Abstract
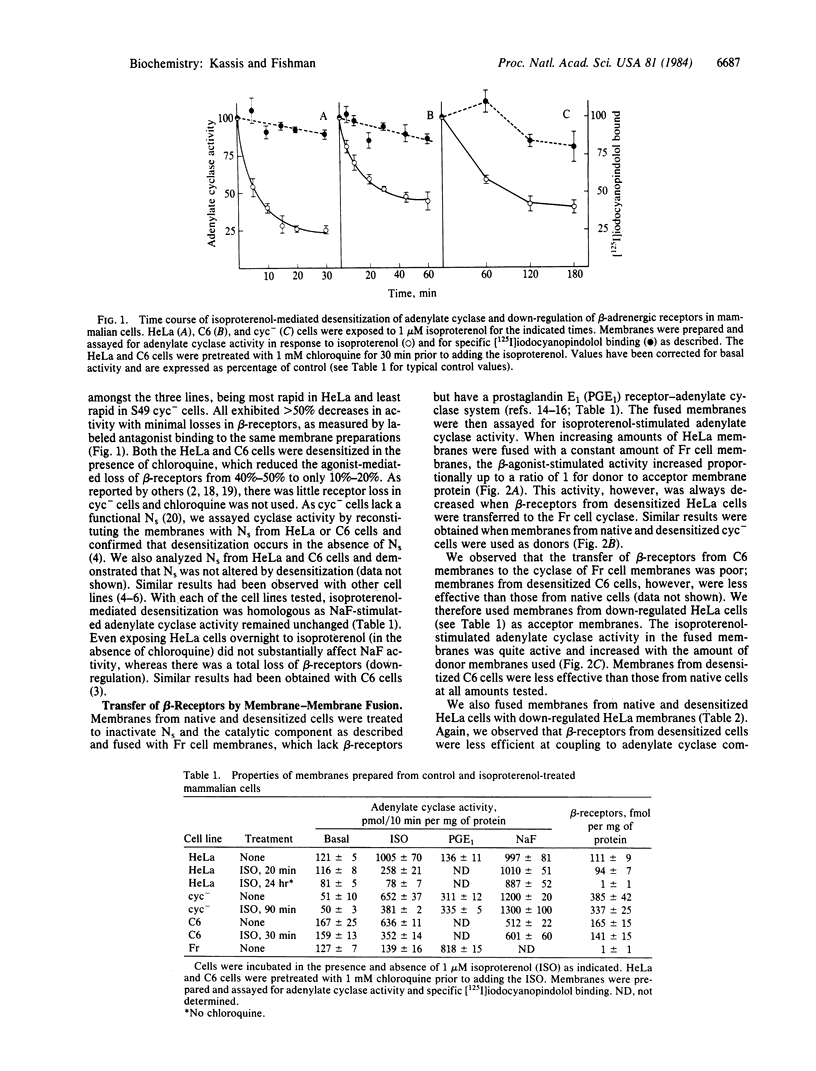
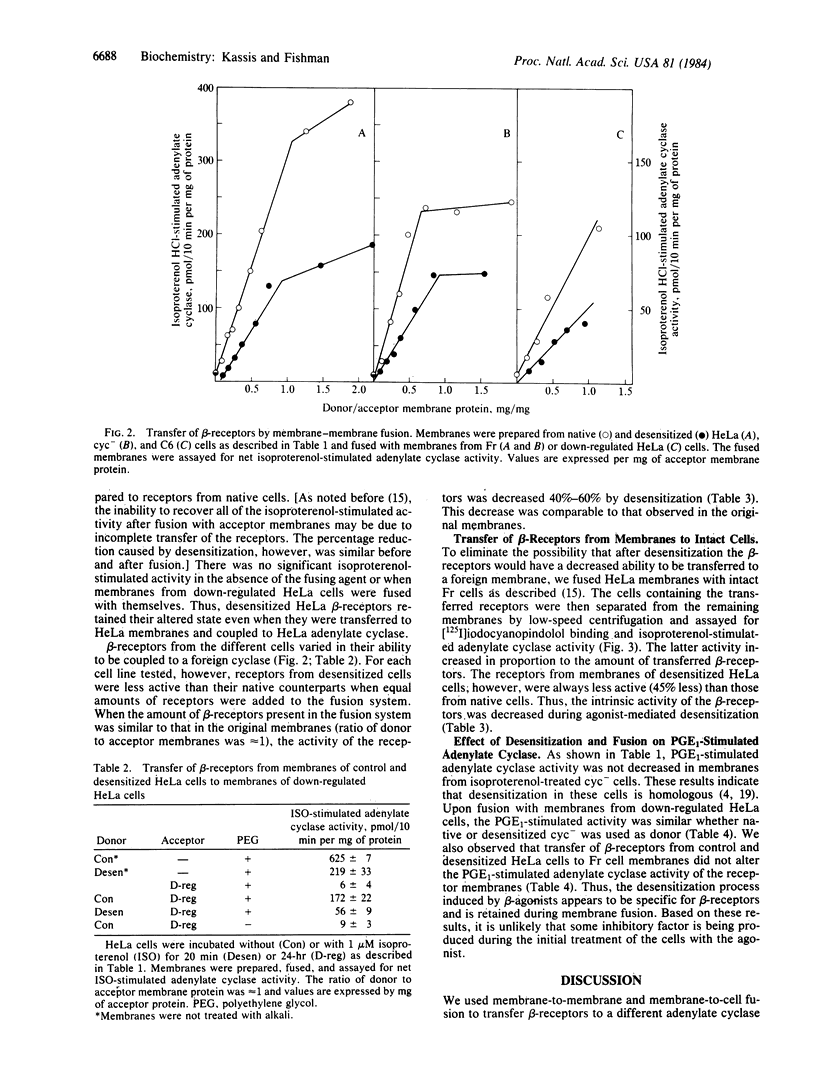
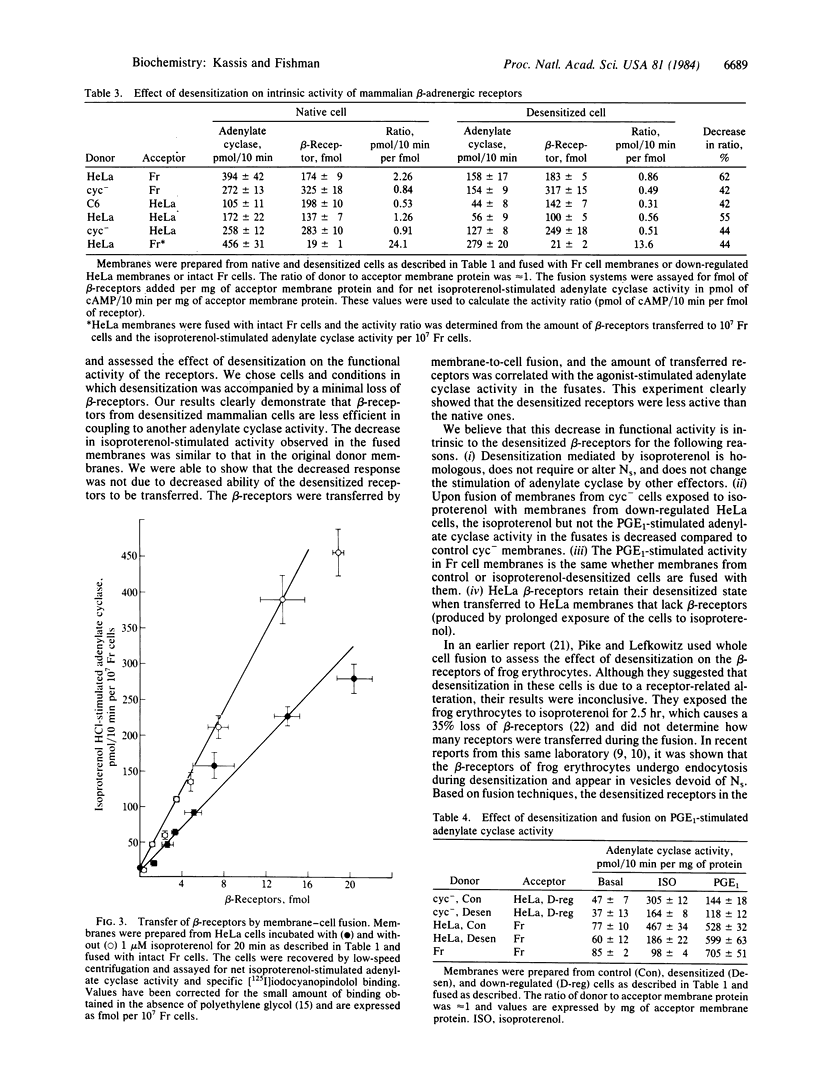
Exposure of several mammalian cell lines to isoproterenol resulted in a desensitization of the beta-adrenergic receptor-adenylate cyclase system in membranes isolated from the cells. Under the experimental conditions chosen, desensitization was accompanied by a minimal loss of beta-receptors. The cells tested included HeLa, S49 cyc- lymphoma, and rat glioma C6. The functional activity of the beta-receptors was determined by coupling them to a foreign adenylate cyclase by membrane fusion. The donor membranes were treated to inactivate the regulatory and catalytic components of adenylate cyclase. The acceptor membranes were from Friend erythroleukemic cells (Fr cells), which lack beta-receptors, and HeLa cells treated overnight with isoproterenol to eliminate their receptors. The fused membranes were assayed for agonist-stimulated activity, which was always reduced when the donor beta-receptors were from the desensitized membranes. The desensitization appeared to be specific for beta-receptors, as the activity of other receptors and cyclase components was not altered. By fusing HeLa membranes with intact Fr cells, we directly measure the intrinsic activity of native and desensitized beta-receptors. For an equal amount of transferred beta-receptors, the activity was 40%-50% lower when the donor membranes were from desensitized cells. Our results clearly indicate that desensitization mediated by a beta-agonist in mammalian cells results in a functional alteration of the beta-receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Mallorga P., Tallman J. F. Catecholamine-induced desensitization of adenylate cyclase in rat glioma C6 cells. Evidence for a specific uncoupling of beta-adrenergic receptors from a functional regulatory component of adenylate cyclase. Mol Pharmacol. 