Abstract
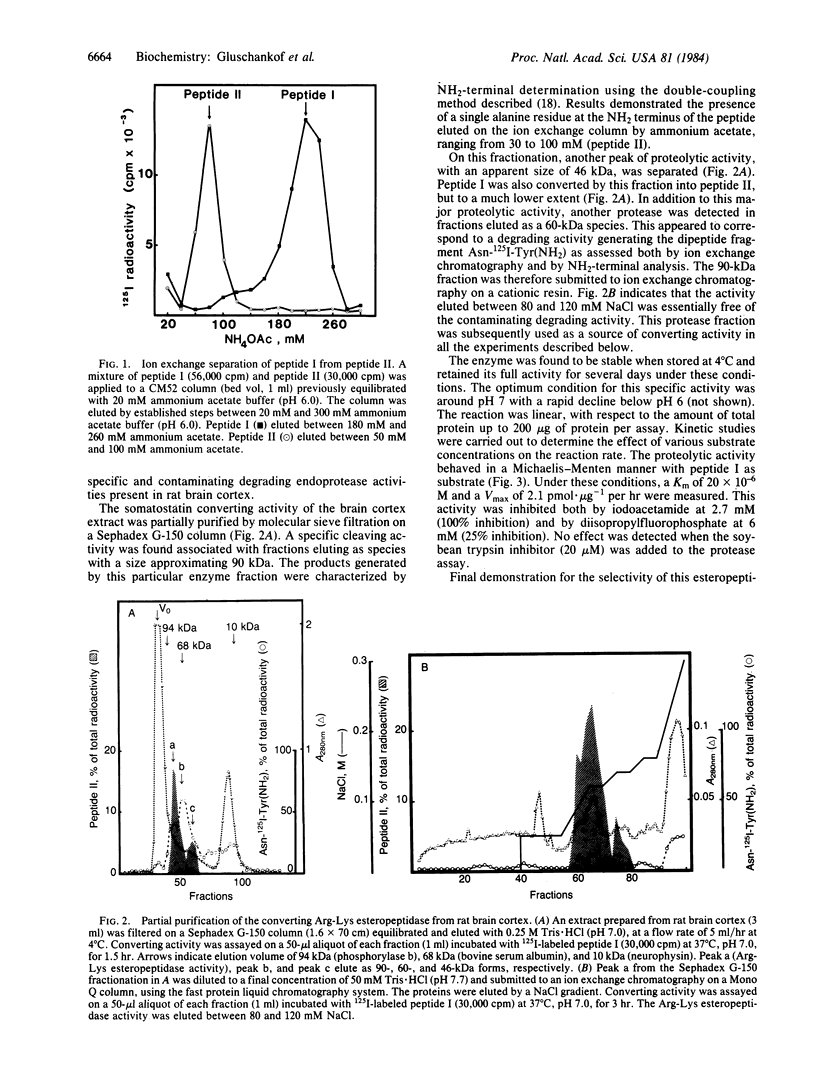
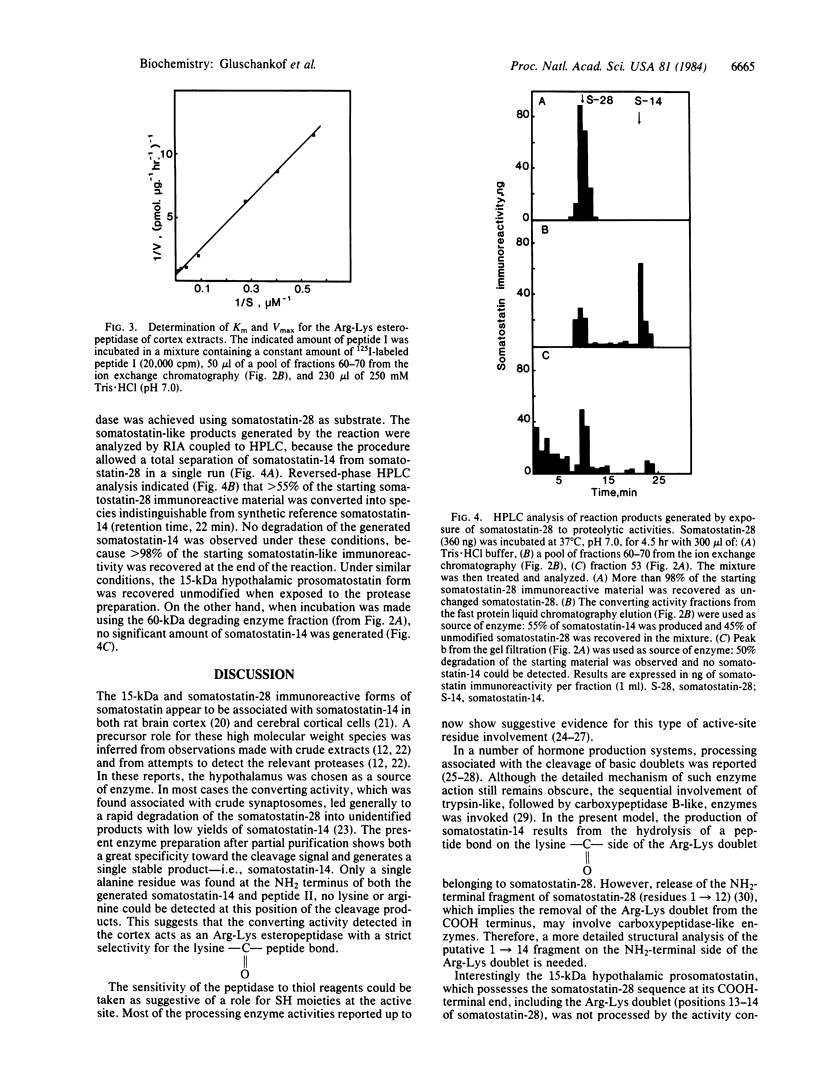
The post-translational proteolytic conversion of somatostatin-14 precursors was studied to characterize the enzyme system responsible for the production of the tetradecapeptide either from its 15-kDa precursor protein or from its COOH-terminal fragment, somatostatin-28. A synthetic undecapeptide Pro-Arg-Glu-Arg-Lys-Ala-Gly-Ala-Lys-Asn-Tyr(NH2), homologous to the amino acid sequence of the octacosapeptide at the putative Arg-Lys cleavage locus, was used as substrate, after 125I labeling on the COOH-terminal tyrosine residue. A 90-kDa proteolytic activity was detected in rat brain cortex extracts after molecular sieve fractionation followed by ion exchange chromatography. The protease released the peptide 125I-Ala-Gly-Ala-Lys-Asn-Tyr(NH2) from the synthetic undecapeptide substrate and converted somatostatin-28 into somatostatin-14 under similar conditions (pH 7.0). Under these experimental conditions, the product tetradecapeptide was not further degraded by the enzyme. In contrast, the purified 15-kDa hypothalamic precursor remained unaffected when exposed to the proteolytic enzyme under identical conditions. It is concluded that this Arg-Lys esteropeptidase from the brain cortex may be involved in the in vivo processing of the somatostatin-28 fragment of prosomatostatin into somatostatin-14, the former species being an obligatory intermediate in a two-step proteolytic mechanism leading to somatostatin-14.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoit R., Böhlen P., Ling N., Briskin A., Esch F., Brazeau P., Ying S. Y., Guillemin R. Presence of somatostatin-28-(1-12) in hypothalamus and pancreas. Proc Natl Acad Sci U S A. 1982 Feb;79(3):917–921. doi: 10.1073/pnas.79.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Willoughby J. O., Brazeau P., Martin J. B. Effects of brain lesions and hypothalamic deafferentation on somatostatin distribution in the rat brain. Endocrinology. 1977 Nov;101(5):1495–1502. doi: 10.1210/endo-101-5-1495. [DOI] [PubMed] [Google Scholar]
