Abstract
Background
Interferon-γ assays based on tuberculosis (TB)-specific antigens have been utilized for diagnosing and ruling out latent TB and active TB, but their utility is still limited for TB incidence countries. The aim of this study is to understand the clinical utility of enzyme-linked immunospot (ELISpot) assays among patients with clinically suspected TB and healthy adults in clinical practices and community-based settings.
Methods
The ELISpot assays (T SPOT.TB, Oxford Immunotec, UK) were prospectively performed in 202 patients. After excluding those with indeterminate results, 196 were included for analysis: 41 were TB patients, 93 were non-TB patients, and 62 were healthy adults.
Results
The sensitivity and negative predictive values of the T SPOT.TB assays for the diagnosis of TB were 87.8% and 89.1%, respectively, among patients with suspected TB. The agreement between the tuberculin skin test (10-mm cutoff) and the T SPOT.TB assay was 66.1% (kappa=0.335) in all participants and 80.0% (kappa=0.412) in TB patients. Among those without TB (n=155), a past history of TB and fibrotic TB scar on chest X-rays were significant factors that yielded positive T SPOT.TB results. There was a significant difference in the magnitude of T SPOT.TB spot counts between TB patients and non-TB patients or healthy adults.
Conclusion
The T SPOT.TB assay appeared to be a useful test for the diagnostic exclusion of TB. A positive result, however, should be cautiously interpreted for potential positives among those without active TB in intermediate TB incidence areas.
Keywords: Enzyme-Linked Immunospot Assay, Interferon-gamma, Tuberculosis
Introduction
The diagnosis and treatment of tuberculosis (TB) is a worldwide problem1. Acid-fast staining of sputum yields a sensitivity of approximately 50-60%, and mycobacterial culture in liquid media usually requires a waiting period of more than 2 weeks before the results are available2. Rapid diagnosis is crucial for effective treatment and control of TB not only in clinical practice but also throughout the community. When microbiological evidence is lacking, the decision to initiate anti-TB treatment can be difficult, especially in areas where the prevalence of TB is considerable.
The TB-specific T-cell interferon (IFN)-γ release assay (IGRA), QuantiFERON-TB, and the T SPOT.TB assays have recently been developed for the diagnosis of TB infection in the peripheral blood3. These assays make use of antigens such as early secreted antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) that are encoded by genes located within the region of difference-1 of the Mycobacterium tuberculosis genome. These antigens are more specific to M. tuberculosis than the purified protein derivatives (PPDs) used in the tuberculin skin test (TST)4. The TST has a lower specificity in populations with high bacilli Calmette-Guerin (BCG) inoculation and non-TB mycobacteria (NTM) exposure because PPD contains a mixture of antigens that are shared among M. tuberculosis, M. bovis, and several NTM5. The T SPOT.TB assay (Oxford Immunotec, Oxford, UK), a type of enzyme-linked immunospot (ELISpot) assay, measures cell-mediated immunity by measuring IFN-γ released from T cells in response to TB-specific antigens such as ESAT-6 and CFP-10. The assay requires a sample of peripheral blood mononuclear cells as well as the antigens ESAT-6 and CFP-10, and the number of T cells producing IFN-γ are detected using an automated ELISpot plate reader6.
We prospectively utilized a commercially available T SPOT. TB assay for diagnosing TB infection in both a clinical practice and community-based setting, and we compared this assay with the clinical characteristics and value of the TST.
Materials and Methods
From June 2008 to June 2010, patients who were clinically suspected of having active TB and healthy adult volunteers were consecutively recruited from Hallym University Hangang Sacred Heart Hospital. The participants were enrolled only after the study protocol was approved by the Ethics Review Committee of the Hallym University Hangang Sacred Heart Hospital, and written informed consent was obtained from all participants. The participants were asked to complete a questionnaire about their previous TB history, contact with patients with active TB, family history, and other underlying conditions.
For TB diagnosis, microbiological and pathological specimens were obtained from the subjects admitted to our hospital who were clinically suspected of having TB. Standard techniques and procedures were employed including chest X-ray, sputum collection, and bronchoscopy. At the same time, approximately 8 mL of peripheral blood was collected from each subject for the T SPOT.TB assays. Thereafter, a TST was performed in which 0.1 mL of tuberculin PPD equivalent to two tuberculin units (RT 23; Statens Serum Institute, Copenhagen, Denmark) was injected intradermally into the volar aspect of the forearm, and the transverse induration diameter was measured 48-72 hours later7. An induration of 10 mm or more was considered a positive test result. In the healthy adults, chest radiography, T SPOT.TB assay, TST, and sputum acid-fast bacilli (AFB) smears and cultures were also performed after a medical history was obtained and a full physical examination was performed.
