Abstract
Background
We sought to better define the role of hematopoietic cell transplantation (HCT) in first remission (CR1) for high-risk pediatric acute myeloid leukemia (AML).
Procedures
Outcomes were compared among patients aged less than 21 years with cytogenetically defined poor-risk AML treated with chemotherapy, matched related (MRD), or unrelated donor (URD) transplantation in CR1. Poor-risk cytogenetics was defined as monosomy 7/del7q, monosomy 5/del 5q, abnormalities of 3q, t(6;9)(p23;q34), or complex karyotype. Included are patients treated on Children’s Oncology Group trials or reported to the Center for International Blood and Marrow Transplant Research from 1989 to 2006.
Results
Of the 233 patients, 123 received chemotherapy, 55 received MRD HCT, and 55 received URD HCT. The 5-year overall survival from the time of consolidation chemotherapy or transplant conditioning was similar: chemotherapy (43% ± 9%), MRD (46% ± 14%), or URD (50% ± 14%), P = 0.99. Similarly, multivariate analysis demonstrated no significant differences in survival [(reference group = chemotherapy); MRD HR 1.08, P = 0.76; URD HR 1.13, P = 0.67] despite lower relapse risk with URD HCT (HR = 0.43, P = 0.01).
Conclusions
Our findings do not provide support for the preferential use of HCT over chemotherapy alone for children with cytogenetically defined poor-risk AML in CR1.
Keywords: acute myeloid leukemia, chemotherapy, hematopoietic cell transplantation, pediatrics
INTRODUCTION
The optimal role of allogeneic hematopoietic cell transplantation (HCT) for pediatric acute myeloid leukemia (AML) in first remission is unclear [1–3]. While HCT more effectively prevents relapse than chemotherapy alone [1,4], much of its benefit is offset by the risk of treatment related mortality (TRM) and late effects [5,6]. Many cooperative groups now limit its use to children at higher risk for relapse [7–9]. For instance, in its ongoing phase III trial, the North American Children’s Oncology Group (COG) assigns only children with high-risk disease, defined by cytogenetics, FLT3 mutation status and by minimal residual disease, to HCT with an HLA matched related (MRD), unrelated donor (URD) or other alternative donor [10].
The benefit of HCT to children with high-risk disease, however, remains uncertain. A cross-study analysis, published in 2008, comprised of COG and the Medical Research Council (MRC) phase III trials, comparing bone marrow transplant (BMT) and chemotherapy alone, failed to demonstrate a survival benefit to children with poor-risk AML treated with BMT [5]. However, this study was limited by relatively small number of poor-risk patients who received BMT.
Therefore, we sought to better understand the role of HCT for pediatric AML patients with high-risk disease in first complete remission (CR1). Using data from COG trials and data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR), we compared clinical outcomes in pediatric patients with poor-risk cytogenetics treated in CR1 with chemotherapy, MRD, or URD HCT.
METHODS
Study Population and Treatments
This analysis includes patients (aged <21 years) who were treated with either HCT or chemotherapy alone for AML with poor-risk cytogenetics in CR1 between 1989 and 2006. Data for patients who received chemotherapy and MRD HCT were obtained from COG trials: CCG 2891, POG 9421, CCG 2961, and AAML03P1 which allocated subjects with a HLA matched family donor to HCT in CR1, regardless of disease risk. The cohorts from trials CCG 2891 and CCG 2,961 have been previously included in a comparison of outcomes by treatment with either chemotherapy or matched-related bone marrow transplant by cytogenetic risk group [5]. The details of treatment on these trials have been previously reported [11–14]. Patients in CR1 proceeded to HCT after the second cycle of induction chemotherapy except those enrolled on AAML03P1, who proceeded to HCT after three cycles of chemotherapy (two cycles of induction and one cycle of intensification) [13].
Data for additional HCT patients with poor-risk AML in CR1 were obtained from the CIBMTR. These patients received either a MRD or URD transplant during the same period as the COG trials. It is plausible that most patients who received URD HSCT were enrolled on the above-mentioned COG trials and received URD HSCT in the absence of a matched sibling. Transplants using any hematopoietic stem cell source were included. URD transplants were categorized by the degree of HLA matching as either matched URD or mismatched URD [15].