- Esch F., Böhlen P., Ling N., Benoit R., Brazeau P., Guillemin R. Primary structure of ovine hypothalamic somatostatin-28 and somatostatin-25. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6827–6831. doi: 10.1073/pnas.77.11.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D. J., Noe B. D., Bauer G. E., Quigley J. P. Characterization of the conversion of a somatostatin precursor to somatostatin by islet secretory granules. Diabetes. 1980 Aug;29(8):593–599. doi: 10.2337/diab.29.8.593. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983 Sep 25;258(18):10950–10955. [PubMed] [Google Scholar]
- Gomez S., Morel A., Nicolas P., Cohen P. Regional distribution of the Mr 15,000 somatostatin precursor, somatostatin-28 and somatostatin-14 in the rat brain suggests a differential intracellular processing of the high molecular weight species. Biochem Biophys Res Commun. 1983 Apr 15;112(1):297–305. doi: 10.1016/0006-291x(83)91830-2. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Joseph-Bravo P., Charli J. L., Sherman T., Boyer H., Bolivar F., McKelvy J. F. Identification of a putative hypothalamic mRNA coding for somatostatin and of its product in cell-free translation. Biochem Biophys Res Commun. 1980 Jun 16;94(3):1004–1012. doi: 10.1016/0006-291x(80)91334-0. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Higher molecular weight forms of immunoreactive somatostatin in mouse hypothalamic extracts: evidence of processing in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6004–6008. doi: 10.1073/pnas.76.11.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Characterization of pro-opiocortin-converting activity in purified secretory granules from rat pituitary neurointermediate lobe. Proc Natl Acad Sci U S A. 1982 Jan;79(1):108–112. doi: 10.1073/pnas.79.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A., Gluschankof P., Gomez S., Fafeur V., Cohen P. Characterization of a somatostatin-28 containing the (Tyr-7, Gly-10) derivative of somatostatin-14: a terminal active product of prosomatostatin II processing in anglerfish pancreatic islets. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7003–7006. doi: 10.1073/pnas.81.22.7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A., Lauber M., Cohen P. Selective processing of the 15 000 Mr prosomatostatin by mouse hypothalamic extracts releases the tetradecapeptide. FEBS Lett. 1981 Dec 28;136(2):316–318. doi: 10.1016/0014-5793(81)80643-6. [DOI] [PubMed] [Google Scholar]
- Morel A., Nicolas P., Cohen P. Evidence for a predominant form of Mr = 15,000 prosomatostatin in the mouse hypothalamus. Relationship with somatostatin-14 and -28. J Biol Chem. 1983 Jul 10;258(13):8273–8276. [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Pradayrol L., Jörnvall H., Mutt V., Ribet A. N-terminally extended somatostatin: the primary structure of somatostatin-28. FEBS Lett. 1980 Jan 1;109(1):55–58. doi: 10.1016/0014-5793(80)81310-x. [DOI] [PubMed] [Google Scholar]
- Robbins R. J., Reichlin S. Somatostatin biosynthesis by cerebral cortical cells in monolayer culture. Endocrinology. 1983 Aug;113(2):574–581. doi: 10.1210/endo-113-2-574. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Huang W. Y., Chang R. C., Arimura A., Redding T. W., Millar R. P., Hunkapiller M. W., Hood L. E. Isolation and structure of pro-somatostatin: a putative somatostatin precursor from pig hypothalamus. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4489–4493. doi: 10.1073/pnas.77.8.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Villarreal J., Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981 Mar 31;20(7):1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Patel Y. C. Processing of somatostatin-28 to somatostatin-14 by rat hypothalamic synaptosomal membranes. Life Sci. 1983 Sep 26;33(13):1241–1247. doi: 10.1016/0024-3205(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Zingg H. H., Patel Y. C. Processing of synthetic somatostatin-28 and a related endogenous rat hypothalamic somatostatin-like molecule by hypothalamic enzymes. Life Sci. 1982 Feb 7;30(6):525–533. doi: 10.1016/0024-3205(82)90265-x. [DOI] [PubMed] [Google Scholar]