A diagnosis of active TB was finally made on the basis of all clinical, radiographic, microbiologic, and histologic information after performing routine diagnostic procedures. AFB culture-positive TB was confirmed if M. tuberculosis was successfully cultured from specimens such as sputum, bronchial washings, body fluid, and other tissues. AFB culture-negative TB was diagnosed by the presence of caseous granuloma based on histology, TB polymerase chain reaction tests, and other biochemical results favoring TB (adenosine deaminase [ADA] level of ≥40 IU/L in the pleural or pericardial effusion; ADA level of ≥10 IU/L in the cerebrospinal fluid). We excluded cases without meeting above criteria, even if those who were suspicious of active TB radiographically.
Fibrotic TB scar was defined when nodulostreaky opacity were evident in the upper lobes based on chest radiography and no significant interval changes were observed in these scars for at least 3 months, and AFB culture findings from sputum samples were negative8.
The IFN-γ ELISpot assay, which is part of T SPOT.TB testing, was performed according to the manufacturer's recommendations, and the test results were assessed by the Centers for Disease Control and Prevention guidelines6. Assays were scored using an ELISpot reader (CTL-ImmunoSpot S4 analyzer; Cellular Technology Ltd., Shaker Heights, OH, USA). In cases where the negative (Nil) control had fewer than or equal to 10 spots, the result was defined as positive if (ESAT-6 stimulated well minus Nil) and/or (CFP-10 stimulated wells minus Nil) greater than or equal to eight spots. If the Nil had greater than 10 spots or positive (mitogen) control had fewer than 20 spots, the result was considered indeterminate. Without meeting above criteria, the result was defined as negative. Available at http://www.oxfordimmunotec.com/USpageInsert. Six participants with indeterminate results of T SPOT.TB assays were excluded in this study.
1. Statistical analysis
The sensitivity, specificity, positive predictive value, and negative predictive value for the diagnosis of active TB were calculated for each test. The continuous variables were compared by Student's t-test or Mann-Whitney U test (if the variables were not normally distributed), whereas the categorical variables were compared using the chi-square test or Fisher's exact test. Agreement between the test results obtained from the TST and T SPOT.TB assays was assessed using kappa coefficients (kappa>0.75, excellent agreement; kappa<0.4, poor agreement; 0.4≤kappa≤0.75, fair to good agreement). A p<0.05 was considered significant. Statistical analyses were performed using dBSTAT software version 4.0 (dBSTAT Inc., Chuncheon, Korea).
Results
In total, 134 admitted subjects who were suspected of having active TB and 62 healthy adults were prospectively recruited in this study. All participants were vaccinated with BCG at a very young age. A past history of TB was noted in 25 (18.7%) patients with suspected TB and in 5 (8.1%) healthy adults (Table 1). Active TB was diagnosed in 41 of the 134 patients with suspected TB (19 were AFB culture positive and 22 were AFB culture negative), although some had risk factors for immunosuppression such as human immunodeficiency virus infection, malignancy, use of immunosuppressive drugs, and end-stage renal disease. Of the 22 patients who were AFB culture negative, 12 (54.5%) had extrapulmonary TB, 5 had TB pleurisy, 3 had TB meningitis, and one patient each had TB pericarditis, TB spondylitis, TB peritonitis, and intestinal TB. The final diagnoses of the 93 non-TB patients were bacterial pneumonia, lung cancer, interstitial fibrosis, bronchiectasis, empyema, and fibrotic TB scars based on chest radiography (Table 2).
Table 1.