Risk Group Definitions
Poor-risk cytogenetics was defined by presence of any of the following abnormalities in the absence of the favorable cytogenetic abnormalities t(8;21)(q22q22), inv(16)(p13;q22), and t(15;17)(q22;q21): monosomy 7, deletion of 7q (del(7q)), monosomy 5, deletions of 5q, abnormalities of 3q, t(6;9)(p23;q34), and complex karyotype: defined as five or more cytogenetic abnormalities [16–18]. The cytogenetic abnormality del(7q) was included because the data collection forms used by the CIBMTR during the study period grouped del(7q) and monosomy 7 together. Therefore, to maintain consistency del(7q) was included as a poor-risk abnormality for the COG cohort.
Statistical Methods
Descriptive characteristics of patient- and disease-related factors were prepared. Differences in proportions were analyzed using the χ2 test or the Fisher’s exact test. Differences in medians were analyzed using the Wilcoxon rank-sum test.
To account for the time to transplantation, overall survival (OS), treatment-related mortality (TRM), and the cumulative incidence of relapse are defined from the end of induction or intensification cycle one (AAML03P1) for the patients treated with either chemotherapy or HCT on the COG studies, or the time of HCT for patients registered through the CIBMTR, to an event. OS is defined as time to death from any cause. The Kaplan–Meier method was used to calculate estimates of OS separately for patients with poor-risk disease treated with either chemotherapy only, MRD HCT, or URD HCT [19]. The log rank test was used to compare OS outcomes [20]. The Cox proportional hazards model was used to estimate hazard ratios (HRs) for OS [20]. Methods of competing events were used to calculate estimates of TRM and relapse [21]. TRM is defined as time to death in CR where relapses were competing events. Relapse is defined as time to relapse where deaths without a relapse were competing events. Gray’s test was used to compare the cumulative incidence or relapse and TRM. Competing risk regression models were used to estimate HRs for TRM and relapse risk in univariate and multivariate analyses [22]. The log-rank test was also used to compare clinical outcomes within treatment groups stratified by time period. Children lost to follow-up were censored at their date of last known contact. All patient outcome data were analyzed using an “as treated” analysis.
The incidence rates of acute and chronic graft versus host disease (GVHD) among the MRD and URD patients were calculated to describe the acute and chronic morbidities associated with MRD and URD for poor-risk patients [23].
Because del(7q) has been shown to confer an improved prognosis compared to monosomy 7 a sensitivity analysis was performed to compare outcomes of del(7q) and monosomy 7 patients among the COG patients for whom it could be distinguished which subjects had del(7q) and which had monosomy 7 [24].
Human Subjects
All COG studies were reviewed and approved by the institutional review boards (IRBs) of participating COG institutions and appropriate written informed consent/assent was obtained before treatment. Consent for reporting/research participation to the CIBMTR was obtained by transplant centers. Separate approvals were granted from the IRB of the Tufts Medical Center/Tufts University School of Medicine for the analysis of de-identified cytogenetic data and from the IRBs of the Medical College of Wisconsin and the National Marrow Donor Program.
RESULTS
Patient, Disease, and Treatment Characteristics
A total of 233 patients were included in this study of which 123 were treated with chemotherapy, 55 with MRD HCT and the remaining 55, with URD HCT (Table I). Children in the chemotherapy group were more likely to be 2 years of age or younger, and to be of African-American race. There was a higher proportion of patients with a high white blood cell (WBC) count at diagnosis in the chemotherapy group. There was a similar distribution of monosomy 5 or del5q, abnormal 3q, and t(6;9) among the treatment groups. Children treated with chemotherapy were more likely to have a complex karyotype and less likely to have monosomy 7 or del(7q).
TABLE I.
