Abstract
Mechanisms underlying ethanol (EtOH)-induced detrusor smooth muscle (DSM) relaxation and increased urinary bladder capacity remain unknown. We investigated whether the large conductance Ca2+-activated K+ (BK) channels or L-type voltage-dependent Ca2+ channels (VDCCs), major regulators of DSM excitability and contractility, are targets for EtOH by patch-clamp electrophysiology (conventional and perforated whole cell and excised patch single channel) and isometric tension recordings using guinea pig DSM cells and isolated tissue strips, respectively. EtOH at 0.3% vol/vol (∼50 mM) enhanced whole cell BK currents at +30 mV and above, determined by the selective BK channel blocker paxilline. In excised patches recorded at +40 mV and ∼300 nM intracellular Ca2+ concentration ([Ca2+]), EtOH (0.1–0.3%) affected single BK channels (mean conductance ∼210 pS and blocked by paxilline) by increasing the open channel probability, number of open channel events, and open dwell-time constants. The amplitude of single BK channel currents and unitary conductance were not altered by EtOH. Conversely, at ∼10 μM but not ∼2 μM intracellular [Ca2+], EtOH (0.3%) decreased the single BK channel activity. EtOH (0.3%) affected transient BK currents (TBKCs) by either increasing frequency or decreasing amplitude, depending on the basal level of TBKC frequency. In isolated DSM strips, EtOH (0.1–1%) reduced the amplitude and muscle force of spontaneous phasic contractions. The EtOH-induced DSM relaxation, except at 1%, was attenuated by paxilline. EtOH (1%) inhibited L-type VDCC currents in DSM cells. In summary, we reveal the involvement of BK channels and L-type VDCCs in mediating EtOH-induced urinary bladder relaxation accommodating alcohol-induced diuresis.
Keywords: smooth muscle, patch-clamp, contractility, detrusor, alcohol
urinary bladder function depends on the contractile status of detrusor smooth muscle (DSM) cells. DSM relaxation allows for bladder expansion during urine storage, whereas its contraction, coupled with the opening of the bladder sphincter, permits urine voiding (3). Multiple ion channels and receptors are expressed in DSM cells regulating bladder function. Among them, the large-conductance voltage- and Ca2+-activated K+ channels (also known as BK, Slo, or KCa1.1 channels) and L-type voltage-dependent Ca2+ channels (VDCCs) are recognized as key regulators of excitability and contractility of DSM (44). Supporting data, provided for DSM studies using rat, guinea pig, mouse, and importantly human tissues and cells (5, 17, 18, 22, 24, 25, 46), demonstrate the role of BK channels in the regulation of the resting membrane potential, modulation of the repolarization phase of DSM action potentials, and generation of spontaneous transient BK currents (TBKCs), also known as spontaneous transient outward currents. For example, the BK channel blockade with either iberiotoxin or charybdotoxin prolonged the action potentials correlating with enhanced contractility (16, 18), whereas the BK channel opener NS11021 reduced action potential generation and DSM contractility in guinea pigs (29). In human DSM, the selective BK channel inhibitor iberiotoxin and activator NS1619 increased and decreased, respectively, the contractility of tissue strips and excitability of freshly isolated DSM cells confirming the important regulatory role of this K+ channel subtype (22, 25). Key characteristics of the BK channels are 1) their large conductance whereby the opening or closure of few channels has pronounced effects on cellular excitability; 2) a dual sensitivity to metabolic factors (e.g., Ca2+ and cAMP-pathways) and membrane voltage; and 3) a close functional interrelationship with VDCCs, which are the primary regulators of contractility mediating the influx of Ca2+ to initiate DSM contractions (3, 24, 44, 46, 54).
Two types of VDCCs (L-type and T-type) have been identified in DSM cells similar to other smooth muscle cell types (1, 13, 48). The lower threshold of activation for the T-type VDCCs determines that they are active at the resting membrane potential of approximately −40 mV and could regulate DSM excitability. The L-type VDCCs activate at more depolarized voltages, and their activity generates the upstroke phase of the DSM action potential allowing for net influx of Ca2+ to initiate contractility (12, 44).
Studies in non-DSM smooth muscle cells, neurons, and cells expressing recombinant BK channels demonstrated various direct effects of ethanol (EtOH) on BK channels and L-type VDCCs. The effects of EtOH on BK channel activity can be stimulatory, inhibitory, or neither. The type of response depends on multivariable factors including the species examined, the tissue or cell type investigated, the molecular composition of BK channel subunits expressed, the EtOH concentration used, and temporal exposure (4, 9, 35, 51). For example, in hypothalamic-neurohypophysial neurons, acute exposure to EtOH increased BK channel activity, whereas chronic 24-h treatment displayed much weaker effects (47). In cerebral artery smooth muscle cells, acute EtOH exposure in the presence of physiologically relevant high intracellular Ca2+ concentrations ([Ca2+]) has been shown to inhibit BK channel activity (6, 7, 31). In contrast, submicromolar concentrations of intracellular [Ca2+] promoted enhancement of BK channel activity (6, 7). In the case of L-type VDCCs, studies on non-DSM smooth muscle cells, neurons, and recombinant, cells demonstrated inhibitory effects of EtOH on the channel activity (10, 33).
In DSM, exposure to EtOH alters bladder function. In vitro studies with rabbit and rat DSM tissue strips revealed that EtOH evoked relaxation of pharmacologically induced contractions (38, 52, 56). In vivo cystometric measurements also demonstrated increased bladder capacity and residual urine volume in rats (56). In humans, clinical observations of acute intoxication-induced urinary retention, and experimental intravesicular or extravesicular EtOH application increased urinary bladder capacity (39, 42). These studies support the notion that EtOH directly modulates the responsiveness of DSM cells; however, the identity of molecular targets, in particular ion channels, remains unknown.
In this study, we investigated the effects of acute EtOH exposure on BK channels and L-type VDCC activity in freshly isolated DSM cells using the patch-clamp technique and isometric tension recordings of isolated DSM strips. We tested the hypothesis that acute EtOH application evokes DSM relaxation in vitro by targeting both BK channels and L-type VDCCs.
MATERIALS AND METHODS
DSM tissue harvesting.
Experiments were conducted in accordance with the Animal Use Protocol No. 1747 reviewed and approved by the University of South Carolina Institutional Animal Care and Use Committee. For this study, 60 adult male Hartley-Albino guinea pigs (300–570 g, average: 429.4 ± 9.8 g) were euthanized with CO2 followed by thoracotomy. Then, the whole bladder was cut above the bladder neck and transferred to a Petri dish containing dissection solution (DS; see Solutions and compounds). The whole bladder was excised, and the mucosa including urothelium was removed. DSM strips (5- to 7-mm long and 2- to 3-mm wide) were prepared for isometric tension recordings and for DSM single cell isolation.
Fresh DSM single cell isolation.
Guinea pig DSM cells were isolated as previously described (23, 41, 54). Briefly, small DSM strips were cut from the mucosa-free DSM specimens and placed in DS (2 ml) supplemented with 1 mg/ml BSA, 1 mg/ml papain, and 1 mg/ml dithiothreitol and incubated for 10–20 min at 37°C. Next, DSM strips were transferred to DS (2 ml) containing 1 mg/ml BSA, 0.5–1 mg/ml collagenase, and 100 μM CaCl2 for 9–15 min at 37°C. After the incubation, DSM strips were washed with fresh DS solution containing BSA. Individual cells were released from the tissue by passing the enzyme-treated DSM strips through a fire-polished Pasteur pipette.
Conventional and perforated whole cell patch-clamp recordings.
Electrophysiological experiments were performed based on methods and procedures previously described (23, 41, 54). Briefly, several drops of the DS containing freshly isolated DSM cells were placed into a recording chamber. After at least 20 min, the cells were washed several times with extracellular solution (see Solutions and compounds). We employed the conventional whole cell technique to record outward K+ currents and amphotericin-perforated whole cell recording method to measure TBKCs and L-type VDCC currents. Whole cell currents were recorded using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP version 10.2 software (Molecular Devices, Union City, CA). An eight-pole Bessel filter 900CT/9L8L (Frequency Devices, Ottawa, IL) was used to filter the recorded currents. The patch-clamp pipettes were prepared from borosilicate glass (Sutter Instruments, Novato, CA) and pulled using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan). The patch-clamp pipettes were fire-polished with a Microforge MF-830 to give a final tip resistance of approximately 3–6 MΩ. In conventional whole cell configuration, DSM cells were held at −60 mV, then depolarized from −80 mV up to +110 mV in 10-mV steps for 400 ms to elicit steady-state outward K+ channel currents, and then stepped back to −80 mV. TBKCs were recorded at −30 mV (corrected for the junction potential of approximately −10 mV). When the L-type VDCC currents were measured, DSM cells were held at −64 or −74 mV and stepped to voltages ranging from −54 to +46 in 10-mV steps for 400 ms and then returned to the holding voltage. Voltages in perforated patch-clamp experiments of Ca2+ currents were corrected for the liquid junction potential of approximately −14 mV. The stable control whole cell current was recorded for at least 6 min, and then EtOH (0.3 or 1%) or nifedipine (3 μM) was applied in the bath. All patch-clamp experiments were carried out at room temperature (22–23°C).
Single-channel recordings.
Isolated DSM cells were allowed to adhere to a glass-plated chamber for at least 30 min and then rinsed with the 140 mM K+ solution containing free Ca2+ concentration of ∼300 nM (WEBMAXC Standard: http://www.stanford.edu/∼cpatton/webmaxcS.htm; Chris Patton; see Solutions and compounds). Single BK channel recordings were done on excised inside-out or outside-out patches using symmetrical 140 mM KCl solution for both the bath and pipette compartments based on previous reports (29, 34, 45) with ∼300 nM, ∼2 μM, or 10 μM free [Ca2+], as indicated. In all excised patch single-channel recordings, the pipette solution contained ∼300 nM free [Ca2+]. The recordings were made with the same equipment as used for whole cell patch-clamp experiments using the single-channel configuration mode. The final tip resistance of electrodes was 6–15 MΩ. All patch-clamp experiments were conducted at room temperature (22–23°C).