Characteristics of the subjects (n=196)
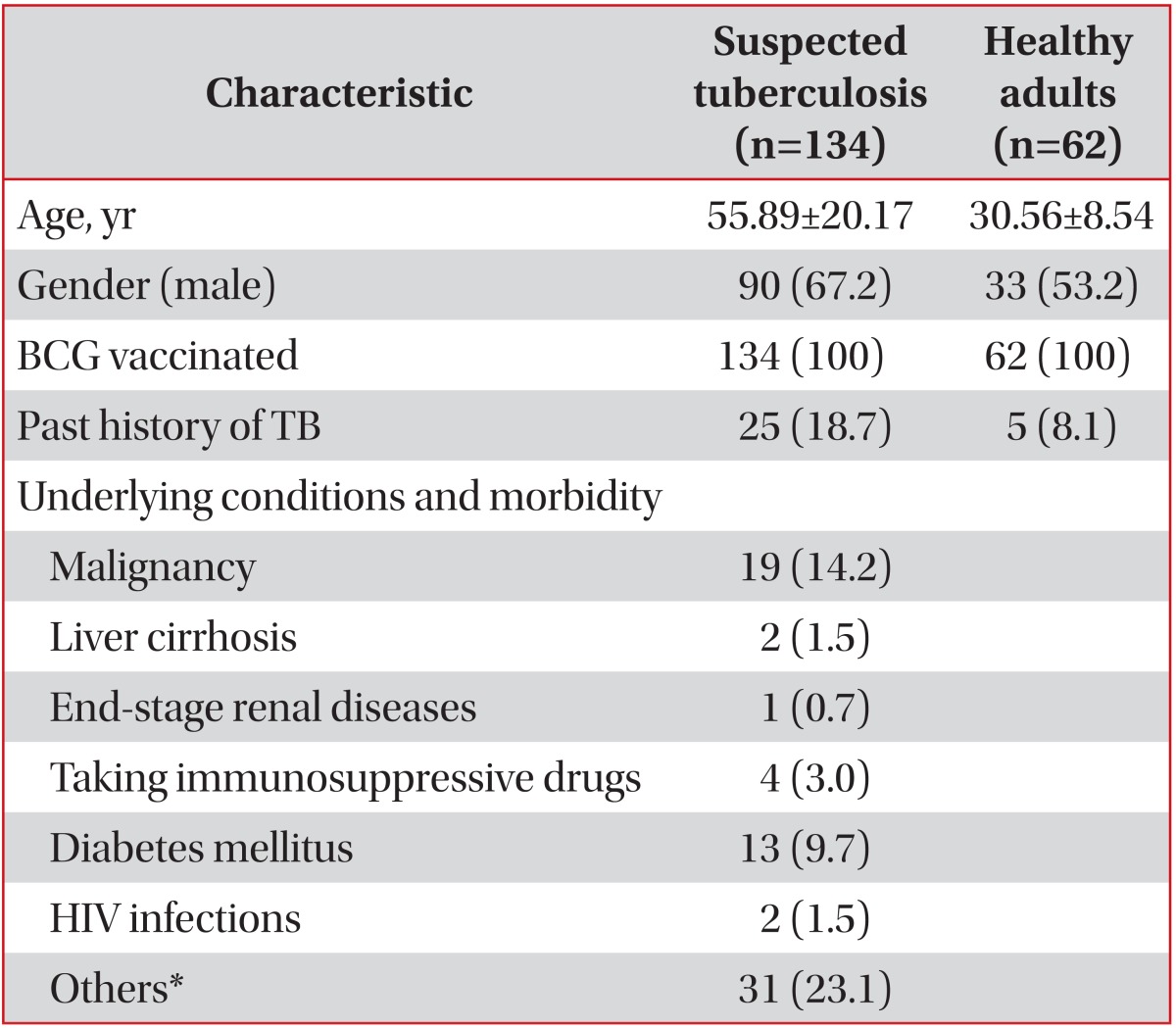
Values are presented as mean±standard deviation or number (%) unless otherwise stated.
*Hypertension, cerebrovascular accident, intracranial hemorrhage.
BCG: bacilli Calmette-Guerin; TB: tuberculosis; HIV: human immunodeficiency virus.
Table 2.
Final diagnoses of the subjects with suspected TB (n=134)
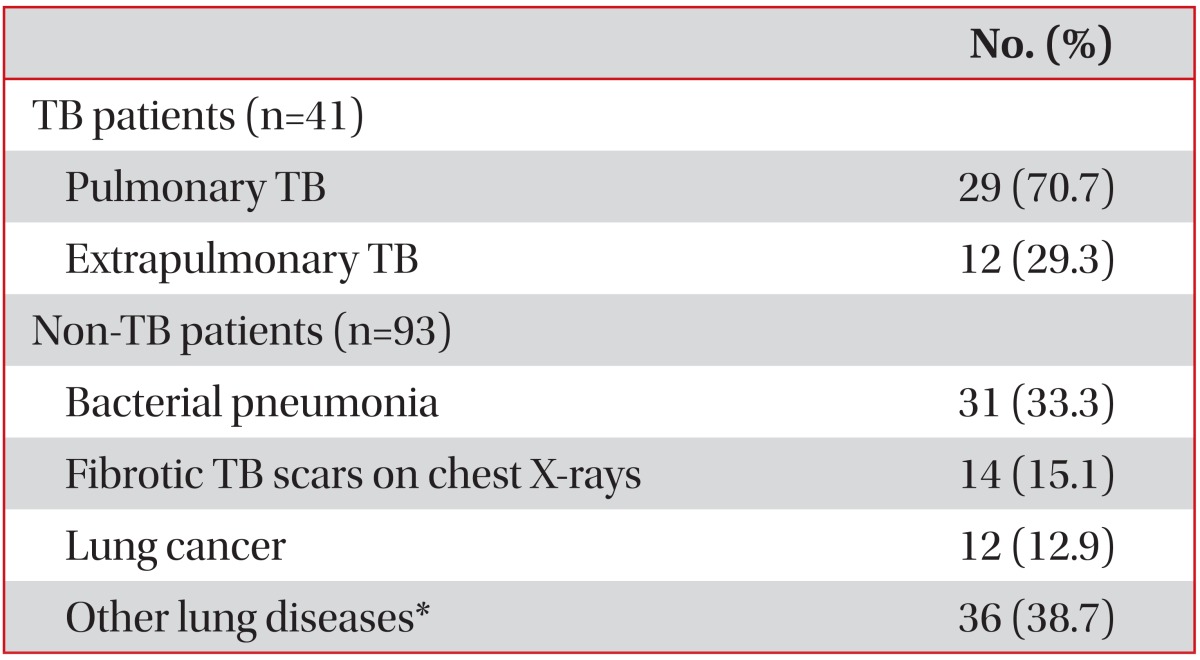
Values are presented as number (%) unless otherwise stated.