Clinical Characteristics of the Study Population
| Characteristic | Chemotherapy, N = 123
|
MRD, N = 55
|
URD, N = 55
|
P | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gender | |||||||
| Male | 58 | 47 | 25 | 45 | 31 | 56 | |
| Female | 65 | 53 | 30 | 55 | 24 | 44 | 0.44 |
| Age at diagnosis | |||||||
| 0–2 yo | 58 | 47 | 13 | 24 | 9 | 16 | <0.001 |
| 3–11 yo | 34 | 28 | 21 | 38 | 24 | 44 | 0.09 |
| 12–21 yo | 31 | 25 | 21 | 38 | 22 | 40 | 0.07 |
| Racea | |||||||
| White | 76 | 70 | 43 | 86 | 47 | 90 | 0.004 |
| African American | 23 | 21 | 4 | 8 | 1 | 2 | 0.002 |
| Other | 10 | 9 | 3 | 6 | 4 | 8 | 0.79 |
| Unknown | 14 | 5 | 3 | ||||
| Ethnicity | |||||||
| Hispanic | 14 | 12 | 3 | 6 | 1 | 2 | 0.07 |
| Not Hispanic | 105 | 88 | 50 | 94 | 52 | 98 | |
| WBC (×103/μl) | |||||||
| <50,000 | 102 | 83 | 49 | 91 | 48 | 96 | 0.04 |
| ≥50,000 | 21 | 17 | 5 | 9 | 2 | 4 | |
| Weight categoryb | |||||||
| Underweight | 3 | 4 | 7 | 14 | 4 | 8 | 0.14 |
| Middleweight | 55 | 74 | 38 | 76 | 41 | 80 | 0.73 |
| Overweight | 16 | 22 | 5 | 10 | 6 | 12 | 0.15 |
| Unknown | 49 | 5 | 4 | ||||
| Monosomy 7 or del7q | |||||||
| Negative | 89 | 72 | 31 | 56 | 23 | 42 | <0.001 |
| Positive | 34 | 28 | 24 | 44 | 32 | 58 | |
| Monosomy 5 or del5q | |||||||
| Negative | 111 | 90 | 50 | 91 | 47 | 85 | 0.57 |
| Positive | 12 | 10 | 5 | 9 | 8 | 15 | |
| Abnormal 3q | |||||||
| Negative | 107 | 87 | 50 | 91 | 52 | 95 | 0.29 |
| Positive | 16 | 13 | 5 | 9 | 3 | 5 | |
| t(6;9) | |||||||
| Negative | 106 | 86 | 43 | 78 | 51 | 93 | 0.09 |
| Positive | 17 | 14 | 12 | 22 | 4 | 7 | |
| Complex cytogenetics | |||||||
| Negative | 54 | 44 | 39 | 71 | 47 | 85 | |
| Positive | 69 | 56 | 16 | 29 | 8 | 15 | <0.001 |
| Study | |||||||
| CIBMTR | 0 | 0 | 27 | 53 | 55 | 100 | |
| POG 9421 | 36 | 29 | 5 | 9 | 0 | 0 | |
| CCG 2891 | 23 | 19 | 8 | 15 | 0 | 0 | |
| CCG 2961 | 30 | 24 | 11 | 20 | 0 | 0 | |
| COG AAML03P1 | 34 | 28 | 4 | 7 | 0 | 0 | |
| Stem cell source | |||||||
| Bone marrow | N/A | 53 | 96 | 41 | 75 | ||
| Peripheral blood | 2 | 4 | 4 | 7 | |||
| Cord blood | 10 | 18 | |||||
| Time period | |||||||
| 1989–1997 | 46 | 37 | 34 | 62 | 22 | 40 | 0.008 |
| 1998–2006 | 77 | 63 | 21 | 38 | 33 | 60 | |
MRD, matched related donor; URD, unrelated donor; yo, years old.
Percentage distribution based on known values.
Weight category definitions: For Age ≥ 2 years old: underweight <5% bone mass index (BMI) percentile; middleweight 5–95% BMI percentile; overweight >95% BMI percentile; for age 1–2 years old: underweight <10% WT/LT percentile; middleweight 10–90% WT/LT percentile; overweight >90% WT/LT percentile; WT/LT, weight for length. Data for height/weight used at time of pre-conditioning or beginning of chemotherapy.
Of the URD transplants, 40% were HLA-matched and 60%, HLA-mismatched. Bone marrow was the predominant graft; peripheral blood stem cells were the graft for 6 transplants, and umbilical cord blood was the graft source for 10 transplants. Three of the cord blood units were 4/6 human leukocyte antigen matched and six of the cord blood units were 5/6 human leukocyte antigen matched. The average cell dose of the cord blood units was 3.6 × 107 total nucleated cells per kilogram. Characteristics of one cord blood unit were not reported.
