JS from other types of infantile nystagmus. Although neural structures responsible for gaze holding are widely distributed, the horizontal pendular nystagmus recorded in four subjects resembles the periodic alternating nystagmus described with lesions of the uvula and nodulus.15,21 These cerebellar structures receive crossed and uncrossed inputs from vestibular, pontine, and inferior olivary nuclei that transmit velocity signals elicited by sustained whole-body rotation (VOR) and full-field motion (OKN).15,21,22 Integration of these velocity signals into eye position underlies stable gaze holding.15,16,23,24 Likewise, the see-saw nystagmus observed in two subjects is associated with abnormalities of the interstitial nucleus of Cajal (INC) located in the midbrain.24–27 The INC is a major component of the neural integrator that converts vertical and torsional eye velocity signals transmitted by crossed and uncrossed inputs into position signals.23,25,28 Selective loss of the crossed inputs in subjects with JS could lead to an imbalance of velocity inputs resulting in instabilities of gaze holding. In support of this notion, histopathologic and neuroimaging studies of subjects with JS indicate defective decussation of the superior cerebellar peduncles and pyramidal tracts.7,13,29–31
Saccadic abnormalities were observed in all subjects. Half either failed to generate saccades or generated spontaneous saccades that showed no consistent relationship with the direction, amplitude or timing of the stimulus. The remaining half generated targeted saccadic eye movements that were either hypometric or hypermetric. Normal infants make hypometric and slow saccades, but accuracy and velocity improve dramatically during the first 6 months of age (Phillips JO, et al. IOVS 1997;38:ARVO Abstract 652).32 The presence of targeted but dysmetric saccades implicates the oculomotor vermis (OMV), caudal fastigial nucleus (CFN), inferior olive, and other saccade-related brain stem structures.14,33,34 Purkinje cells in the OMV provide only inhibitory inputs to the CFN. During a saccade, there are complex interactions of OMV and CFN neurons that lead to acceleration or deceleration of the saccade at various points in its trajectory. Therefore, lesions of these structures can produce hypometric or hypermetric saccades. However, the presence of lesions would not explain the absence of targeted saccades observed in half of our subjects. Slowing and absence of saccades implicates structures downstream from the cerebellum, especially burst neurons in the pontine and medullary reticular formation and extraocular motor neurons. Misdirection of saccades possibly implicates abnormalities of crossed connections between these brain stem structures but may reflect attentional deficits or abnormalities of other saccade-related cortical regions.35,36 The motor errors observed in JS may be further compounded by the loss of climbing fiber input to the Purkinje cells. The inferior olivary nuclei, known to be hypoplastic in JS, are the only source of climbing fiber inputs to the Purkinje cells, and their activity may signal errors in eye position after saccades.37,38
Smooth-pursuit eye movements were abolished or gains severely reduced in all subjects. Abnormalities of smooth pursuit were not simply due to age-related immaturities of motion processing and attention. Infants display smooth eye movement from 4 month of age at velocities ranging from 8 to 24 deg/s, respectively.39 Such profound deficits in smooth pursuit likely arise from distributed abnormalities of the cerebellar and brain stem structures responsible for generating smooth pursuit. Visual cortical areas involved in motion processing project to and encode the dorsolateral pontine nuclei (DLPN) and nucleus reticularis tegmenti pontis (NRTP) with velocity information.40–44 These pontine nuclei then relay velocity signals to the cerebellar structures including the OMV, flocculus, and paraflocculus.45–47 which in turn project to vestibular nuclei. The absence of smooth pursuit in some of our subjects is consistent with abolished smooth pursuit after complete cerebellectomy in the monkey.48 The presence of smooth pursuit with reduced gains in the remaining subjects parallels the gain reductions reported with localized lesions of the OMV49,50 or flocculus/paraflocculus in the cerebellum51 and the DLPN in the brain stem.52 Abnormalities of cortical motion processing provide another explanation in the subset of subjects with retinal degeneration, since smooth pursuit depends on normal foveal inputs. When smooth pursuit gain is low, the ocular motor system can adapt by substituting a saccade or head movement to track the target. Clinically, we observed that subjects who could generate saccades in the appropriate direction relied more on saccadic tracking, whereas those who could not depended on head movements.