*Interstitial fibrosis, bronchiectasis, solitary pulmonary nodule, empyema, chronic obstructive pulmonary diseases.
TB: tuberculosis.
The sensitivity of the T SPOT.TB assay for the diagnosis of active TB in TB patients was 87.8%. The specificity of the assay was 44.1% in non-TB patients and 75.8% in healthy adults. The positive and negative predictive values of the assay were 40.9% and 89.1%, respectively, in TB patients and non-TB patients (Table 3).
Table 3.
Diagnostic validity of T SPOT.TB assays (n=196)
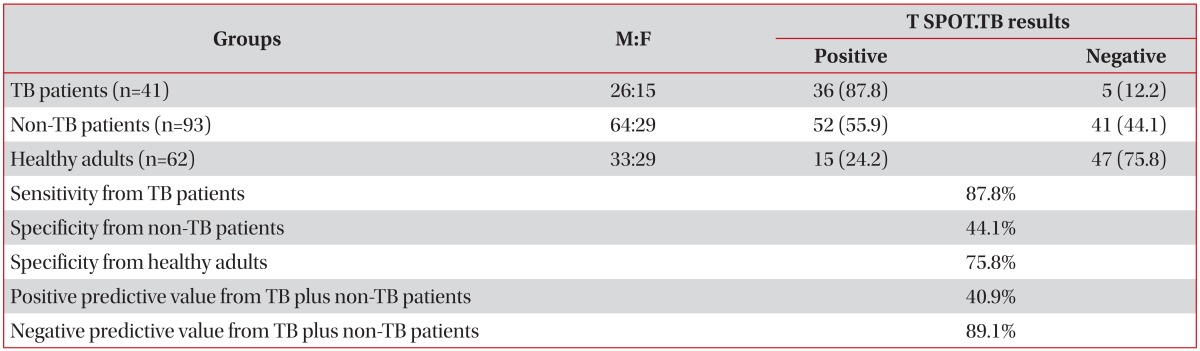
Values are presented as number (%) unless otherwise stated.
TB: tuberculosis.
Until the age of 60, the positive rates of the T SPOT.TB assay significantly increased with age in all participants (p<0.001) and in those who did not have TB (p<0.001) (Table 4).
Table 4.