Results of Univariate Analysis
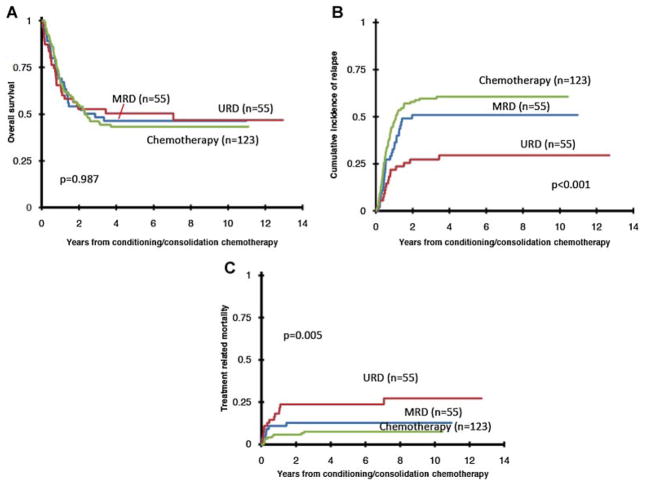
The results of the univariate analysis are shown in Table II and Figure 1. Overall survival from the start of conditioning or consolidation chemotherapy was not significantly different for those treated with chemotherapy alone (43 ± 9% at 5 years), MRD HCT (46 ± 14%), or URD HCT (50 ± 14%), (P = 0.99, Fig. 1). The pattern of treatment failure, however, differed by treatment group. Relapse rates were significantly lower and TRM rates were significantly higher with URD HCTs. The 5-year cumulative incidence of relapse was 61 ± 9%, 51 ± 13%, and 30 ± 12%, after chemotherapy, MRD, and URD transplant respectively (P ≤ 0.001). The corresponding 5-year TRM rates were 7 ± 5%, 13 ± 9%, and 23 ± 11% (P = 0.005). African-American race and a WBC count ≥50,000/μl at diagnosis were associated with significantly lower OS and higher relapse risk. Among the COG cohort for which patients with del(7q) (n = 28) and monosomy 7 (n = 17) could be identified, the OS (50 ± 19% vs. 41 ± 24%, P = 0.54) was not significantly different.
TABLE II.
Univariate Analysis of Patient- and Treatment-Related Factors Associated With OS, TRM and RR
| OS
|
TRM
|
RR
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Treatment group | ||||||||||
| Poor-risk: chemotherapya | 123 | 1 | 1 | 1 | ||||||
| Poor-risk: MRD | 55 | 0.97 | 0.63–1.51 | 0.90 | 1.79 | 0.66–4.82 | 0.25 | 0.74 | 0.48–1.14 | 0.17 |
| Poor-risk: URD | 55 | 0.97 | 0.62–1.51 | 0.89 | 3.77 | 1.65–8.62 | 0.002 | 0.37 | 0.21–0.64 | <0.001 |
| Age at diagnosis | ||||||||||
| 3–11 yoa | 79 | 1 | 1 | 1 | ||||||
| 0–2 yo | 80 | 0.86 | 0.55–1.34 | 0.49 | 0.37 | 0.12–1.18 | 0.09 | 1.11 | 0.71–1.71 | 0.65 |
| 12–21 yo | 74 | 1.49 | 0.98–2.27 | 0.07 | 1.81 | 0.83–3.97 | 0.14 | 0.98 | 0.63–1.53 | 0.93 |
| Race | ||||||||||
| Not African Americana | 183 | 1 | 1 | 1 | ||||||
| African American | 28 | 2.15 | 1.34–3.44 | 0.001 | 0.82 | 0.24–2.77 | 0.75 | 2.58 | 1.61–4.12 | <0.001 |
| Ethnicity | ||||||||||
| Not Hispanica | 207 | 1 | 1 | 1 | ||||||
| Hispanic | 18 | 0.72 | 0.35–1.48 | 0.38 | 0.40 | 0.06–2.83 | 0.36 | 0.81 | 0.40–1.64 | 0.55 |
| WBC at diagnosis | ||||||||||
| <50,000a | 199 | 1 | 1 | 1 | ||||||
| ≥50,000 | 28 | 1.99 | 1.23–3.23 | 0.005 | 0.53 | 0.12–2.31 | 0.39 | 1.93 | 1.08–3.46 | 0.03 |
| Weight category | ||||||||||
| Not overweighta | 148 | 1 | 1 | 1 | ||||||
| Overweight | 27 | 1.07 | 0.62–1.87 | 0.80 | 1.52 | 0.56–4.12 | 0.41 | 0.83 | 0.45–1.53 | 0.55 |
| Cytogenetics: monosomy 7 or del7q | ||||||||||
| Negativea | 143 | 1 | 1 | 1 | ||||||
| Positive | 90 | 1.01 | 0.70–1.44 | 0.98 | 2.14 | 1.04–4.39 | 0.04 | 0.91 | 0.62–1.31 | 0.60 |
| Cytogenetics: complexity ≥5 | ||||||||||
| Negativea | 140 | 1 | 1 | 1 | ||||||
| Positive | 93 | 0.88 | 0.61–1.27 | 0.50 | 0.44 | 0.19–1.01 | 0.05 | 0.98 | 0.68–1.42 | 0.92 |
| Time period | ||||||||||
| 1989–1997a | 102 | 1 | 1 | 1 | ||||||
| 1998–2006 | 131 | 0.84 | 0.59–1.20 | 0.34 | 0.92 | 0.45–1.88 | 0.86 | 0.84 | 0.58–1.20 | 0.34 |
OS, overall survival; TRM, treatment-related mortality; RR, relapse risk; HR, hazard ratio; CI, confidence interval; MRD, matched related donor; URD, unrelated donor; yo, years old.