1981 Sep;20(2):310–318. [PubMed] [Google Scholar]
- Frederich R. C., Jr, Waldo G. L., Harden T. K., Perkins J. P. Characterization of agonist-induced beta-adrenergic receptor-specific desensitization in C62B glioma cells. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(2):103–118. [PubMed] [Google Scholar]
- Green D. A., Clark R. B. Adenylate cyclase coupling proteins are not essential for agonist-specific desensitization of lymphoma cells. J Biol Chem. 1981 Mar 10;256(5):2105–2108. [PubMed] [Google Scholar]
- Green D. A., Friedman J., Clark R. B. Epinephrine desensitization of adenylate cyclase from cyc- and S49 cultured lymphoma cells. J Cyclic Nucleotide Res. 1981;7(3):161–172. [PubMed] [Google Scholar]
- Harden T. K., Cotton C. U., Waldo G. L., Lutton J. K., Perkins J. P. Catecholamine-induced alteration in sedimentation behavior of membrane bound beta-adrenergic receptors. Science. 1980 Oct;210(4468):441–443. doi: 10.1126/science.6254143. [DOI] [PubMed] [Google Scholar]
- Hertel C., Staehelin M., Perkins J. P. Evidence for intravesicular beta-adrenergic receptors in membrane fractions from desensitized cells: binding of the hydrophilic ligand CGP-12177 only in the presence of alamethicin. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(2):119–128. [PubMed] [Google Scholar]
- Iyengar R., Bhat M. K., Riser M. E., Birnbaumer L. Receptor-specific desensitization of the S49 lymphoma cell adenylyl cyclase. Unaltered behavior of the regulatory component. J Biol Chem. 1981 May 25;256(10):4810–4815. [PubMed] [Google Scholar]