Positive rates of T SPOT.TB assays according to age
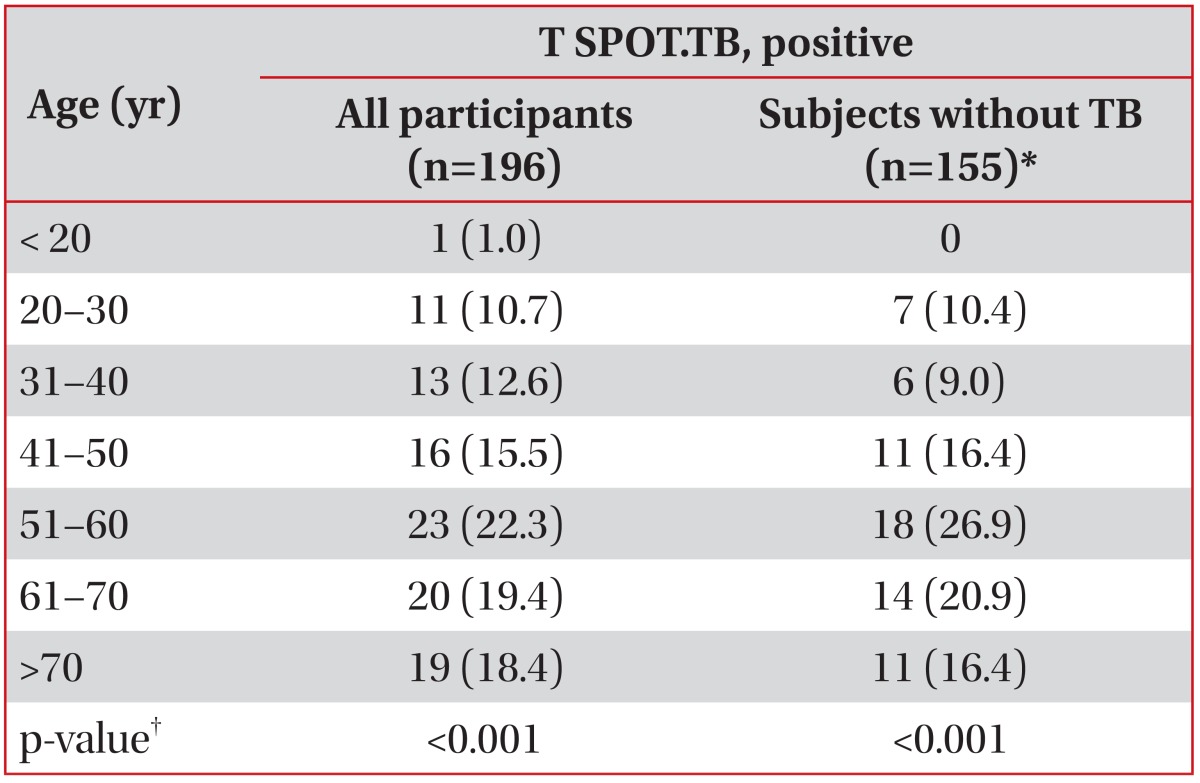
Values are presented as number (%).
*Non-TB patients plus healthy adults. †Linear-by-linear association.
TB: tuberculosis.
A comparison of the results from T SPOT.TB assays in non-TB patients and healthy adults (n=155) is shown in Table 5. The positive rate of the T SPOT.TB assay was significantly higher in the subjects who had a past history of TB and fibrotic TB scars visible on chest X-ray than in those who did not have (p=0.001 and p=0.001, respectively). With the exception of hypertension (p=0.004), underlying conditions and comorbidities did not appear to have effects on the results of T SPOT. TB assays. However, hypertension did not lead to significant differences between the 2 groups after the data was adjusted for age.
Table 5.
Comparison of the results from T SPOT.TB assays among subjects without TB (n=155)*
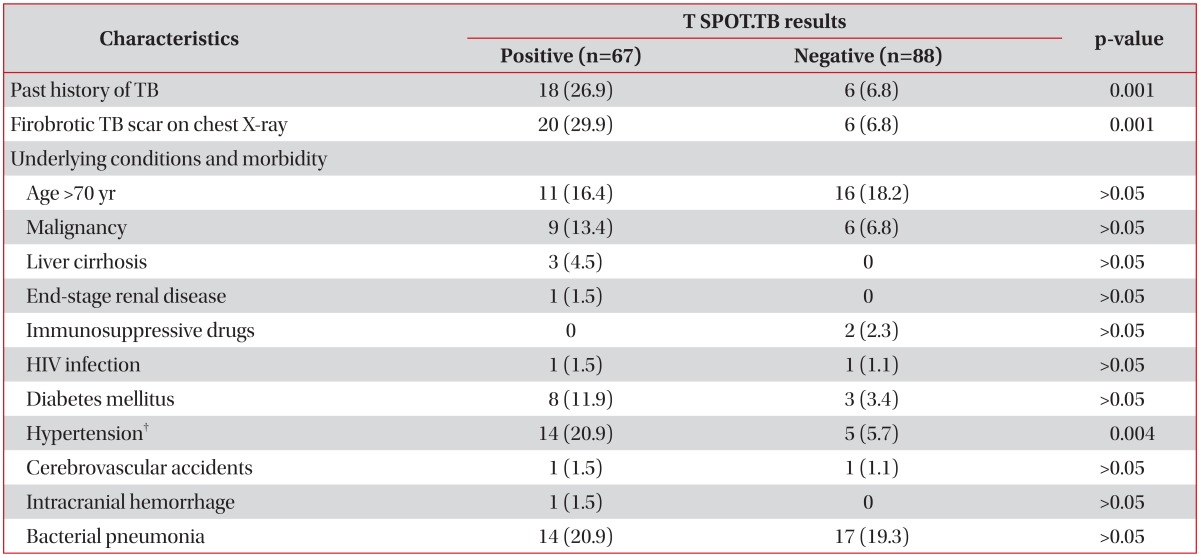
Values are presented as number (%).