Identifies reference group.
Fig. 1.
Overall survival (A), relapse risk (B), and treatment related mortality (C) by treatment group.
Results of Multivariate Analysis
The results of multivariate analysis are shown in Table III. Consistent with the results of univariate analysis, there were no differences in overall survival between the three treatment groups. The only factor significantly associated with overall survival was patient race; the effect of patient race was independent of treatment type. Overall mortality risks were higher in African Americans. Other factors such as age, WBC at diagnosis, and treatment period were not significantly associated with survival.
TABLE III.
Multivariate Analysis of Clinical Outcomes
| OS
|
TRM
|
RR
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Treatment group | ||||||||||
| Poor-risk: chemotherapya | 109 | 1 | 1 | 1 | ||||||
| Poor-risk: MRD | 49 | 1.08 | 0.65–1.80 | 0.76 | 1.32 | 0.38–4.62 | 0.66 | 0.82 | 0.50–1.34 | 0.42 |
| Poor-risk: URD | 47 | 1.13 | 0.66–1.92 | 0.67 | 2.66 | 0.93–7.62 | 0.07 | 0.43 | 0.23–0.81 | 0.01 |
| Race | ||||||||||
| Not African Americana | 178 | 1 | 1 | 1 | ||||||
| African American | 27 | 2.02 | 1.20–3.40 | 0.009 | 1.35 | 0.32–5.75 | 0.69 | 2.01 | 1.22–3.30 | 0.01 |
| Age at diagnosis | ||||||||||
| 3–11 yoa | 72 | 1 | 1 | 1 | ||||||
| 0–2 yo | 64 | 0.94 | 0.56–1.55 | 0.80 | 0.66 | 0.19–2.26 | 0.51 | 0.98 | 0.60–1.58 | 0.92 |
| 12–21 yo | 69 | 1.45 | 0.92–2.29 | 0.11 | 2.01 | 0.85–4.73 | 0.11 | 0.94 | 0.58–1.54 | 0.82 |
| WBC at diagnosis | ||||||||||
| <50,000a | 179 | 1 | 1 | 1 | ||||||
| ≥50,000 | 26 | 1.54 | 0.90–2.65 | 0.12 | 0.65 | 0.12–3.37 | 0.61 | 1.52 | 0.81–2.86 | 0.19 |
| Time period | ||||||||||
| 1989–1997a | 89 | 1 | 1 | 1 | ||||||
| 1998–2006 | 116 | 0.90 | 0.61–1.34 | 0.62 | 0.94 | 0.41–2.15 | 0.88 | 0.84 | 0.57–1.25 | 0.39 |
OS, overall survival; TRM, treatment-related mortality; RR, relapse risk; HR, hazard ratio; CI, confidence interval; MRD, matched related donor; URD, unrelated donor; yo, years old.
Identifies reference group.
Unlike the results of univariate analysis, there were no statistically significant differences in TRM risks between the three treatment groups (Table III). In a competing risks regression model relapse risks were lower after URD transplantation (HR 0.43, P = 0.01) but not MRD transplantation (HR 0.82, P = 0.42) when compared to chemotherapy only.
Results by Time Period
Because of changes in clinical practice during the time period over which patients in this study were treated, OS, RR, and TRM results were stratified by time period within each treatment group and compared (Table IV). There was no significant difference in OS, RR, or TRM within the treatment groups by time period. There was a non-significant reduction in TRM and improvement in OS for those treated with URD over time.
TABLE IV.