Isometric DSM tension recordings.
The isometric DSM tension recording experiments were conducted as previously described (23, 41, 54). DSM strips were clipped between a stationary mount and a force-displacement transducer and then placed in tissue baths filled with Ca2+ containing physiological saline solution (PSS) thermostatically controlled at 37°C. DSM strips were stretched to an initial ∼10 mN and washed with fresh PSS every 15 min for 45–60 min. The neuronal Na+ channel blocker tetrodotoxin at 1 μM was then added into the bath to attenuate neuronal activity. Increasing EtOH concentrations (0.01–1%) were applied at 10 to 12-min intervals into the baths in the absence or presence of paxilline (1 μM), a selective BK channel inhibitor. Paxilline was preapplied for at least 45 min, and the effect of EtOH was determined in the presence of the BK channel blocker only when a stable baseline was achieved.
Solutions and compounds.
The DS solution with or without supplemented Ca2+ (100 μM) had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, pH 7.3, adjusted with NaOH. In patch-clamp experiments for single-channel recordings, the symmetrical K+ solution used contained (in mM): 140 KCl, 1.08 MgCl2, 5 EGTA, and 3.16 CaCl2, adjusted to pH 7.2 with NaOH (calculated [Ca2+]-free was ∼300 nM). In single-channel experiments requiring ∼2 or 10 μM free [Ca2+], CaCl2 was increased to 4.516 or 4.943 mM, respectively. In conventional whole cell experiments measuring outward K+ currents, the extracellular (bath) solution composition was as follows (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH; and the pipette solution was the same as used in the single-channel experiments. TBKCs were recorded with the perforated whole cell patch-clamp technique under conditions of the bath solution being the same as in the conventional whole cell experiments, and the following pipette solution components (in mM): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA with the pH adjusted to 7.2 with NaOH. For perforated whole cell patch-clamp experiments measuring L-type VDCC currents, the bath solution contained the following (in mM): 10 TEA-Cl, 6 CsCl, 124 NaCl, 1 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose (pH 7.4 adjusted with CsOH), and pipette solution supplemented with freshly dissolved (every 1–2 h) 200–300 μg/ml amphotericin-B (in mM): 110 CsOH, 110 aspartate (free), 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, and 30 CsCl (pH 7.2 adjusted with CsOH). The Ca2+-containing PSS for DSM contractility studies was prepared daily and had the following composition (in mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 11 glucose, and aerated with 95% O2-5% CO2 to obtain pH 7.4. Papain was obtained from Worthington (Lakewood, NJ), and dithiothreitol, collagenase type II, and paxilline were from Sigma-Aldrich (St. Louis, MO). Paxilline was dissolved as a stock solution in DMSO and diluted to the indicated concentrations. The final DMSO concentration in the bath did not exceed 0.05%.
Data analysis and statistics.
Clampfit software version 10.2 and GraphPad Prism version 4.03 (La Jolla, CA) were used for analysis of patch-clamp data. Whole cell BK currents were analyzed before and after the addition of EtOH as means of steady-state currents over the last 200 ms for each voltage. The amplitude of inward L-type VDCC currents was obtained by measuring peak negative inward current for each voltage step. The constants for half-maximum activation (V0.5) of L-type VDCC currents were calculated using the Boltzmann sigmoidal fit (GraphPad Prism) to the G/Gmax vs. voltage relationship. In the case of single BK channel recordings, the values for open single-channel probability of each patch (NPo) were obtained using the build-in algorithm in Clampfit, which calculates NPo as (To)/(To + Tc), where To and Tc correspond, respectively, to total open time and total closed time during the recording interval. Single BK channel currents were either filtered off-line with the Gaussian build-in option (11 coefficients) in Clampfit or not filtered before analysis. In excised patch-clamp experiments, the unitary BK channel conductance (determined from the current-voltage relationship) and the effects on Po at +40 mV were typically determined in the same patches. First, the unitary current amplitudes were measured at various voltages in the absence of EtOH. Second, the patches were stepped to +40 mV and the effects of EtOH were determined over 5- to 10-min intervals before and after its application. Third, in the continued presence of EtOH the unitary current amplitudes were recorded again at various voltages. When examining the single BK channel activity at other voltages, the analysis interval was ∼1–3 min for voltages of +80 mV and below and 15–60 s for +90 mV and higher. The graphs for mean single BK channel amplitude-voltage and Po-voltage were plotted in GraphPad Prism by respectively fitting linear regression (slope providing the conductance). The total number of BK channels present (N#) in each patch was determined at +80 to +100 mV. The determination of channel Po was done by dividing the open single-channel probability of each patch (NPo) by the number of ion channels present in the patch (N#). The single-channel amplitudes were calculated from all-point histograms using the Gaussian distribution function to qualify the values for closed and open states. The open dwell time constants were obtained in Clampfit using the two-term exponential equation from curve fitting of open-dwell time histograms. This function also provided the relative fraction of events for each component. To estimate the actual number of events for each component, the relative fraction was multiplied by the total number of events detected during the analysis of pre- and EtOH-treated intervals of 5–10 min. For normalization, the τ constant and the number of events values were expressed as fold increases over the pre-EtOH control taken as 1. TBKCs were analyzed using the threshold of 7.5 pA, which is three times the BK channel unitary current of 2.5 pA at −30 mV (21, 43), and the event detection function in Clampfit.
Isometric DSM tension recordings were measured with MyoMed software (Med Associates, St. Albans, VT). MiniAnalysis software (Synaptosoft, Decatur, GA) was used for data analysis of DSM phasic contraction amplitude and muscle force integral (determined by integrating the area under the curve of the phasic contractions) over the last 5-min interval before the addition of a higher concentration of EtOH. Increasing concentrations of EtOH were added at 10 to 12-min intervals. GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) was used for statistical analysis, and CorelDraw Graphic Suite software (Corel, Ottawa, ON, Canada) and Microsoft Powerpoint (Seattle, WA) for data illustration. The data are summarized as means ± SE (n = number of different DSM patches, cells, or tissue strips; N = number of guinea pigs) and compared using paired or unpaired Student's t-test. A P value of <0.05 was considered statistically significant.
RESULTS
EtOH increases whole cell BK currents in freshly isolated native DSM cells.
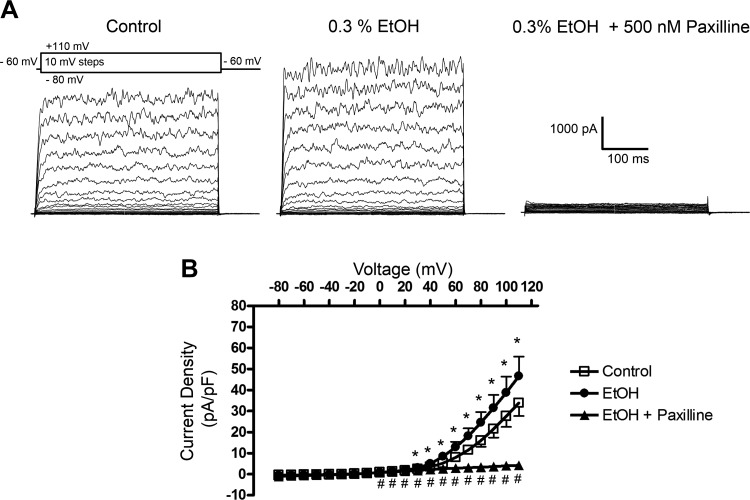
To examine whether EtOH modulates BK channel function, we first employed the conventional whole cell patch-clamp method with [Ca2+] of ∼300 nM and 2 mM for the pipette (intracellular) and the bath (extracellular), respectively. We utilized this approach rather than the perforated patch-clamp technique since we were interested in addressing the direct effects of EtOH on BK currents under the conditions of experimentally controlled intracellular Ca2+ levels. As shown in Fig. 1, whole cell currents were evoked by changing the voltage from −80 to +110 mV in steps of 10 mV in 11 cells (N = 10). The DSM cells had a mean capacitance of 43.1 ± 2.9 pF (n = 11, N = 10). This protocol allowed us to record the whole cell BK currents, especially at voltages above +30 mV. They were characterized by the noisy current profile indicative of channel openings with large conductance. The addition of EtOH at 0.3% significantly enhanced the responses measured at +30 mV and above (Fig. 1). For example, at +40 and +110 mV, the mean outward current density significantly increased by 1.43-fold (from 3.49 ± 0.47 to 4.96 ± 0.86 pA/pF) and 1.36-fold (from 33.81 ± 6.2 to 46.58 ± 9.30 pA/pF), respectively (n = 11, N = 10, P < 0.05). These experiments suggest that in DSM cells, EtOH directly enhances the activity of the BK channels. This was further supported by the experiments in which, in the continued presence of EtOH, the selective BK channel blocker paxilline (500 nM) was applied. Paxilline inhibited currents at 0 mV and above (P < 0.05, Fig. 1B) consistent with the previous observations in DSM cells showing contribution of BK channels at voltages of ∼0 to +10 mV and above (19, 22). For instance, at +40 and +110 mV the mean current density value significantly decreased to 2.18 ± 1.28 pA/pF and to 4.18 ± 5.69 pA/pF (n = 6, N = 6, P < 0.05), respectively. These results indicate that the majority of the outward K+ current recorded under the experimental conditions was carried by the BK channels and was also sensitive to the modulation by EtOH.
Fig. 1.