HOKN under binocular viewing was uniformly abnormal. Although immature in normal infants, binocular OKN can be elicited in infants and reaches gains (eye velocity/grating velocity) near 1.0 for velocities up to 34 deg/s by 6 months of age.53,54 The slow component of optokinetic (OKN) responses was either absent (two subjects) or had reduced gains, especially at higher stimulus velocities (eight subjects). OKN is a reflexive conjugate eye movement that stabilizes gaze in response to retinal image motion induced by motion in the environment. As a result of its link to motion stimuli, OKN shares pathways involved with the processing of smooth pursuit and vestibular signals.15,55,56 The reduced OKN gains observed in most of our subjects in whom OKN was recorded over relatively short intervals may reflect functional abnormalities of the flocculus and paraflocculus. Complete absence of slow-phase HOKN in two subjects implicates all cerebellar or brain stem structures in the OKN pathway.57–59 Similar to other investigators, we observed in three of five subjects that during horizontal OKN the eyes conjugately drifted to extreme orbital position where they remained locked up for up to 10 seconds.4,7 Inability of these subjects to initiate or generate targeted saccades that recenter the eye in primary gaze may underlie this phenomenon.
The finding of normal angular VOR in some subjects, indicates that in this subset of subjects the direct VOR pathways are intact.15 The direct VOR includes the vestibular end organs that encode angular head velocity, interneurons in the vestibular nucleus, and extraocular motoneurons.15 The low VOR gains and abnormal phase relationships in the remaining subset of subjects implicate any of these structures or functional abnormalities of the vestibulocerebellum. The VOR is subserved by pathways through the flocculus and paraflocculus that terminate on target neurons in the brain stem.60,61 Previous investigators reported that visually enhanced VOR elicited by rapid head thrust or passive head rotation at lower velocities was qualitatively normal in JS.4,7 The disparate results probably reflect the fact that we quantified angular VOR in the dark, unlike previous studies in which visually enhanced VOR in the light was assessed qualitatively.
In general, the MRI studies were consistent with the observed abnormalities of conjugate eye movements. The three patients below 1 year of age did not generate volitional (smooth pursuit or saccades) or reflexive eye movements (VOR and OKN). Although the gains for smooth pursuit, saccades, and HOKN are low, children in this age group normally generate each of these eye movements, and the VOR gain approaches 1.0.20,32 The lack of reflexive eye movements is consistent with functional abnormalities within the brain stem and argues against delays in maturation of the ocular motor system. Although the MRI showed pontine hypoplasia in only one of these infants, the eye movement recordings revealed functional evidence of brain stem abnormalities in all three infants. As further evidence, each of these patients came to medical attention early in life because of respiratory difficulties, hypotonia and failure to thrive. In our laboratory, children older than 1 year normally show adultlike gains in all conjugate eye movements. Therefore, the presence of voluntary and reflexive eye movements showing mild to moderate reductions in gains is consistent with involvement of midline cerebellar structures, with relative sparing of brain stem structures. In comparison, only two of the three patients with severely reduced gains in volitional and reflexive eye movements had MRI evidence of pontine hypoplasia. By providing a functional measure of each conjugate eye movement, oculomotor testing provided information about specific neural structures within the cerebellum and brain stem that were below the detection threshold of the MRI. The consistent correspondence between the reductions in gain across eye movement subtypes reflects the spatial proximity and shared functions of oculomotor structures within the cerebellum and brain stem.
In earlier reports, JS was considered to be a distinct clinical entity. More recently, this disorder has been found to be genetically heterogeneous with mutations in NPHP1, AHI1, CEP290, RPGRIPI, MKS3, and CCD2A, identified in approximately 40% of patients.62–69 Each genotypic variant of JS may be associated with distinctive developmental and functional abnormalities of the cerebellum and brain stem. Although these genetic disorders are heterogeneous, they share a functional abnormality of the primary cilium/basal body organelle. To describe these genetic disorders, which share overlapping features, the term Joubert syndrome and related disorders (JSRDs) is used. In this study, quantitative analysis of eye movements indicated large individual differences between subjects with JS that were not delineated by MRI. Future studies may benefit from the combined use of a multivariate discriminant analysis of the ocular motor phenotype in a larger cohort of affected patients and higher resolution MRI to help characterize the neuroanatomic and functional abnormalities associated with specific genotypes of JS.
Acknowledgments
Supported by an unrestricted grant from the Peter LeHaye, Barbara Anderson, and William O. Rogers Endowment Funds.