Comparison of Clinical Outcomes Within Treatment Group by Time Period
| Treatment group | N | Time period | OSa | P | Relapseb | P | TRM | P |
|---|---|---|---|---|---|---|---|---|
| Poor risk: chemotherapy | 46 | 1989–1997 | 39 ± 14% | 0.64 | 65 ± 14% | 0.49 | 7 ± 7% | 0.72 |
| 77 | 1998–2006 | 46 ± 12% | 58 ± 12% | 8 ± 6% | ||||
| Poor risk: MRD | 34 | 1989–1997 | 47 ± 17% | 0.80 | 53 ± 17% | 0.51 | 9 ± 10% | 0.26 |
| 21 | 1998–2006 | 46 ± 22% | 48 ± 22% | 19 ± 17% | ||||
| Poor risk: URD | 22 | 1989–1997 | 36 ± 21% | 0.11 | 32 ± 20% | 0.67 | 36 ± 21% | 0.17 |
| 33 | 1998–2006 | 60 ± 17% | 27 ± 16% | 15 ± 13% |
OS, overall survival; RR, relapse risk; TRM, treatment-related mortality; MRD, matched related donor; URD, unrelated donor.
All outcomes are rates at 5 years.
Relapse refers to cumulative incidence of relapse.
Graft Versus Host Disease
As expected, rates of GVHD were higher among URD HCT recipients than MRD recipients. The rates of grade II–IV acute GVHD were 27% and 45% among the MRD and URD groups, respectively (P = 0.06). The rates of chronic GVHD were 17% and 39%, respectively, (P = 0.01). Nearly half of the URD HCT recipients with chronic GVHD had extensive disease.
Causes of Death
There were a total of 30 deaths attributed to TRM (chemotherapy, n = 9, MRD, n = 7, and URD n = 14). There were no predominant causes of death in any of the groups; causes included infection, interstitial pneumonitis, adult respiratory distress syndrome and GVHD and organ failure (HCT only).
DISCUSSION
This study demonstrates that for children with AML in CR1 with poor-risk cytogenetic abnormalities, treatment with HCT or chemotherapy alone on COG trials CCG 2891, POG 9421, CCG 2961, and AAML03P1 achieve comparable OS. Our observations confirm and extend the findings of others [5,25]. In an earlier report from the COG [5], nine recipients of MRD HCT were compared to 38 recipients of chemotherapy alone and significant differences in survival were not observed between the two poor-risk groups. In the recent report from the Berlin-Frankfurt-Munster group (AML-BFM98) [25], a subgroup analysis of 30 patients with poor-risk disease as defined by the COG study also did not observe significant survival differences among those with (n = 11) and without a matched sibling donor (n = 19). We defined poor-risk cytogenetics to be as consistent as possible with the COG’s earlier study [5] but there are distinctions between the definitions used. We included del7q and t(6;9) as poor risk abnormalities and included only cytogenetics to define poor risk; we did not use the blast count at day-15 in our risk definition. Several trials led by the pediatric cooperative groups worldwide have utilized MRD HCT for children with poor-risk cytogenetic abnormalities [1,7,13,25], and others have assigned treatment with MRD, URD, or other alternative donor HCT for these high-risk patients in the absence of a clear advantage for HCT over chemotherapy [8–10,26,27]. The current analysis challenges the convention that HCT should be offered for AML patients in CR1 with poor-risk cytogenetics as the only adverse risk factor.
Given that consolidation chemotherapy, MRD HCT, and URD HCT, appear to offer children with poor-risk cytogenetics similar hope for survival, it is important to consider health related quality of life and cost. The recent report from the BFM study group highlights the burden of toxicity associated with the transplantation procedure as compared to chemotherapy alone and in the absence of a survival advantage after MRD or URD HSCT [25]. Further, transplant recipients are at risk for chronic GVHD the risk of which varies by donor source and degree of donor-recipient HLA-match. In severe cases, chronic GVHD can be associated with compromise of physical function, and altered health-related quality of life (HRQL) [28]. Chronic GVHD is also a risk factor for late mortality for those that survive at least 2 years after HCT [29]. There is insufficient evidence to draw conclusions regarding the costs of treatment with chemotherapy compared to HCT with AML [30]. A pediatric specific cost-effectiveness analysis is needed.
Although there was no significant difference in survival, our findings highlight the protection against relapse that can be achieved through allogeneic HCT, specifically URD HCT. Recipients of URD HCT had nearly half the relapse risk than the children treated with chemotherapy. Unfortunately, the protection against relapse for URD HCT recipients was offset by higher risk of TRM. While one could debate about the relative merits of a graft-versus-leukemia effect from transplantation [31], TRM is high even with substantial improvements in supportive care in the current era [32,33] and higher TRM diminishes the anti-leukemia benefit.