Enhancement of large conductance Ca2+-activated K+ (BK) channel outward currents by ethanol (EtOH) in freshly isolated native detrusor smooth muscle (DSM) cells. A: representative recordings obtained using the conventional whole cell patch-clamp configuration illustrating an increase in BK channel outward currents and current inhibition following the addition of the selective BK channel inhibitor paxilline at 500 nM. B: current-voltage relationships for control (pre-EtOH, n = 11, N = 10), 0.3% EtOH (n = 11, N = 10), and 500 nM paxilline (n = 6, N = 6) added in the presence of 0.3% EtOH (where n = number of different DSM cells; N = number of guinea pigs). *P < 0.05, statistical significance for comparison between control (pre-EtOH) and EtOH; and #P < 0.05, statistical significance for EtOH and EtOH + paxilline.
EtOH positively modulates the activity of single BK channels in freshly isolated native DSM cells in the presence of low intracellular [Ca2+].
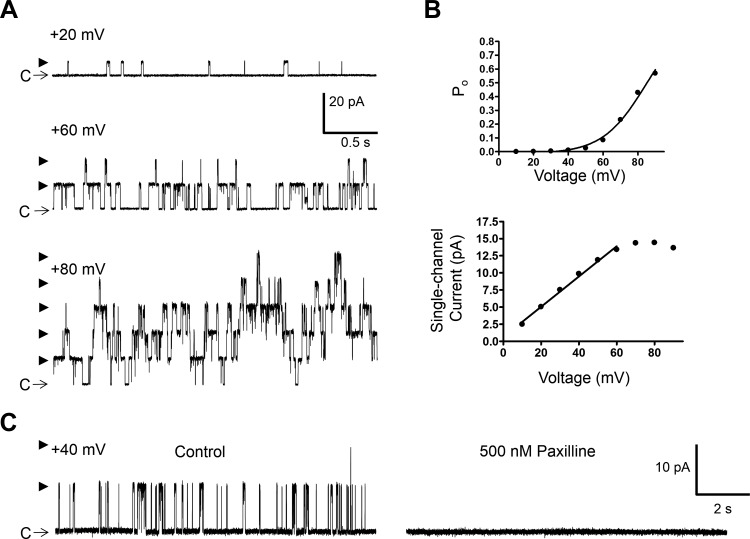
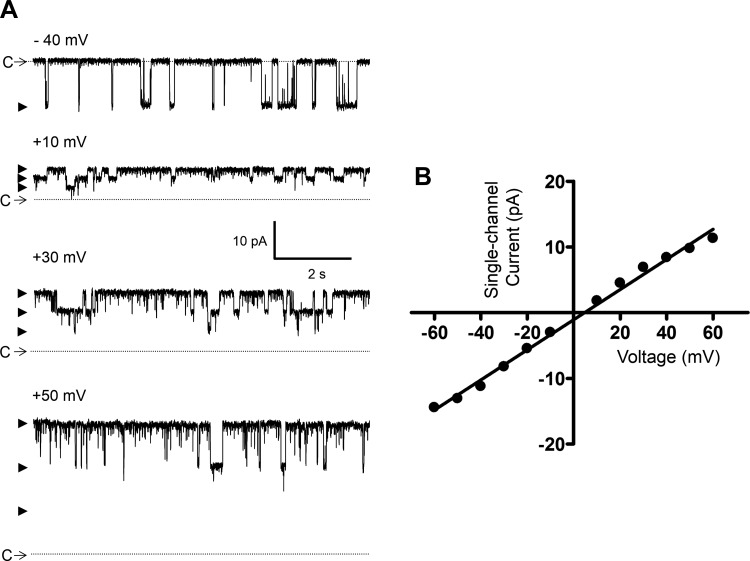
To determine whether EtOH directly modulates the activity of BK channels, the single-channel recordings were conducted by the cell-free excised patch-clamp method, both inside-out and outside-out configurations. In all experiments, a symmetrical high KCl solution containing 140 mM KCl and ∼300 nM free [Ca2+] was used for pipette and bath compartments. Under these experimental conditions, single BK channel currents were recorded at potentials above +20 mV as exemplified in Fig. 2, A and B. In total, single BK channel currents were measured in 14 outside-out and 16 inside-out patches (Table 1). There were no differences in the single-channel conductance and Po values measured for the two excised configurations. As indicated in Table 1, the mean single-channel conductance values were ∼210 pS. At +20 mV, the mean Po of ∼0.002 increased by approximately eightfold at +40 mV and ∼75-fold at +60 mV (normalized to Po at +20 mV) indicating strong voltage dependence. To further confirm that the measured single-channel currents were indeed mediated by BK channels, the effect of the selective BK channel inhibitor paxilline (500 nM) was examined in seven patches (5 inside-out and 2 outside-out, N = 3) at the Vh of +40 mV. A representative experiment is shown in Fig. 2C. Before the paxilline application, the control Po was 0.0045 ± 0.0015 (n = 7, N = 3) and the single-channel current amplitude 8.8 ± 0.9 pA (n = 7, N = 3). Paxilline (500 nM) inhibited all of the single-channel activity (Fig. 2C). In the 3-min recording following the application of paxilline, there were no single-channel openings.
Fig. 2.
Excised patch single BK channel recording from a freshly isolated native DSM cell in the presence of low intracellular Ca2+ concentration ([Ca2+]). A: representative single BK channel currents measured at the indicated voltages demonstrating an increase in channel activity with depolarization. C depicts the closed channel state and arrowheads the open channel states. B: single-channel current-voltage and open probability (Po)-voltage graphs for the patch represented by traces in A. C: single BK channel activity measured at +40 mV before and after the addition of paxilline (500 nM) showing inhibition of the BK channel opening. Data and recordings in A–C were obtained from the same inside-out patch using symmetrical 140 mM K+ solution with ∼300 nM free [Ca2+].
Table 1.
Single BK channel parameters in excised membrane patches derived from freshly isolated DSM cells measured at ∼300 nM [Ca2+]
| Inside-Out (n = 16, N =12) | Outside-Out (n = 14, N =12) | Combined Excised Patches (n = 30, N =18) | |
|---|---|---|---|
| Conductance, pS | 212.6 ± 4.8 | 206.8 ± 5.4 | 210.0 ± 3.5 |
| Po at +20 mV | 0.00206 ± 0.00109 | 0.00097 ± 0.00034 | 0.00155 ± 0.00060 |
| Po at +40 mV | 0.00584 ± 0.00187 | 0.00891 ± 0.00368 | 0.00727 ± 0.00197 |
| Po at +60 mV | 0.0428 ± 0.0150 | 0.0601 ± 0.0228 | 0.0509 ± 0.0132 |
| Normalized Po at +20 mV | 1 | 1 | 1 |
| Normalized Po at +40 mV | 7.7 ± 1.7 | 8.2 ± 1.0 | 7.9 ± 1.0 |
| Normalized Po at +60 mV | 76.9 ± 20.4 | 70.2 ± 13.1 | 73.8 ± 12.3 |
Values are means ± SE; n = number of different detrusor smooth muscle (DSM) patches; N = number of guinea pigs. Po, open channel probability.
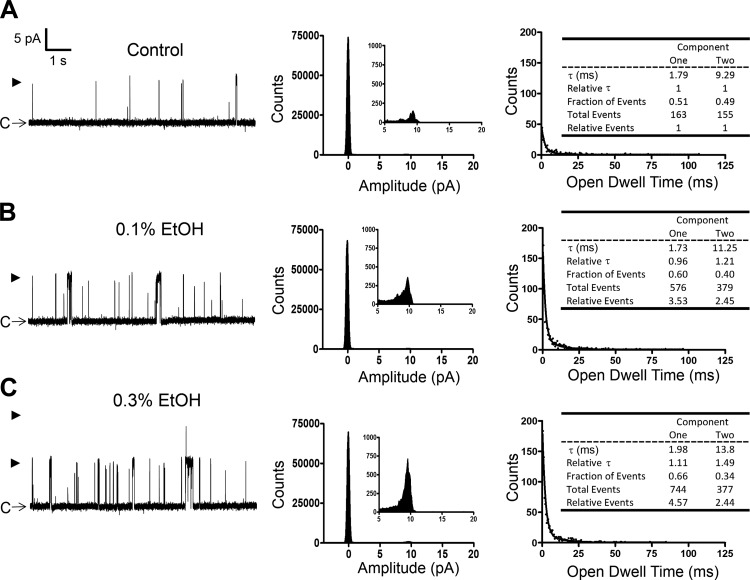
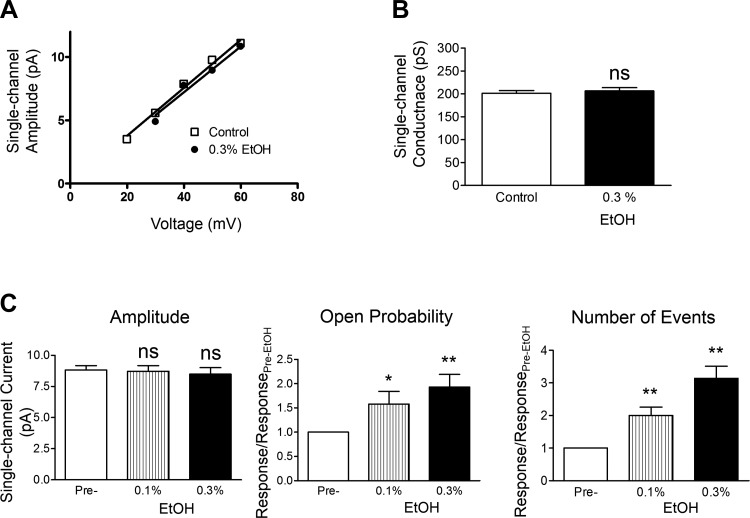
In subsequent experiments, the effects of EtOH at 0.1 and 0.3% were examined on single BK channel currents measured at +40 mV. A representative recording from this series of experiments is shown in Fig. 3A, whereas Fig. 4 provides the summary data for the mean single BK channel unitary conductance, Po, current amplitude, and total number of events in the absence or presence of EtOH. As summarized, EtOH at 0.1 and 0.3% caused significant enhancement of Po and increased the number of events but did not significantly change the single BK channel current amplitude. The single-channel conductance was determined from the slope of the single BK channel current-voltage relationship as exemplified in Fig. 4A and summarized in Fig. 4B. Consistent with the lack of an effect on the single BK channel amplitude, the mean single-channel conductance values were not significantly different in the absence or presence of EtOH. Figure 3 (histograms at right) further illustrates the effect of EtOH on the open dwell time constants. Similar to a previous study (8), we fitted the open dwell time histograms with the two-component function. This revealed two types of channel openings: one with a shorter time constant of ∼1–2 ms and the other of ∼10 ms. For each of the two time constants, we obtained the relative fraction of activities and the total number of events. For normalization purposes, we expressed the values as fold increase over the pre-EtOH control levels. In the example shown in Fig. 3, the two open dwell time constants at 0.3% EtOH increased by 1.1- and 1.5-fold and the relative events by 4.6- and 2.4-fold, respectively, for components 1 and 2. Table 2 provides the summary data for the effects of 0.1 and 0.3% EtOH on open channel dwell time parameters. These data show that EtOH changed the proportion of the BK channel openings favoring the channel activity with the shorter dwell time constant (i.e., component 1). This was accomplished by an increase in the number of events for this component. As summarized in Table 2, at 0.1 and 0.3% EtOH, the number of events for component 1 increased on average by ∼2.3- and 5-fold, respectively. In comparison, the incidence of component 2 increased by 1.6- and 1.8-fold for 0.1 and 0.3% EtOH, respectively. At 0.3% EtOH there were ∼1.5- and ∼1.8-fold increases in the dwell time constants for components 1 and 2, respectively. The increases in the dwell time constants and overall number of events underlie the observed enhancement of the Po (Table 2 and Fig. 4). Collectively, these single-channel recordings reveal that EtOH in the presence of low intracellular [Ca2+] of ∼300 nM positively modulates the activity of BK channels by increasing Po, the incidence of single-channel events, and open dwell time constants.