- Kassis S., Fishman P. H. Different mechanisms of desensitization of adenylate cyclase by isoproterenol and prostaglandin E1 in human fibroblasts. Role of regulatory components in desensitization. J Biol Chem. 1982 May 10;257(9):5312–5318. [PubMed] [Google Scholar]
- Kassis S., Henneberry R. C., Fishman P. H. Induction of catecholamine-responsive adenylate cyclase in HeLa cells by sodium butyrate. Evidence for a more efficient stimulatory regulatory component. J Biol Chem. 1984 Apr 25;259(8):4910–4916. [PubMed] [Google Scholar]
- Neufeld G., Schramm M., Weinberg N. Hybridization of adenylate cyclase components by membrane fusion and the effect of selective digestion by trypsin. J Biol Chem. 1980 Oct 10;255(19):9268–9274. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Lefkowitz R. J. Use of cell fusion techniques to probe the mechanism of catecholamine-induced desensitization of adenylate cyclase in frog erythrocytes. Biochim Biophys Acta. 1980 Oct 15;632(3):354–365. doi: 10.1016/0304-4165(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear M., Insel P. A., Melmon K. L., Coffino P. Agonist-specific refractoriness induced by isoproterenol. Studies with mutant cells. J Biol Chem. 1976 Dec 10;251(23):7572–7576. [PubMed] [Google Scholar]
- Stadel J. M., De Lean A., Mullikin-Kilpatrick D., Sawyer D. D., Lefkowitz R. J. Catecholamine-induced desensitization in turkey erythrocytes: cAMP mediated impairment of high affinity agonist binding without alteration in receptor number. J Cyclic Nucleotide Res. 1981;7(1):37–47. [PubMed] [Google Scholar]