*Non-TB patients plus healthy adults. †There was no significant difference after the data were adjusted for age.
TB: tuberculosis; HIV: human immunodeficiency virus.
The positive rate of the T SPOT.TB assays was significantly higher in TB patients compared to non-TB patients and healthy adults (p<0.001 and p<0.001, respectively). No differences were observed in the TST results of TB and non-TB patients (p>0.05), TB patients and healthy adults (p>0.05), and non-TB patients and healthy adults (p>0.05) (Table 6). The agreement between T SPOT.TB assays and TST was 66.1% (kappa=0.335) among all participants and 58.1% (kappa=0.237) among healthy adults. However, the overall agreement was 80.0% (kappa=0.412) among TB patients and 72.7% (kappa=0.453) among non-TB patients (Table 7).
Table 6.
Comparison of the results from the TST and T SPOT.TB assay

Values are presented as number (%).
*TB patients versus non-TB patients, TB patients versus healthy adults (p<0.001 and p<0.001, respectively); non-TB patients versus healthy adults (p<0.001). †TB patients versus non-TB patients, TB patients versus healthy adults (p>0.05 and p>0.05, respectively); non-TB patients versus healthy adults (p>0.05).
TST: tuberculin skin test; TB: tuberculosis.
Table 7.
Agreement between the TST and T SPOT.TB assay
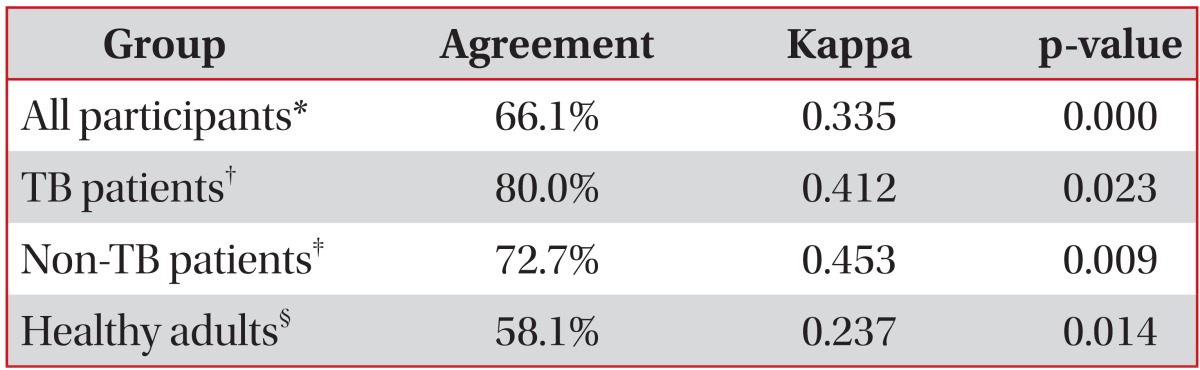
*Data were available for 115 participants. †Data were available for 20 patients. ‡Data were available for 33 patients. §Data were available for 62 subjects.
TST: tuberculin skin test; TB: tuberculosis.
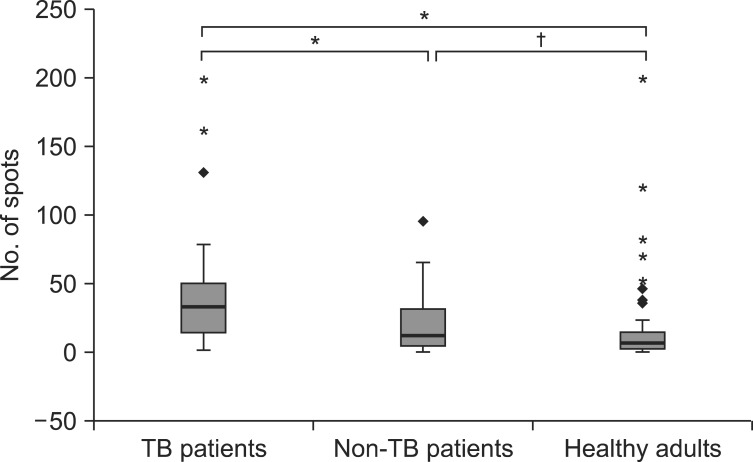
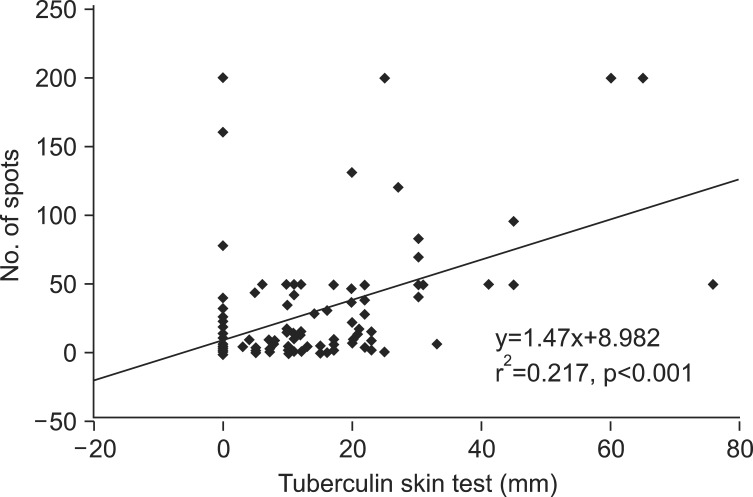
The mean number of spot-forming cells was 40.1-41.3 in TB patients, 18.9-19.1 in non-TB patients, and 26.2-51.5 in healthy adults. The number of spot-forming cells was significantly higher in TB patients compared with non-TB patients and healthy adults (p<0.001 and p<0.001, respectively). No difference was observed in the number of spots between non-TB patients and healthy adults (p>0.05) (Figure 1). Regression analysis demonstrated a good positive correlation between the number of spot-forming cells in the T SPOT.TB assay and the size of the induration in the TST (r2=0.217, p<0.001) (Figure 2).