Fig. 3.
EtOH-induced enhancement of single BK channel currents in an excised patch from a freshly isolated native DSM cell in the presence of low intracellular [Ca2+]. A–C: representative recordings from a single experiment (outside-out configuration at +40 mV) depicting the single BK channel currents measured before (A) and after the application of EtOH at 0.1% (B) and 0.3% (C) under the recording condition of ∼300 nM free [Ca2+] in symmetrical 140 mM K+ solution. Middle: all point-histograms (analyzed interval of 30 s) for each condition. Right: summarized histograms for open-dwell times with the fitted line using the 2-component exponential function (exponential probability in Clampfit). Insets: channel parameters calculated for each condition. This example shows increases in the number of channel openings, especially component 1 (shorter open dwell time constant) and open dwell time for component 2 (longer dwell time constant). C depicts the closed channel state and arrowheads the open channel states.
Fig. 4.
Effects of EtOH on single BK channel activity in excised patches from native DSM cells under low intracellular [Ca2+]. A: an example of current-voltage relationship before and after the addition of 0.3% EtOH measured in the same excised patch. The represented slope single BK channel conductance values were 189 pS for pre-EtOH control and 181 pS for 0.3% EtOH. B: summary for the single-channel conductance in 9 excised patch experiments showing no significant change in the mean single BK channel conductance following the application of 0.3% EtOH (N = 9, P > 0.05). C: summary data for the effects of EtOH on the open channel probability, the number of events, and the single BK channel amplitude at +40 mV. Each data-point is n = 13–16 (N = 11–13). Data in A–C were obtained with ∼300 nM free [Ca2+] in symmetrical 140 mM K+ solution. Statistically significant effects were noted for Po and the number of events as indicated; *P < 0.05, **P < 0.01 (vs. pre-EtOH control values).
Table 2.
Summary of the effects by EtOH on single BK channel parameters
| Pre-EtOH (n = 13, N =10) | 0.1% EtOH (n = 11, N =8) | 0.3% EtOH (n = 10, N =8) | |
|---|---|---|---|
| Component 1 | |||
| Fraction of events | 0.37 ± 0.05 | 0.42 ± 0.06† | 0.63 ± 0.06† |
| Relative fold increase in number of events | 1 | 2.34 ± 0.36† | 5.01 ± 0.78‡ |
| τ, ms | 1.43 ± 0.15 | 1.58 ± 0.18 | 2.15 ± 0.36* |
| Relative fold increase in τ | 1 | 1.21 ± 0.15 | 1.46 ± 0.13† |
| Component 2 | |||
| Fraction of events | 0.63 ± 0.06 | 0.58 ± 0.06† | 0.37 ± 0.06† |
| Relative fold increase in number of events | 1 | 1.56 ± 0.21* | 1.78 ± 0.32* |
| τ, ms | 9.96 ± 0.90 | 10.26 ± 0.78 | 18.27 ± 3.50* |
| Relative fold increase in τ | 1 | 1.02 ± 0.09 | 1.77 ± 0.28* |
Values are means ± SE; n = number of different DSM patches; N = number of guinea pigs. BK channel, large conductance Ca2+-activated K+ channel; EtOH, ethanol. Comparison versus control (pre-EtOH) values:
P < 0.05,
P < 0.01,
P < 0.001.
EtOH induces differential effects on single BK channel activity in the presence of elevated intracellular [Ca2+]: no significant change at ∼2 μM and inhibition at ∼10 μM.
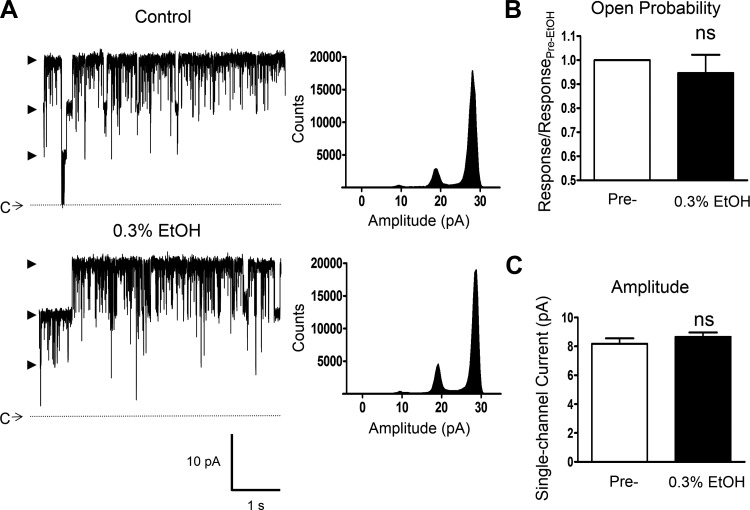
Since previous reports in non-DSM cells indicated that the effect of EtOH may depend on intracellular [Ca2+] (4, 8, 30), additional single BK channel recordings using the inside-out configuration under moderately elevated intracellular free [Ca2+] of ∼2 μM were performed. In the control condition (without EtOH), the single BK channel activity was very robust as illustrated in Fig. 5. Single BK channel openings were observed at negative voltages and increased with depolarization. At +40 mV, the mean single BK channel open probability was 0.84 ± 0.03 (n = 14, N = 9, P < 0.001 vs. Po at ∼300 nM [Ca2+], see Table 1). In 12 patches (N = 8), current-voltage relationships were determined, as exemplified in Fig. 5, A and B, revealing the mean single BK channel unitary conductance of 228.3 ± 7.0 pS under these experimental conditions used, which was not significantly different (P > 0.05) from the value obtained for ∼300 nM free [Ca2+] (see Table 1). Subsequently, the effect of EtOH at 0.3% was determined at +40 mV and revealed no statistically significant changes in the channel open probability nor in the single BK channel unitary current (n = 11, N = 8, P > 0.05 for both parameters; Fig. 6, A–C).
Fig. 5.
Excised patch single BK channel recording from a freshly isolated native DSM cell in the presence of moderately elevated intracellular [Ca2+]. A: representative single BK channel currents measured with the inside-out configuration using 140 mM K+ solution containing ∼2 μM free [Ca2+] for the intracellular (bath) compartment illustrating high degree of channel activity, the enhancement with depolarization, and multiple channel openings at negative voltages such as −40 mV (as shown). C and the dotted line depict the closed channel state and arrowheads the open channel states. B: single-channel current-voltage relationship for the patch represented by traces in A.
Fig. 6.
Lack of an effect by EtOH on native DSM single BK channel activity under the condition of moderately elevated intracellular [Ca2+]. A: representative recordings from a single experiment (inside-out configuration at +40 mV) with K+ solution containing ∼2 μM free [Ca2+] for the intracellular (bath) compartment before and after the application of 0.3% EtOH. Shown are also all point-histograms (analyzed interval of 30 s) for each condition. C depicts the closed channel state along with the dotted line and arrowheads the open channel states. B and C: summaries for the single BK channel open probability (B) and single BK channel current amplitude (C) in 11 inside-out excised patch experiments showing no significant changes in the mean values following the application of 0.3% EtOH in the presence of ∼2 μM intracellular free [Ca2+] (N = 8, P > 0.05); ns, nonsignificant vs. pre-EtOH control values.