Footnotes
The work presented in this manuscript was performed at Children's Hospital and the University of Washington Medical Centers.
Disclosure: A.H. Weiss, None; D. Doherty, None; M. Parisi, None; D. Shaw, None; I. Glass, None; JO. Phillips, None
References
- 1.Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969;19(9):813–825. doi: 10.1212/wnl.19.9.813. [DOI] [PubMed] [Google Scholar]
- 2.Boltshauser E, Isler W. Joubert syndrome: episodic hyperpnea, abnormal eye movements, retardation and ataxia, associated with dysplasia of the cerebellar vermis. Neuropadiatrics. 1977;8(1):57–66. doi: 10.1055/s-0028-1091505. [DOI] [PubMed] [Google Scholar]
- 3.King MD, Dudgeon J, Stephenson JB. Joubert's syndrome with retinal dysplasia: neonatal tachypnoea as the clue to a genetic brain-eye malformation. Arch Dis Child. 1984;59(8):709–718. doi: 10.1136/adc.59.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert SR, Kriss A, Gresty M, Benton S, Taylor D. Joubert syndrome. Arch Ophthalmol. 1989;107(5):709–713. doi: 10.1001/archopht.1989.01070010727035. [DOI] [PubMed] [Google Scholar]
- 5.Maria BL, Boltshauser E, Palmer SC, Tran TX. Clinical features and revised diagnostic criteria in Joubert syndrome. J Child Neurol. 1999;14(9):583–590. doi: 10.1177/088307389901400906. discussion 590–1. [DOI] [PubMed] [Google Scholar]
- 6.Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15(5):511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- 7.Maria BL, Hoang KB, Tusa RJ, et al. “Joubert syndrome” revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol. 1997;12(7):423–430. doi: 10.1177/088307389701200703. [DOI] [PubMed] [Google Scholar]
- 8.Maria BL, Quisling RG, Rosainz LC, et al. Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol. 1999;14(6):368–376. doi: 10.1177/088307389901400605. [DOI] [PubMed] [Google Scholar]
- 9.Quisling RG, Barkovich AJ, Maria BL. Magnetic resonance imaging features and classification of central nervous system malformations in Joubert syndrome. J Child Neurol. 1999;14(10):628–35. doi: 10.1177/088307389901401002. discussion 669–672. [DOI] [PubMed] [Google Scholar]
- 10.Maria BL, Bozorgmanesh A, Kimmel KN, Theriaque D, Quisling RG. Quantitative assessment of brainstem development in Joubert syndrome and Dandy-Walker syndrome. J Child Neurol. 2001;16(10):751–758. doi: 10.1177/088307380101601008. [DOI] [PubMed] [Google Scholar]
- 11.Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125(2):125–34. doi: 10.1002/ajmg.a.20437. discussion 117. [DOI] [PubMed] [Google Scholar]
- 12.Friede RL, Boltshauser E. Uncommon syndromes of cerebellar vermis aplasia. I: Joubert syndrome. Dev Med Child Neurol. 1978;20(6):758–763. doi: 10.1111/j.1469-8749.1978.tb15307.x. [DOI] [PubMed] [Google Scholar]
- 13.Yachnis AT, Rorke LB. Neuropathology of Joubert syndrome. J Child Neurol. 1999;14(10):655–659. doi: 10.1177/088307389901401006. discussion 669–672. [DOI] [PubMed] [Google Scholar]
- 14.Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- 15.Leigh RJ, Zee DS. The Neurology of Eye Movements. 3rd ed. Oxford University Press; New York: 1999. [Google Scholar]
- 16.Robinson FR, PhD, Phillips JO, PhD, Weiss AH. Animal oculomotor data illuminate cerebellum-related eye movement. In: LeDoux M, editor. Animal Models of Movement Disorders. Elsevier; New York: 2004. pp. 1–21. [Google Scholar]
- 17.Moore AT, Taylor DS. A syndrome of congenital retinal dystrophy and saccade palsy: a subset of Leber's amaurosis. Br J Ophthalmol. 1984;68(6):421–431. doi: 10.1136/bjo.68.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sztriha L, Al-Gazali LI, Aithala GR, Nork M. Joubert's syndrome: new cases and review of clinicopathologic correlation. Pediatr Neurol. 1999;20(4):274–281. doi: 10.1016/s0887-8994(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 19.Hodgkins PR, Harris CM, Shawkat FS, et al. Joubert syndrome: long-term follow-up. Dev Med Child Neurol. 2004;46(10):694–699. doi: 10.1017/s0012162204001161. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JO, Backous DD. Evaluation of vestibular function in young children. Otolaryngol Clin North Am. 2002;35(4):765–790. doi: 10.1016/s0030-6665(02)00062-2. [DOI] [PubMed] [Google Scholar]