- Stadel J. M., Nambi P., Shorr R. G., Sawyer D. F., Caron M. G., Lefkowitz R. J. Catecholamine-induced desensitization of turkey erythrocyte adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3173–3177. doi: 10.1073/pnas.80.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel J. M., Strulovici B., Nambi P., Lavin T. N., Briggs M. M., Caron M. G., Lefkowitz R. J. Desensitization of the beta-adrenergic receptor of frog erythrocytes. Recovery and characterization of the down-regulated receptors in sequestered vesicles. J Biol Chem. 1983 Mar 10;258(5):3032–3038. [PubMed] [Google Scholar]
- Strulovici B., Stadel J. M., Lefkowitz R. J. Functional integrity of desensitized beta-adrenergic receptors. J Biol Chem. 1983 May 25;258(10):6410–6414. [PubMed] [Google Scholar]
- Su Y. F., Harden T. K., Perkins J. P. Catecholamine-specific desensitization of adenylate cyclase. Evidence for a multistep process. J Biol Chem. 1980 Aug 10;255(15):7410–7419. [PubMed] [Google Scholar]
- Waldo G. L., Northup J. K., Perkins J. P., Harden T. K. Characterization of an altered membrane form of the beta-adrenergic receptor produced during agonist-induced desensitization. J Biol Chem. 1983 Nov 25;258(22):13900–13908. [PubMed] [Google Scholar]
- Wessels M. R., Mullikin D., Lefkowitz R. J. Differences between agonist and antagonist binding following beta-adrenergic receptor desensitization. J Biol Chem. 1978 May 25;253(10):3371–3373. [PubMed] [Google Scholar]
- Wessels M. R., Mullikin D., Lefkowitz R. J. Selective alteration in high affinity agonist binding: a mechanism of beta-adrenergic receptor desensitization. Mol Pharmacol. 1979 Jul;16(1):10–20. [PubMed] [Google Scholar]