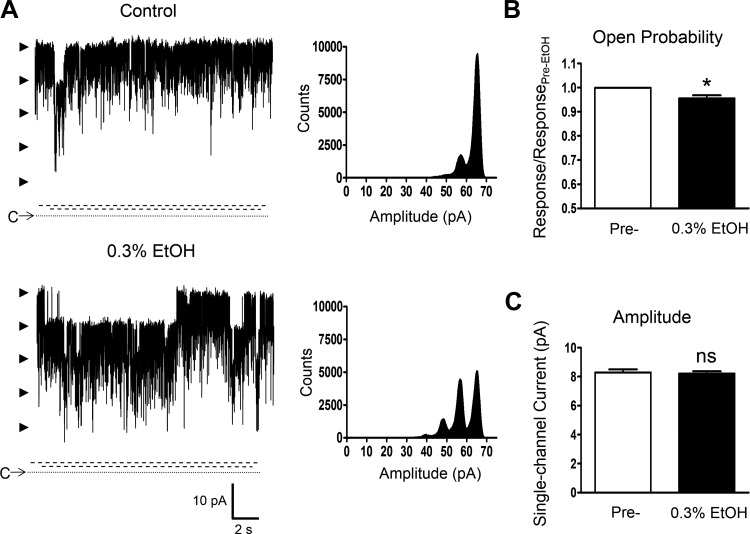
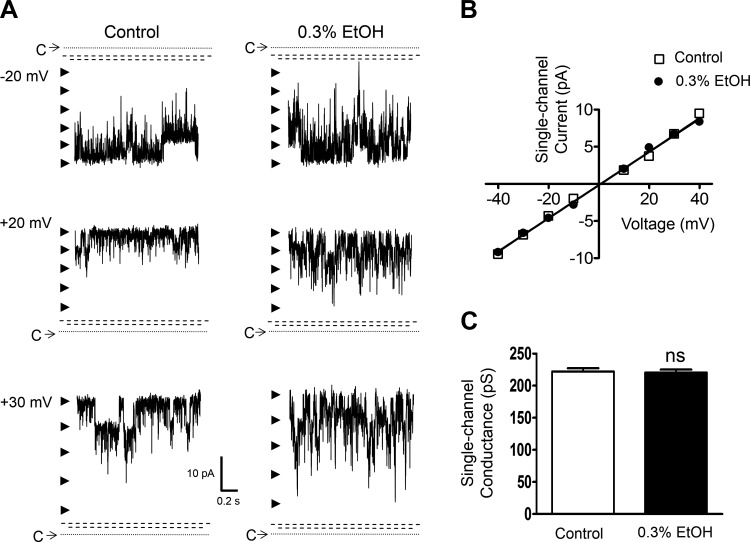
Next, the effect of EtOH (0.3%) was assessed in the presence of ∼10 μM intracellular free [Ca2+], which corresponds to a relatively high level of cytosolic Ca2+. As shown in Fig. 7, A–C, under this condition at +40 mV, EtOH caused a significant reduction of Po (n = 8, N = 4, P < 0.05) and had no effect on the unitary current amplitude (P > 0.05). The Po values were 0.817 ± 0.038 and 0.783 ± 0.037 in the absence and in the presence of EtOH, respectively (P < 0.05). Thus EtOH reduced the single BK channel activity on average by Po of ∼0.04, which corresponds approximately to the activity of untreated BK channels measured at +60 mV under the condition of ∼300 nM free [Ca2+] (Table 1). In addition, the unitary single BK channel conductance was not altered by EtOH at ∼10 μM intracellular free [Ca2+] (Fig. 8, A–C). The values were 222.3 ± 5.3 pS for the control and 220.3 ± 6.2 pS with EtOH (n = 8, N = 4, P > 0.05). The pre-EtOH control conductance values in the presence of ∼10 μM were not significantly different (P > 0.05) from those obtained at ∼ 300 nM (Table 1) and ∼ 2 μM [Ca2+]. These experiments reveal dependence on intracellular [Ca2+] for the effects of EtOH on DSM BK channels, specifically enhancement of channel activity at ∼300 nM, no significant modulation at ∼2 μM, and a decrease in Po at ∼10 μM.
Fig. 7.
EtOH-induced reduction of native DSM single BK channel activity under the condition of highly elevated intracellular [Ca2+]. A: representative recordings from a single experiment (inside-out configuration at +40 mV) with K+ solution containing ∼10 μM free [Ca2+] for the intracellular (bath) compartment before and after the application of 0.3% EtOH. Shown are also all point-histograms (analyzed interval of 30 s) for each condition. C and the dotted line depict the closed channel state, arrowheads the open channel states, and the parallel dashed lines breaks in the y-axis as there were no single channel events detected within the limits. B and C: Summaries for single BK channel open probability (B) and single BK channel current amplitude (C) in 8 inside-out excised patch experiments showing a significant decrease in the open probability, but no effect on the single BK channel unitary amplitude following the application of 0.3% EtOH in the presence of ∼10 μM intracellular free [Ca2+] (N = 4); *P < 0.05; ns, nonsignificant effect (P > 0.05) vs. pre-EtOH control values.
Fig. 8.
Lack of an effect by EtOH on native DSM single BK channel unitary conductance under the condition of highly elevated intracellular [Ca2+]. A: representative recordings from a single experiment (inside-out configuration at +40 mV) with K+ solution containing ∼10 μM free [Ca2+] for the intracellular (bath) compartment before (left) and after (right) the application of 0.3% EtOH at −20, +20, and +30 mV. C and the dotted line depict the closed channel state, arrowheads the open channel states, and the dashed parallel dashed lines breaks in the y-axis as no single channel events were recorded within the limits. B: unitary current-voltage relationship for the same patch depicted in A, and also in Fig. 7A, in the presence or absence of 0.3% EtOH. Represented slope single conductance values were 227 pS for pre-EtOH control and 223 pS for 0.3% EtOH. C: summary of the unitary conductance values in the presence or absence of 0.3% EtOH in 8 inside-out excised patch experiments in the presence of ∼10 μM intracellular free [Ca2+] (N = 4); ns, nonsignificant effect (P > 0.05) vs. pre-EtOH control values.
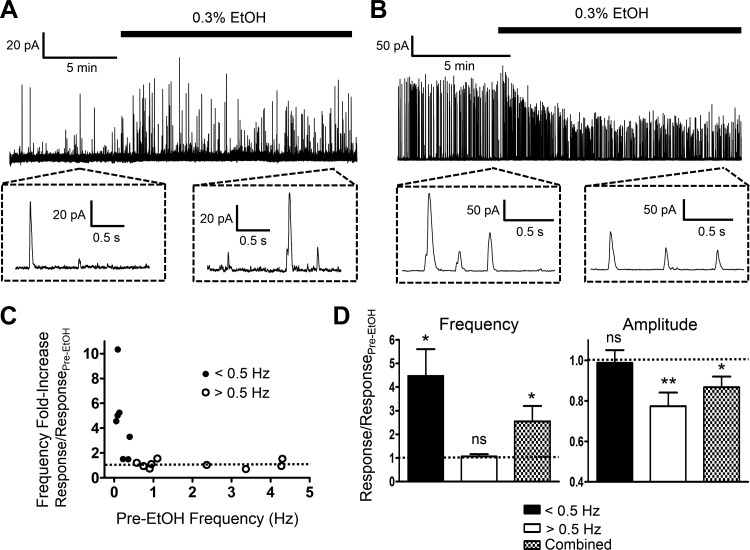
EtOH modulates TBKCs by either increasing the frequency or decreasing the amplitude depending on basal level of activity.
DSM cells exhibit robust TBKC activity, which regulates excitability of these cells (44). Whole cell perforated patch-clamp recordings were obtained in 17 cells (N = 13) at Vh of −30 mV with a mean cell capacitance of 45.5 ± 2.3 pF and a frequency and amplitude of TBKCs of 1.2 ± 0.4 Hz and 26.0 ± 6.0 pA, respectively. This high number of experiments was needed since DSM cells showed differential responses to EtOH. In certain DSM cells, as exemplified in Fig. 9A, EtOH induced enhancement of TBKCs by increasing their frequency. In other cells, shown in Fig. 9B, the primary effect of EtOH appeared on the TBKC amplitude. A key characteristic that determined how DSM cells responded was the TBKC frequency. As summarized in Fig. 9, C and D, DSM cells exhibiting the basal level of TBKC frequency of <0.5 Hz responded to EtOH with increases in frequency, whereas those above this threshold did not. The analysis of the basal pre-EtOH control characteristics of these two types of cells, which we refer to as low- and high-frequency TBKC DSM cells, revealed ∼10-fold separation for the mean frequency, 0.17 ± 0.05 (n = 8, N = 8) vs. 2.1 ± 0.5 Hz (n = 9, N = 7), and no statistically significant differences in the amplitude of TBKCs and cell capacitance. The values for low (n = 8, N = 8) and high (n = 9, N = 7) frequency TBKC DSM cells were, respectively, 15.9 ± 1.5 and 34.9 ± 10.8 pA (P > 0.05) for the amplitude, and 41.2 ± 2.7 and 49.3 ± 3.1 pF (P > 0.05) for the cell capacitance. Low-frequency TBKC DSM cells responded to 0.3% EtOH by an average of approximately fourfold increase in the frequency and no effect on the amplitude (Fig. 9D). In contrast, 0.3% EtOH in high-frequency TBKC DSM cells caused inhibition of the amplitude of TBKCs to ∼0.8-fold of the control and no effect on the frequency (Fig. 9D). The specific effects of EtOH depending on the basal level of activity would not have been obvious if all data for DSM cells were analyzed together. Both amplitude and frequency of TBKCs showed overall significant effects (n = 17, N = 13, P < 0.05; Fig. 9D).
Fig. 9.
Regulation of transient BK currents (TBKCs) by EtOH in native DSM cells. A and B: representative recordings in DSM cells, which exhibited a low (A) or a high (B) level of basal TBKC activity, showing differential responses to 0.3% EtOH. Insets depict TBKCs on an expanded time scale. C: summary graph of the increase in TBKC frequency vs. basal activity for each of the 17 recorded cells (N = 13). Cells with the basal activity of TBKCs of <0.5 Hz (low-frequency TBKC DSM cells) showed enhancement in the frequency. Dotted line represents the control level. D: summary of the effects of 0.3% EtOH on the frequency and amplitude of TBKCs for low-frequency TBKC DSM cells defined by the threshold activity level of <0.5 Hz (n = 8, N = 8), high-frequency TBKC DSM cells with >0.5 Hz (n = 9, N = 7), and for all DSM cells combined (n = 17, N = 13). For both the frequency and amplitude of TBKCs, the y-axis describes the fold increase over pre-EtOH control. Dotted lines represent the control levels. Statistically significant effects were noted as shown; *P < 0.05; **P < 0.01; and ns, nonsignificant vs. pre-EtOH control values.