- 21.Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228(4696):199–202. doi: 10.1126/science.3871968. [DOI] [PubMed] [Google Scholar]
- 22.Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J Comp Neurol. 1994;349(1):51–72. doi: 10.1002/cne.903490105. [DOI] [PubMed] [Google Scholar]
- 23.Fukushima K, Kaneko CR, Fuchs AF. The neuronal substrate of integration in the oculomotor system. Prog Neurobiol. 1992;39(6):609–639. doi: 10.1016/0301-0082(92)90016-8. [DOI] [PubMed] [Google Scholar]
- 24.Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Res. 1999;39(25):4286–4295. doi: 10.1016/s0042-6989(99)00142-x. [DOI] [PubMed] [Google Scholar]
- 25.Helmchen C, Rambold H, Fuhry L, Buttner U. Deficits in vertical and torsional eye movements after uni- and bilateral muscimol inactivation of the interstitial nucleus of Cajal of the alert monkey. Exp Brain Res. 1998;119(4):436–452. doi: 10.1007/s002210050359. [DOI] [PubMed] [Google Scholar]
- 26.Kanter DS, Ruff RL, Leigh RJ, Modic M. See-saw nystagmus and brainstem infarction: MRI findings. Neuroophthalmology. 1987;7(5):279–283. doi: 10.3109/01658108708996003. [DOI] [PubMed] [Google Scholar]
- 27.Halmagyi GM, Aw ST, Dehaene I, Curthoys IS, Todd MJ. Jerkwaveform see-saw nystagmus due to unilateral meso-diencephalic lesion. Brain. 1994;117:789–803. doi: 10.1093/brain/117.4.789. [DOI] [PubMed] [Google Scholar]
- 28.Crawford JD, Cadera W, Vilis T. Generation of torsional and vertical eye position signals by the interstitial nucleus of Cajal. Science. 1991;252(5012):1551–1553. doi: 10.1126/science.2047862. [DOI] [PubMed] [Google Scholar]
- 29.Parisi MA, Pinter JD, Glass IA, et al. Cerebral and cerebellar motor activation abnormalities in a subject with Joubert syndrome: functional magnetic resonance imaging (MRI) study. J Child Neurol. 2004;19(3):214–218. [PubMed] [Google Scholar]
- 30.Poretti A, Boltshauser E, Loenneker T, et al. Diffusion tensor imaging in Joubert syndrome. AJNR Am J Neuroradiol. 2007;28(10):1929–1933. doi: 10.3174/ajnr.A0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widjaja E, Blaser S, Raybaud C. Diffusion tensor imaging of midline posterior fossa malformations. Pediatr Radiol. 2006;36(6):510–517. doi: 10.1007/s00247-006-0146-x. [DOI] [PubMed] [Google Scholar]
- 32.Aslin RN, Salapatek P. Saccadic localization of visual targets by the very young human infant. Percept Psychophys. 1975;17:293–302. [Google Scholar]
- 33.Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol. 1993;70(5):1723–1740. doi: 10.1152/jn.1993.70.5.1723. [DOI] [PubMed] [Google Scholar]
- 34.Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70(5):1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- 35.Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3(12):952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- 36.Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142(4):439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- 37.Eccles JC, Llinas R, Sasaki K. The excitatory synaptic action of climbing fibers on the Purkinje cells of the cerebellum. J Physiol (Lond) 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soetedjo R, Fuchs AF. Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci. 2006;26(29):7741–7755. doi: 10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips JO, Finocchio DV, Ong L, Fuchs AF. Smooth pursuit in 1-to 4-month-old human infants. Vision Res. 1997;37(21):3009–3020. doi: 10.1016/s0042-6989(97)00107-7. [DOI] [PubMed] [Google Scholar]
- 40.Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5(3):825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May JG, Andersen RA. Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Exp Brain Res. 1986;63(2):265–278. doi: 10.1007/BF00236844. [DOI] [PubMed] [Google Scholar]
- 42.Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60(3):940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- 43.Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271(4):493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- 44.Mustari MJ, Fuchs AF, Wallman JR. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol. 1988;60(2):664–686. doi: 10.1152/jn.1988.60.2.664. [DOI] [PubMed] [Google Scholar]