There is no “standard” definition of poor-risk cytogenetics in pediatric AML [34]. We chose to define poor-risk cytogenetics according to the definitions used by the MRC and the COG at the times the studies were conducted so that the results would complement the results of previous studies comparing the treatment effect of BMT compared to chemotherapy by cytogenetic risk group [5,16,25]. We therefore included complex cytogenetics (≥5 cytogenetic abnormalities) in our definition of poor-risk although complex cytogenetics has been found to be associated with a poor prognosis by some pediatric study groups [18,34] but not others [17]. We additionally included t(6;9) in our definition of poor-risk as this translocation has been associated with inferior survival [17,18]. However, there are several other cytogenetic abnormalities that should be considered in future definitions of poor-risk. Specific MLL rearrangements have been identified as high-risk by the partner chromosome that translocates with 11q23 [35]. Other poor-risk abnormalities include abnormal 12p, t(7;12)(p36;q13) and t(5;11)(q35;p15.5) [17,18,34]. Due to the data collection forms used at the time to the transplants we were unable to identify these abnormalities in subjects drawn from the CIBMTR.
A limitation is that we were unable to distinguish between monosomy 7 and del(7q) abnormalities in all of the patients in our cohort. Although we explored the differences in survival among monosomy 7 and del(7q) patients for which we could differentiate the specific cytogenetic abnormality (the COG patients) in a sensitivity analysis, del(7q) has been demonstrated to be an intermediate risk abnormality and should be classified as such in future analyses [24].
There are several limitations when performing retrospective comparisons but we have performed a carefully controlled analysis. When comparing three different treatment options it is important to ensure comparable groups of patients. We adjusted for imbalances in patient characteristics in the three groups through the conduction of a multivariate survival analysis. In addition, the start time for analyses was the start of consolidation for the chemotherapy only group and for the HCT group, start of transplant conditioning. We remind the reader that the favorable survival reported here for this high risk group of pediatric AML patients should not be compared to reports of overall survival from the diagnosis AML. Another limitation is the assignment of HLA-match for the URD cohort. Newer techniques of HLA typing and the wide spread adoption of allele-level HLA typing in recent years has lowered TRM risks after URD HCT. During the course of this study, less stringent HLA-matching guidelines were employed.
Because of the changes in HLA-typing and other treatment practices over time that have likely contributed to improvements in survival for children with AML treated with both chemotherapy and HCT we considered the effect of time period of treatment in our analysis. We included time period as a variable in both the univariate and multivariate analyses but did not find a significant association of time period with OS, RR, or TRM in either analysis. We further explored the effect of time period by comparing OS, RR, and TRM within each treatment group by time period. Although OS improved and TRM decreased in more recent years for those treated with URD HCT the improvement was not statistically significant. However, the comparisons of outcomes by time period within treatment group are limited by the small numbers of patients in each treatment group, particularly for those treated with MRD or URD HCT.
High-risk AML is no longer defined solely by cytogenetics, but is also defined by response to therapy, minimal residual disease status at the end of induction, and the presence of molecular mutations such as FLT3 mutations with high allelic ratios [34]. Our analysis included patients treated over a 15-year time period during which the COG trials’ risk stratification evolved which prohibited us from testing for these relatively recent definitions of high risk AML. Minimal residual disease status and FLT3 mutation status was not collected at the time most of the patients in this analysis were treated. We caution the reader to remember that our findings apply only to those with poor risk cytogenetics, not all high-risk AML patients. The size of the current analysis is a strength. Previous analyses and reviews have been limited in their ability to compare treatments for these patients given that they comprise a small subset of patients on any one study [1,5,12,25]. To our knowledge this is the largest description of pediatric AML patients with poor-risk cytogenetics. As data accrues, the role of HCT in the presence of adverse risk factors such as response to induction therapy and the presence of molecular mutations should be examined. In the mean time, our observations do not support the routine use of HCT in CR1 for AML in children and adolescents with poor-risk cytogenetics and should only be offered in the setting of carefully controlled clinical trials designed to incorporate the relatively new adverse risk factors.
Acknowledgments
Grant sponsor: National Cancer Institute; Grant number: KM1 CA 156726; Grant sponsor: Public Health Service; Grant number: U24-CA76518; Grant Sponsors: National Heart Lung and Blood Institute; National Institute of Allergy and Infectious Diseases; National Institutes of Health
This work was funded in part by a KM1 award received by Dr. Kelly from the National Cancer Institute (KM1 CA 156726, PI: H. Selker) and Public Health Service grant (U24-CA76518, PI: M.M. Horowitz) from the National Cancer Institute, the National Heart Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge and thank Janet Cowan, PhD, Tufts Medical Center, who assisted with the analysis of cytogenetic data and Rebecca Burns, research assistant in the Institute for Clinical Research and Health Policy Studies at Tufts Medical Center, who assisted with the preparation of the manuscript.