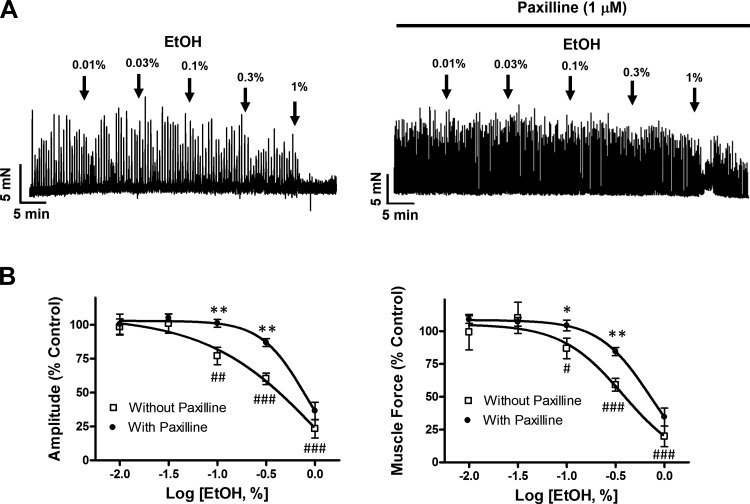
EtOH attenuates DSM spontaneous phasic contractions by BK channel-dependent and BK channel-independent mechanisms.
To examine the effects on DSM contractility, increasing concentrations of EtOH (0.01–1%) were applied to strips exhibiting spontaneous phasic contractions. EtOH inhibited DSM spontaneous phasic contraction amplitude and muscle force in a concentration-dependent manner (Fig. 10). At the maximal concentration of 1%, EtOH decreased DSM spontaneous phasic contraction amplitude and muscle force by 70.2 ± 10.3 and 72.3 ± 11.4%, respectively (n = 8, N = 4, P < 0.001 for both; Fig. 10B).
Fig. 10.
Attenuation of EtOH-induced DSM relaxation of DSM isolated tissue strips by the selective BK channel inhibitor paxilline. A: representative traces depicting the inhibitory effect of EtOH (0.01 to 1%) on spontaneous phasic contractions of DSM strips in the absence or presence of paxilline (1 μM). B: cumulative concentration-response curves for EtOH, without or with paxilline (1 μM), on DSM spontaneous phasic contraction amplitude and muscle force. ##P < 0.01 and ###P < 0.001, statistical differences for the comparison of DSM strips in the absence (without) paxilline vs. the pre-EtOH control level, i.e., the comparison of responses indicated by □ vs. the control of 100%. *P < 0.05 and ***P < 0.001, statistical differences for the effect of EtOH in the absence of paxilline (1 μM) vs. in its presence for each concentration of EtOH. Each data point in B is n = 8–17, N = 4–8.
Because BK channels are known to critically regulate DSM contractility (44), we further tested the effects of EtOH under the condition of BK channel blockade with paxilline. We found that paxilline (1 μM) attenuated the EtOH inhibitory effects on DSM phasic contraction amplitude and force (Fig. 10B). These results provide evidence that BK channels are involved in mediating the EtOH inhibitory effects on the spontaneous phasic contractions of DSM isolated strips. However, in the presence of paxilline EtOH was still able to cause DSM relaxation. In particular, at 1% EtOH, the effects were comparable in the absence or presence of paxilline. This points to the involvement of an additional non-BK channel-dependent mechanism, which we address below.
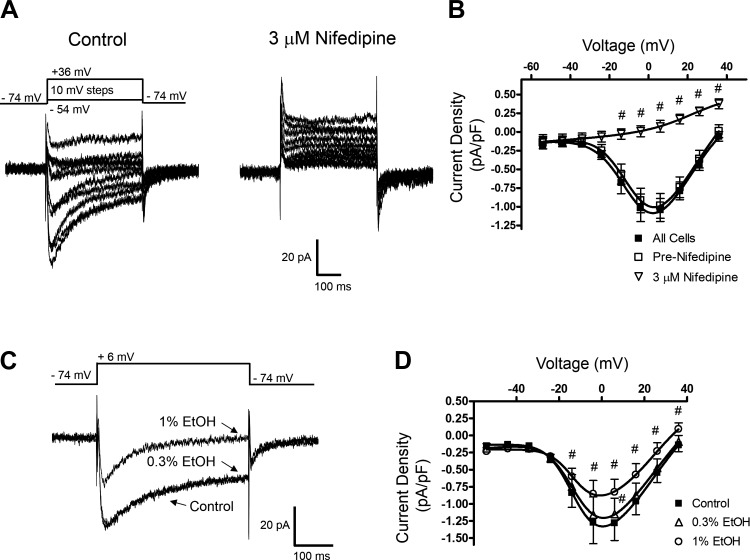
EtOH inhibits L-type VDCC currents in freshly isolated native DSM cells.
Reports indicate that in various non-DSM cells EtOH inhibits L-type VDCC channel activity (10, 33). Here, we examined whether EtOH could attenuate DSM VDCC activity and thus contribute to DSM relaxation. We recorded L-type VDCC currents using the whole cell perforated patch-clamp technique and solutions appropriate to isolate VDCC currents (see Solutions and compounds). In response to the voltage protocol in which cells were stepped from −74 to +36 mV in steps of 10 mV, the currents peaked at ∼+5 mV. At +6 mV, the average inward current density was −1.02 ± 0.2 pA/pF (n = 13, N = 9). The cell capacitance and the half-maximum constant for activation (V0.5) averaged 44.8 ± 3.4 pF and −7.5 ± 1.7 mV (n = 13, N = 9), respectively. This profile suggests that these inward currents were due to opening of L-type VDCC channels. To confirm this, we examined the effect of the selective L-type VDCC inhibitor nifedipine. Nifedipine (3 μM) completely inhibited the inward current. At +6 mV, the values before and after nifedipine were −0.99 ± 0.16 pA/pF and 0.07 ± 0.08 pA/pF (n = 5, N = 5, P < 0.001; Fig. 11). This demonstrates that L-type VDCCs were primarily responsible for the inward current recorded.
Fig. 11.
EtOH-induced inhibition of L-type VDCC currents recorded with the perforated whole cell patch-clamp method in freshly isolated native DSM cells. A: representative currents measured before and after the addition of the L-type VDCC channel inhibitor nifedipine (3 μM) evoked by depolarizing steps as indicated. B: current-voltage relationships for DSM cells in the absence or presence of nifedipine (3 μM). The label “All cells” indicates responses recorded in 11 cells (N = 10). “Pre-Nifedipine” and “3 μM Nifedipine” curves were measured in the same 6 cells (N = 5). Significant inhibition by nifedipine was observed for the voltages shown (#P < 0.05). C: examples of whole cell current traces in response to the specified protocol showing the effect of EtOH at 0.3 and 1%. The protocol used was the same as in A; for simplicity traces evoked by a single step causing maximum inward L-type VDCC currents are shown. D: summary of the inhibitory effect of EtOH at 0.3 and 1% on current-voltage relationship with significant reductions (#P < 0.05) in the DSM L-type VDCC current as indicated. Each data point is n = 6, N = 6.
The effect of EtOH was further examined on the L-type VDCC current. As shown in Fig. 11, EtOH at 0.3% had minimal effects on the L-type VDCC current (P > 0.05 for all voltages except for +6 mV). The subsequent increase to 1% EtOH significantly inhibited the L-type VDCC peak current for measured voltages at −14 mV and above. When measured at +6 mV, the current density decreased from the pre-EtOH control level of −1.28 ± 0.26 pA/pF to −1.15 ± 0.13 pA/pF (n = 6, N = 5, P < 0.05) at 0.3% EtOH and further to −0.83 ± 0.19 pA/pF (n = 6, N = 5, P < 0.001) at 1% EtOH. The constant for half-maximum activation was not significantly altered: −9.5 ± 2.7 mV and −6.5 ± 2.1 mV in the presence or absence of 1% EtOH, respectively (n = 6, N = 5, P > 0.05). This series of experiments indicates that in DSM cells EtOH inhibited L-type VDCCs in the concentration range producing non-BK channel-dependent relaxation. This provides support that EtOH mediates relaxation of DSM also by inhibiting L-type VDCCs when applied at higher concentrations (>0.3% EtOH).
DISCUSSION
The data presented here reveal that BK channels and L-type VDCCs are molecular targets for EtOH, thus supporting their role in mediating alcohol-induced relaxation of DSM. EtOH at concentrations as low as 0.1%, corresponding to blood alcohol concentration (BAC) achieved in moderate-heavy drinking, caused attenuation of DSM contractility. EtOH at 0.3% in the presence of ∼300 nM intracellular free [Ca2+] enhanced the whole cell outward BK current and at 0.1 and 0.3% increased the Po of single BK channel currents. The single BK channel recordings further revealed open-state-dependent effects of EtOH causing an increase in the number of events and open dwell time constants. In contrast, at 2 μM intracellular free [Ca2+] no statistically significant effects were observed for the open channel probability and single BK channel currents; whereas in the presence of 10 μM intracellular free [Ca2+], the open channel probability was significantly decreased demonstrating a strong dependence of the EtOH effects on [Ca2+]. The effect of EtOH (0.3%) on TBKCs depended on the basal level of activity in the frequency. Low-frequency TBKC DSM cells, those with the frequency <0.5 Hz, responded to EtOH by an increase in the frequency and no effect on the amplitude, whereas high-frequency TBKC cells showed no change in the frequency and a decrease in the amplitude of TBKCs. In the presence of the BK channel blocker paxilline, the inhibitory effects of EtOH on DSM spontaneous phasic contraction amplitude and muscle force, and on whole cell BK currents were attenuated supporting the involvement of these channels in mediating the DSM relaxation. However, EtOH at 1% evoked similar DSM relaxation in the presence or absence of the selective BK channel inhibitor paxilline. L-type VDCC blockade by EtOH likely plays a role in relaxation, as in isolated DSM cells these Ca2+ currents were directly inhibited by EtOH. Our experiments collectively support that EtOH-induced relaxation of DSM involves at least enhancement of BK channel activity and blockade of L-type VDCCs.