- 45.Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41(3):733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol. 1994;72(6):2714–2728. doi: 10.1152/jn.1994.72.6.2714. [DOI] [PubMed] [Google Scholar]
- 47.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63(5):1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 48.Westheimer G, Blair SM. Oculomotor defects in cerebellectomized monkeys. Invest Ophthalmol. 1973;12(8):618–621. [PubMed] [Google Scholar]
- 49.Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiol. 1997;78(2):848–859. doi: 10.1152/jn.1997.78.2.848. [DOI] [PubMed] [Google Scholar]
- 50.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83(4):2047–2062. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 51.Zee DS, Yamazaki A, Butler PH, Gucer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- 52.May JG, Keller EL, Suzuki DA. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol. 1988;9(3):952–977. doi: 10.1152/jn.1988.59.3.952. [DOI] [PubMed] [Google Scholar]
- 53.Schor CM, Narayan V, Westall C. Postnatal development of optokinetic after nystagmus in human infants. Vision Res. 1983;23(12):1643–1647. doi: 10.1016/0042-6989(83)90178-5. [DOI] [PubMed] [Google Scholar]
- 54.Roy M-S, Lachapelle P, Lepore F. Maturation of the optokinetic nystagmus as a function of the speed of stimulation in fullterm and preterm infants. Clin Vis Sci. 1989;4(4):357–366. [Google Scholar]
- 55.Fuchs AF, Mustari MJ. The optokinetic response in primates and its possible neuronal substrate. Rev Oculomot Res. 1993;5:343–369. [PubMed] [Google Scholar]
- 56.Mustari MJ, Fuchs F, Kaneko CR, Robinson FR. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol. 1994;349(1):111–128. doi: 10.1002/cne.903490108. [DOI] [PubMed] [Google Scholar]
- 57.Heinen SJ, Keller EL. The function of the cerebellar uvula in monkey during optokinetic and pursuit eye movements: singleunit responses and lesion effects. Exp Brain Res. 1996;110(1):1–14. doi: 10.1007/BF00241368. [DOI] [PubMed] [Google Scholar]
- 58.Kaneko CR. Eye movement deficits following ibotenic acid lesions of the nucleus prepositus hypoglossi in monkeys II. Pursuit, vestibular, and optokinetic responses. J Neurophysiol. 1999;81(2):668–681. doi: 10.1152/jn.1999.81.2.668. [DOI] [PubMed] [Google Scholar]
- 59.Bense S, Janusch B, Vucurevic G, Bauermann T, et al. Brainstem and cerebellar fMRI-activation during horizontal and vertical optokinetic stimulation. Brain Res. 2006;174(2):312–323. doi: 10.1007/s00221-006-0464-0. [DOI] [PubMed] [Google Scholar]
- 60.Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- 61.Lisberger SG, Pavelko AT, Broussard DM. Responses during eye movements of brain stem neurons that receive monosynaptic inhibition from the flocculus and ventral paraflocculus in monkeys. J Neurophysiol. 1994;72:909–927. doi: 10.1152/jn.1994.72.2.909. [DOI] [PubMed] [Google Scholar]
- 62.Ferland RJ, Eyaid W, Collura RV, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36(9):1008–1013. doi: 10.1038/ng1419. published correction in Nat Genet. 2004; 36(10):1126. [DOI] [PubMed] [Google Scholar]
- 63.Dixon-Salazar T, Silhavy JL, Marsh SE, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75(6):979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parisi MA, Doherty D, Eckert ML, et al. AHI1 mutations cause both retinal dystrophy and renal cystic disease in Joubert syndrome. J Med Genet. 2006;43(4):334–339. doi: 10.1136/jmg.2005.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sayer JA, Otto EA, O'Toole JF, et al. The centrosomal protein nephrocystin-6 is mutated in syndrome and activates transcription factor ATF4. Nat Genet. 2006;38(6):674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 66.Baala L, Romano S, Khaddour R, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80(1):186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delous M, Baala L, Salomon R, Laclef C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39(7):875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 68.Arts HH, Doherty D, van Beersum SE, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39(7):882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 69.Gorden NT, Arts HH, Parisi MA, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83(5):559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]