Figure 1.
Boxplot showing the number of spot-forming cells in subjects with positive T SPOT.TB results. *p<0.001, †p>0.05. TB: tuberculosis.
Figure 2.
Regression analysis of the number of spot-forming cells and the size of the induration between the T SPOT.TB assay and the tuberculin skin test.
Discussion
A great deal of effort is needed to confirm a diagnosis of TB in routine clinical practice. Invasive procedures including bronchoscopy and biopsy may also be needed to obtain infected specimens or tissues for microbiological and pathological examinations. There is a delay in diagnosis and initiation of therapy because mycobacterial culture requires several weeks before a final result can be obtained. Therefore, a rapid, sensitive, and specific diagnostic test is needed because the TST is regarded as unreliable and is neither sensitive nor specific for diagnosing TB, especially in areas with an intermediate TB burden8-10. We investigated the diagnostic utility of the newly introduced T cell-based ELISpot (T SPOT.TB) assay in patients with clinically suspected TB and healthy adults. The T SPOT. TB assay is another form of IGRA using antigens specific to M. tuberculosis. The T SPOT.TB assay demonstrated 87.8% sensitivity for diagnosing TB in patients with confirmed TB. In addition, the negative predictive value of the T SPOT.TB assay was 89.1%. Considering that the diagnostic specificity of the TST has been confounded in people vaccinated with BCG11, the T SPOT.TB assay could be more useful in the diagnosis and exclusion of active TB. Although the specificity of the T SPOT. TB assay in healthy adults (75.8%) was slightly higher than that in non-TB patients (44.1%), this low specificity is expected to limit its clinical application in routine practice. A few factors should be considered in order to adequately interpret this low specificity. Latent TB has a high prevalence, which is as high as 30% among Koreans12 and probably contributes a decrease in the specificity of the T SPOT.TB assay. This study was focused on understanding the utility of the T SPOT.TB assay for patients who were clinically suspected of having TB; thus, we focused on unselected patients, which could have introduced a selection bias. This is one reason why the specificity of the T SPOT. TB assay was lower in non-TB patients than in healthy adults.
We noted that positive rates of T SPOT.TB assays in non-TB patients and healthy adults proportionally increased up to the age of 60, and thereafter, we noted a slight decrease, due to maybe senescence. A significant proportion (43.2%) of those who did not have TB had a positive T SPOT.TB result. A past history of TB and fibrotic TB scars visible on a chest X-ray turned out to be important factors that yielded positive T SPOT.TB results. To understand our results correctly, we must consider the clinical limitations of an area where BCG vaccination is mandatory and the prevalence of latent TB infection is considerable. ELISpot results correlate significantly with TB exposure, and show a strong positive correlation with exposure intensity13,14. For each hour that room air is shared with an active TB case, the odds of a positive result for ELISpot increased by 1.0514. In our interpretation of the results, we must consider that our population has been heavily exposed to active TB in the community-based setting, especially during the ages of 20 to 60 as their age increases.
The agreement between the TST and the T SPOT.TB assay was poor among all participants and healthy adults (kappa=0.335 and kappa=0.237, respectively), and was relatively fair among TB and non-TB patients (kappa=0.412 and kappa=0.453, respectively). The ELISpot assay is known to be less affected by BCG status13,15. Most previous studies have reported a modest agreement (60-80%) between the TST and IGRA. However, BCG vaccination appeared to be associated with a specific pattern of discordance based on a review of domestic studies9. Compared with the TST, The T SPOT.TB assay is known to correlate significantly better with increasing TB exposure. The T cell responses to ESAT-6 and CFP-10 are also not completely specific for infection with M. tuberculosis complex and may result from certain NTM infections. Therefore, we must use caution when accepting the results of the T SPOT.TB assay because positive results may suggest past exposure to TB or NTM infection.