In acute EtOH intoxication, BAC may reach 0.5% and the loss of bladder control often occurs at ∼0.3% (37). At EtOH concentrations of 0.5–1%, various types of ion channels are affected, among them are BK channels and L-type VDCCs (9). The evidence for their involvement has been obtained in studies investigating neuronal or cardiovascular cells (4, 9). Additional insights have been provided in recombinant expression systems (10, 30). In contrast, relatively little work has been carried out in the urinary bladder. To our knowledge, no previous study addressed whether BK channels and L-type VDCCs are molecular targets for EtOH in DSM. Prior studies with DSM tissue strips demonstrated the EtOH-induced relaxation of carbachol-, purinergic agonist-, and electrical field stimulation-evoked DSM contractions (38, 52, 56). In this study, EtOH induced relaxation of DSM spontaneous phasic contractions by attenuating phasic contraction amplitude and muscle force at concentrations of 0.1% and higher. The observed DSM relaxation, hence, occurred at physiologically relevant BAC. Furthermore, the EtOH-mediated effects were attenuated by paxilline, a selective BK channel inhibitor, providing strong evidence for a role of BK channels.
To confirm that BK channels directly mediate EtOH-induced effects in DSM, whole cell patch-clamp and excised patch (both inside-out and outside-out configurations) single-channel recordings were carried out. In the case of single BK channel recordings in the presence of ∼300 nM free [Ca2+] for both pipette and bath, we have combined all of the data regardless of the configuration used since EtOH crosses the plasma membrane through simple diffusion (50). At the steady state, EtOH in the vicinity of the recorded patch should distribute at comparable levels intracellularly and extracellularly. There were no statistical differences, under the experimental conditions of this study, between the two inside- and outside-out configurations used (Table 1). Both whole cell (Fig. 1) and single-channel (Figs. 2 and 3) recordings showed that EtOH potentiated the activity of the BK channels. The enhancement by EtOH appeared to be dependent on the basal level of BK channel (current) activity. Only when the activity was not very low, an increase in the BK channel current occurred in the presence of ∼300 nM intracellular free [Ca2+] (Fig. 1). Since in the whole cell configuration direct and indirect effects of EtOH are difficult to distinguish, the data obtained with the excised patches clearly demonstrate the direct modulation of BK channels by EtOH. To our knowledge, no previous study investigated the effects of EtOH on DSM electrical properties with any configuration of the patch-clamp technique. In non-DSM cells, similar to our findings, acute EtOH exposure increased the whole cell BK currents in a subset of dorsal root ganglion neurons (15) and recombinant BK channels (mSlo) expressed in Xenopus laevis oocytes (30). The latter study also demonstrated the dependency of EtOH-mediated responses on intracellular Ca2+ levels. In the absence of Ca2+ or in the presence of high concentrations (≥10 μM), no change or inhibition, respectively, was observed. In our study, we noted similar effects of EtOH (0.3%) for Po observing no significant change for ∼2 μM (Fig. 6) and an inhibition for ∼10 μM intracellular [Ca2+] (Fig. 7) when measured with the inside-out configuration. The unitary single BK channel amplitude or the slope conductance was insensitive to EtOH at ∼2 or 10 μM intracellular free [Ca2+] (Figs. 6–8). Thus, in DSM cells, the effect of EtOH on BK channels is also dependent on [Ca2+]. In the presence of ∼2 or 10 μM intracellular free [Ca2+], it was not possible to reliably calculate open dwell time constants due to frequent simultaneous BK channel openings and high Po. Other investigators using excised patches derived from neuronal, recombinant, and GH3 cells with the [Ca2+] ranging from 100 nM to 10 μM reported EtOH-induced enhancements in the Po, increases in the open dwell time constant, and decreases in the closed dwell time constant without changing the single-channel conductance (8, 15, 26, 30). In our study, in excised patches derived from DSM cells at ∼300 nM intracellular free [Ca2+] where the Po was low, we also identified an enhancement of the Po, an increase in the open dwell time constants and no change in single BK channel conductance. The effects of EtOH on the open dwell time in DSM patches were different from those described previously. Unlike in the recombinant cells expressing mSlo in oocytes, where either decreases in mean open dwell time constant or increases in the longest time constant were seen at ∼0.3% (∼50 mM) (8, 30), in DSM patches at ∼300 nM free [Ca2+] we report increases for both shorter and longer time constants (Table 2). We also noted an increase in the proportion of dwell time events favoring the shorter time constant, in contrast to recombinant mSlo channels (8, 30), as well as an overall increase in the incidence of BK channel openings. The differences in the single BK channel properties may be due to species differences, mouse vs. guinea pig, or our experimental approach using native DSM cells vs. recombinant mSlo (8, 30).
TBKCs constitute a fundamental mechanism, which regulates excitability of DSM cells and other cell types (44). They are evoked by Ca2+ sparks, localized releases of subplasmalemmal Ca2+ via sarcoplasmic reticulum (SR) ryanodine receptors (20). In the current-clamp mode measuring membrane potential, Ca2+ spark-BK channel events manifest as spontaneous transient hyperpolarizations (STHs). STHs provide a hyperpolarizing influence regulating smooth muscle excitability. Indeed, DSM cells show a high degree of correlation for activities of TBKCs and STHs (55). The mechanisms regulating activity of TBKCs are of considerable interest. Previous studies have demonstrated that in DSM cells enhancement of the PKA signaling pathway via inhibition of phosphodiestareses (54, 55) or activation of muscarinic subtype 3 receptors with carbachol (40) enhanced or attenuated TBKCs, respectively. In addition, the β3-adrenergic agonist BRL37344 (24) and the nonselective adrenergic receptor agonist isoproterenol (46) increased TBKC activity in DSM cells. In this study, we identified that another mechanism for TBKC regulation involves EtOH. Interestingly, the manner by which EtOH altered DSM TBKCs depended the basal level of TBKC activity, in particular the frequency. DSM cells with the basal frequency of <0.5 Hz, referred to as low-frequency TBKC DSM cells, responded to EtOH (0.3%) with an increase in the frequency and no effect on the amplitude of TBKCs. In contrast, high-frequency TBKC DSM cells (>0.5 Hz) showed differential effects, no effect on the frequency, and a decrease in the amplitude of TBKCs (Fig. 9). A potential explanation might be related to the basal level of [Ca2+] present in SR of DSM cells. In cerebral artery smooth muscle cells, EtOH increased the [Ca2+] load of the SR (31). Thus it may be that low-frequency TBKC DSM cells have relatively low SR [Ca2+] load and EtOH by increasing the SR [Ca2+] load allows for faster Ca2+ sparks and an increase in the TBKC frequency. In the case of high-frequency TBKC DSM cells, the high basal SR [Ca2+] load probably cannot be further increased. The fact that the amplitude of TBKCs was decreased in high-frequency TBKC DSM cells suggests that [Ca2+] within the Ca2+ sparks reached high concentrations of ∼10 μM. Such concentrations have been reported for Ca2+ sparks in smooth muscle cells (20, 57). In excised patches in the presence of ∼10–20 μM intracellular [Ca2+], EtOH caused inhibition of single BK channel open probability, as observed by us (Fig. 7) and others (30, 31). Since low-frequency TBKC DSM cells responded to EtOH without a significant effect on the amplitude, the [Ca2+] of Ca2+ spark perhaps reaches low micromolar levels in these cells. This would be consistent with the lack of an effect of EtOH on DSM single BK channel activity at 2 μM [Ca2+] (Fig. 6). The effects of EtOH in high-frequency TBKC DSM cells resemble those previously reported for cerebral arteries (31). In both cell types the amplitudes were decreased; however, the frequency was only attenuated in the vascular myocytes. This difference, the presence of low-frequency TBKC DSM cells, and enhancement of BK channel activity by EtOH at the low intracellular [Ca2+] could contribute to alcohol-mediated relaxation of the urinary bladder.