Footnotes
Conflict of interest: Nothing to declare.
Presented in part at the 2012 American Society of Hematology Meeting.
References
- 1.Niewerth D, Creutzig U, Bierings MB, et al. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- 2.Chen AR, Alonzo TA, Woods WG, et al. Current controversies: Which patients with acute myeloid leukaemia should receive a bone marrow transplantation? An American view. Br J Haematol. 2002;118:378–384. doi: 10.1046/j.1365-2141.2002.03701.x. [DOI] [PubMed] [Google Scholar]
- 3.Creutzig U, Reinhardt D. Current controversies: Which patients with acute myeloid leukaemia should receive a bone marrow transplantation? A European view. Br J Haematol. 2002;118:365–377. doi: 10.1046/j.1365-2141.2002.03697.x. [DOI] [PubMed] [Google Scholar]
- 4.Oliansky DM, Rizzo JD, Aplan PD, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: An evidence-based review. Biol Blood Marrow Transplant. 2007;13:1. doi: 10.1016/j.bbmt.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Horan JT, Alonzo TA, Lyman GH, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: The Children’s Oncology Group. J Clin Oncol. 2008;26:5797–5801. doi: 10.1200/JCO.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker KS, Bresters D, Sande JE. The burden of cure: Long-term side effects following hematopoietic stem cell transplantation (HSCT) in children. Pediatr Clin North Am. 2010;57:323. doi: 10.1016/j.pcl.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with Acute Myeloid Leukaemia: Medical Research Council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: The AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. [Accessed March 18, 2013.];Bortezomib and sorafenib in patients with newly diagnosed acute myeloid leukemia with or without mutations. http://www.clinicaltrials.gov/ct2/show/NCT01371981. Published June 12, 2011.
- 11.Becton D, Dahl GV, Ravindranath Y, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: A report from the children’s oncology group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia. Cancer. 2011;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 14.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: A report from the Children’s Cancer Group. Blood. 1996;87:4979–4989. [PubMed] [Google Scholar]
- 15.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: Revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 17.Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 18.von Neuhoff C, Reinhardt D, Sander A, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010;28:2682–2689. doi: 10.1200/JCO.2009.25.6321. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J R Stat Soc. 1958;53:457–481. [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972:187–220. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York, NY: John Wiley and Sons; 1980. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J R Stat Soc. 1999;94:496–509. [Google Scholar]
- 23.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–1112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 24.Hasle H, Alonzo TA, Auvrignon A, et al. Monosomy 7 and deletion 7q in children and adolescents with acute myeloid leukemia: An international retrospective study. Blood. 2007;109:4641–4647. doi: 10.1182/blood-2006-10-051342. [DOI] [PubMed] [Google Scholar]
- 25.Klusmann JH, Reinhardt D, Zimmermann M, et al. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: Results from the AML-BFM 98 study. Haematologica. 2012;97:21–29. doi: 10.3324/haematol.2011.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukemia: Results of the AML02 multicenter trial. Lancet Oncol. 2010;11:543. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Children’s Oncology Group. [Accessed March 27, 2013.];Combination chemotherapy with or without gemtuzumab in treating young patients with newly diagnosed acute myeloid leukemia. http://www.clinicaltrials.gov/ct2/show/NCT00372593. Published September 6, 2006.
- 28.Dahllöf G, Hingorani SR, Sanders JE. Late effects following hematopoietic cell transplantation for children. Biol Blood Marrow Transplant. 2008;14:88–893. doi: 10.1016/j.bbmt.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashfaq K, Yahaya I, Hyde C, et al. Clinical effectiveness and cost-effectiveness of stem cell transplantation in the management of acute leukaemia: A systematic review. Health Technology Assessment. 2010;14:1–167. doi: 10.3310/hta14540. [DOI] [PubMed] [Google Scholar]
- 31.Weisdorf D, Zhang MJ, Arora M, et al. Graft vs. host disease induced graft vs. leukemia effect: Greater impact on relapse and disease-free survival following reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18:1727–1733. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: How much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creutzig U, van den Heuvel-Eibrink M, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: Recommendations from an international expert panel. Blood. 2012;120:3187–3205. doi: 10.1182/blood-2012-03-362608. [DOI] [PubMed] [Google Scholar]
- 35.Balgobind BV, Raimondi SC, Harbott J, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: Results of an international retrospective study. Blood. 2009;114:2489–2496. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]