The number of spot-forming cells was significantly increased in TB patients compared with non-TB patients and healthy adults. However, the quantitative results of T SPOT. TB assays overlapped considerably between TB patients and those who did not have TB. Therefore, T SPOT.TB should be limited in routine clinical practice to establish or exclude a diagnosis of TB because the assay is not able to distinguish active TB from latent TB16. Tuberculin reactivity caused by BCG vaccination generally wanes over the time. Unfortunately, there is no reliable method currently available to distinguish a positive TST reaction from a natural mycobacterial infection. However, reactions with an induration of more than 20 mm are not likely caused by BCG17. This study showed that there was a good positive correlation between the number of spot-forming cells and the size of the induration for both the T SPOT.TB and the TST in all participants. We believe that this phenomenon represents the immune response of subjects who were exposed to M. tuberculosis.
It may be argued whether the participants in this study were appropriate in terms of the routine clinical practice and the community-based setting. Five (8.1%) of the healthy adults had a past history of TB. We did not reorganize the admitted patients according to immune status. The TST was performed on all healthy adults but not on all TB and non-TB patients. However, the sputa of 5 healthy adults with a past history of TB did not yield any microbiological evidence of TB. There were no significant differences in the positive rates for the TST and T SPOT.TB assay according to underlying conditions and comorbidity, which were likely due to the small sample size. Considering that previous domestic studies have reported a positive TST rate (≥10 mm of induration) ranging from 66.7% to 78% among active TB patients and from 21.4% to 51% among subjects with a low risk of TB9,10, we believe that the participants in our study did not deviate much from our clinical and community setting and did not affect the outcome of this study.
In conclusion, the relatively high sensitivity and negative predictive value of the T SPOT.TB assay, which is based on the TB-specific antigens ESAT-6 and CFP-10, make it a useful clinical tool for the diagnosis and exclusion of active TB. However, the test results should be very cautiously interpreted for patients with a past history of TB, fibrotic TB scars visible on chest X-ray and old ages because many of these factors yield false positive T SPOT.TB assays results in clinical practice and community-based settings. This is especially true in areas with an intermediate burden of TB and considerable prevalence of latent TB.
Acknowledgements
The authors would like to thank the volunteers who agreed to participate in this study.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Aber VR, Allen BW, Mitchison DA, Ayuma P, Edwards EA, Keyes AB. Quality control in tuberculosis bacteriology. 1. Laboratory studies on isolated positive cultures and the efficiency of direct smear examination. Tubercle. 1980;61:123–133. doi: 10.1016/0041-3879(80)90001-x. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 4.Gooding S, Chowdhury O, Hinks T, Richeldi L, Losi M, Ewer K, et al. Impact of a T cell-based blood test for tuberculosis infection on clinical decision-making in routine practice. J Infect. 2007;54:e169–e174. doi: 10.1016/j.jinf.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jasmer RM, Nahid P, Hopewell PC. Clinical practice: latent tuberculosis infection. N Engl J Med. 2002;347:1860–1866. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 7.Sokal JE. Editorial: measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 8.Kang YA, Lee HW, Hwang SS, Um SW, Han SK, Shim YS, et al. Usefulness of whole-blood interferon-gamma assay and interferon-gamma enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007;132:959–965. doi: 10.1378/chest.06-2805. [DOI] [PubMed] [Google Scholar]
- 9.Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005;293:2756–2761. doi: 10.1001/jama.293.22.2756. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006;28:24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 11.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 12.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 13.Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–2021. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 14.Richeldi L, Ewer K, Losi M, Bergamini BM, Roversi P, Deeks J, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004;170:288–295. doi: 10.1164/rccm.200403-307OC. [DOI] [PubMed] [Google Scholar]
- 15.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 16.Janssens JP, Roux-Lombard P, Perneger T, Metzger M, Vivien R, Rochat T. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur Respir J. 2007;30:722–728. doi: 10.1183/09031936.00028507. [DOI] [PubMed] [Google Scholar]
- 17.McKay A, Kraut A, Murdzak C, Yassi A. Determinants of tuberculin reactivity among health care workers: Interpretation of positivity following BCG vaccination. Can J Infect Dis. 1999;10:134–139. doi: 10.1155/1999/749765. [DOI] [PMC free article] [PubMed] [Google Scholar]