Effects of EtOH on visceral smooth muscle contractility and the underlying mechanisms involved appear to be tissue-dependent. In DSM, similar to the intestine and corpus cavernosum, EtOH induced relaxation of isolated smooth muscle tissue strips (11, 28, 32). In the intestine, the excitation-contraction coupling mechanism for EtOH-mediated inhibition of contractility has been shown to involve smooth muscle hyperpolarization consistent with the activation of K+ channels (32). However, it still remains to be established if these K+ channels are indeed the BK channels. In contrast, preliminary evidence in the gastrointestinal tract has been provided for intermediate conductance Ca2+ activated (IK) channels playing a role in mediating responses to EtOH on contractility (11). In DSM, although IK channels are expressed, they do not contribute to excitability and contractility based on the lack of an effect by the selective IK channel inhibitor TRAM-34 in guinea pig and human DSM (2, 41). Our study points to DSM BK channels being directly modulated by EtOH. In corpus cavernosum smooth muscle, a role for BK channels has also been identified in EtOH-mediated relaxation, but the provided evidence suggested a potential indirect modulation via increase of intracellular Ca2+ levels (28). In cerebral arteries, comparable concentrations of EtOH caused vasoconstriction relating to increases in cerebral blood pressure after binge drinking (6, 7). In this tissue, inhibition of BK channels by EtOH mediates this contractile effect, dependent on the presence of the BK channel regulatory β1-subunit (6, 7) and also on inhibition of TBKCs due to reduction of Ca2+ sparks and observed direct inhibition of BK channels at high [Ca2+] reached during high amplitude TBKCs, estimated to be ∼10 μM (20, 57). As a result, attenuation of the negative feedback loop mediated by BK channels on cerebral artery smooth muscle excitability leads to depolarization, increase in [Ca2+] influx, and vasoconstriction (31). In contrast, DSM responded differently to the same concentrations of EtOH by relaxation. Our study provides evidence that EtOH-induced attenuation of DSM contractility involves BK channel modulation via two distinct mechanisms. First, under submicromolar [Ca2+], present for most of the time in DSM cells, EtOH enhanced the activity of BK channels assessed by conventional whole cell (Fig. 1) and excised patch single-channel recordings (Figs. 3 and 4). Second, EtOH increased the TBKC frequency in DSM cells exhibiting a low level of basal TBKC activity (Fig. 9). Although a differential effect of EtOH was observed in the high-frequency TBKC DSM cells, in whole DSM tissue low-frequency DSM cells may predominate and drive overall responsiveness to EtOH. Importantly, our report identifies novel mechanisms involving BK channels in mediating the inhibitory effects of EtOH on DSM contractility and adds to the consideration of tissue or cell type specificity in elucidating the pathways underlying responses to EtOH.
When BK channels were blocked with the selective BK channel inhibitor paxilline, EtOH at the highest concentration tested of 1% induced similar DSM relaxation in the presence or absence of paxilline. This suggests that at such relatively high concentrations of EtOH, BK channels are unlikely to be the primary mediators of the EtOH inhibitory effects on DSM contractility. Since L-type VDCCs are key regulators of DSM excitability and contractility (12, 48), and in non-DSM cells, EtOH has been shown to inhibit these channels (10, 31, 33), we investigated the effect of EtOH on L-type VDCC currents in DSM cells. We used the perforated whole cell patch-clamp technique since this method preserves the intracellular signaling pathways and molecules with minimal current run-down. To our knowledge, our study is the first to record DSM L-type VDCC currents with the perforated patch-clamp technique using conditions ideally suited to optimally record Ca2+ currents. Unlike previous reports (21, 41, 46) that used K+-based solutions and thus contained contaminating K+ channel currents, the recordings of our study were carried out in the presence of external TEA and Cs+-containing solutions to minimize the influence of K+ conductance and maximize the ability to record stable L-type VDCC currents. We further analyzed the VDCC voltage dependency identifying the V0.5 constant of approximately −7 mV and the peak current occurring at approximately +5 mV, expected for the L-type VDCCs, and demonstrated inhibition of the currents by the selective L-type VDCC blocker nifedipine. Our report further extends the characterization of VDCC currents provided by the conventional whole cell patch-clamp method (14, 27, 48). Although DSM cells also express T-type VDCC currents (49) under our recording conditions, their contribution was negligible. DSM L-type VDCC currents were inhibited ∼33% by 1% EtOH without significantly changing the constant for half maximum activation. The attenuation of L-type VDCC activity was likely due to EtOH decreasing the channel Po, shortening the open dwell time without altering the single-channel conductance as well as promoting steady-state inactivation as observed in neurohypophysial terminals and PC-12 cells (36, 53). Thus inhibition of L-type VDCC currents and potentially another mechanism or multiple, yet to be identified, could explain the BK channel-resistant DSM relaxation in response to EtOH.
In summary, our study reveals that both BK channels and L-type VDCCs are molecular targets for EtOH in DSM. We provide evidence that the enhancement of BK channel activity and inhibition of L-type VDCC currents by EtOH are involved in mediating the alcohol-induced relaxation of the urinary bladder. The effects on BK channels occurred at physiologically relevant BAC levels during moderate-to-heavy acute alcohol intake. The EtOH-mediated DSM relaxation allows for expansion of the urinary bladder resulting in higher urine storage capacity accommodating alcohol-induced diuresis.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-084284 (to G. V. Petkov) and a University of South Carolina Research Foundation Advanced Support Program for Innovative Research Excellence-I Grant (to J. Malysz and G. V. Petkov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M. and G.V.P. conception and design of research; J.M., S.A.A., and A.P. performed experiments; J.M., S.A.A., A.P., and G.V.P. analyzed data; J.M., A.P., and G.V.P. interpreted results of experiments; J.M., S.A.A., A.P., and G.V.P. prepared figures; J.M. and G.V.P. drafted manuscript; J.M., S.A.A., A.P., and G.V.P. edited and revised manuscript; J.M., S.A.A., A.P., and G.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Shankar Parajuli, Dr. Kiril Hristov, Dr. Wenkuan Xin, Dr. Ning Li, Amy Smith, and Vitor Leite Fernandes for critical evaluation of the manuscript and Amy Smith, Dr. Qiuping Cheng, Dr. Shankar Parajuli, and Dr. Ning Li for assistance with tissue dissection or preparation of freshly isolated DSM cells.
REFERENCES
- 1.Abernethy DR, Soldatov NM. Structure-functional diversity of human L-type Ca2+ channel: perspectives for new pharmacological targets. J Pharmacol Exp Ther 300: 724–728, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Afeli SA, Rovner ES, Petkov GV. SK but not IK channels regulate human detrusor smooth muscle spontaneous and nerve-evoked contractions. Am J Physiol Renal Physiol 303: F559–F568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson KE. Treatment-resistant detrusor overactivity–underlying pharmacology and potential mechanisms. Int J Clin Pract Suppl 151: 8–16, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res 31: 1625–1632, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β-Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukiya AN, Liu J, Dopico AM. The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett 583: 2779–2784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukiya AN, Vaithianathan T, Kuntamallappanavar G, Asuncion-Chin M, Dopico AM. Smooth muscle cholesterol enables BK beta1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler Thromb Vasc Biol 31: 2410–2423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dopico AM, Anantharam V, Treistman SN. Ethanol increases the activity of Ca2+-dependent K+ (mslo) channels: functional interaction with cytosolic Ca2+. J Pharmacol Exp Ther 284: 258–268, 1998 [PubMed] [Google Scholar]
- 9.Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev 61: 98–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl DE, Tietz EI. Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors. J Pharmacol Exp Ther 337: 301–311, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagundes DS, Grasa L, Arruebo MP, Plaza MA, Murillo MD. Ca2+-activated K+ channels involved in duodenal dismotility induced by ethanol. Alcohol Alcohol 42: 291–295, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154: 3–13, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Fry CH, Sui G, Wu C. T-type Ca2+ channels in non-vascular smooth muscles. Cell Calcium 40: 231–239, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ganitkevich VY, Isenberg G. Depolarization-mediated intracellular calcium transients in isolated smooth muscle cells of guinea-pig urinary bladder. J Physiol 435: 187–205, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruss M, Henrich M, Konig P, Hempelmann G, Vogel W, Scholz A. Ethanol reduces excitability in a subgroup of primary sensory neurons by activation of BKCa channels. Eur J Neurosci 14: 1246–1256, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Heppner TJ, Layne JJ, Pearson JM, Sarkissian H, Nelson MT. Unique properties of muscularis mucosae smooth muscle in guinea pig urinary bladder. Am J Physiol Regul Integr Comp Physiol 301: R351–R362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360–C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakab M, Schmidt S, Grundbichler M, Paulmichl M, Hermann A, Weiger T, Ritter M. Hypotonicity and ethanol modulate BK channel activity and chloride currents in GH4/C1 pituitary tumour cells. Acta Physiol (Oxf) 187: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kajioka S, Nakayama S, McMurray G, Abe K, Brading AF. Ca2+ channel properties in smooth muscle cells of the urinary bladder from pig and human. Eur J Pharmacol 443: 19–29, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kam SC, Chae MR, Kim JY, Choo SH, Han DH, Lee SW. Effects of ethanol on the tonicity of corporal tissue and the intracellular Ca2+ concentration of human corporal smooth muscle cells. Asian J Androl 12: 890–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298: R378–R384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Vaithianathan T, Manivannan K, Parrill A, Dopico AM. Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol Pharmacol 74: 628–640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci USA 101: 18217–18222, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu G, Sarr MG, Szurszewski JH. Effects of ethyl alcohol on canine jejunal circular smooth muscle. Dig Dis Sci 42: 2403–2410, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Mah SJ, Fleck MW, Lindsley TA. Ethanol alters calcium signaling in axonal growth cones. Neuroscience 189: 384–396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malysz J, Rovner ES, Petkov GV. Single-channel biophysical and pharmacological characterization sof native human large conductance calcium-activated potassium channels in freshly isolated detrusor smooth muscle cells. Pflügers Arch 465: 965, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, Bonci A, Treistman SN, Chandler LJ. Sizing up ethanol-induced plasticity: the role of small and large conductance calcium-activated potassium channels. Alcohol Clin Exp Res 33: 1125–1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullikin-Kilpatrick D, Treistman SN. Inhibition of dihydropyridine-sensitive Ca++ channels by ethanol in undifferentiated and nerve growth factor-treated PC12 cells: interaction with the inactivated state. J Pharmacol Exp Ther 272: 489–497, 1995 [PubMed] [Google Scholar]
- 37.National Institutes of Health (US) Information about Alcohol. Bethesda, MD: Biological Sciences Curriculum Study. NIH Curriculum Supplement Series [Internet], http://www.ncbi.nlm.nih.gov/books/NBK20364/ (accessed Feb. 6, 2013), 2007 [Google Scholar]
- 38.Ohmura M, Kondo A, Saito M. Effects of ethanol on responses of isolated rabbit urinary bladder and urethra. Int J Urol 4: 295–299, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Ost D, Van der Aa F, De RD. Intravesical ethanol 10% in saline is not an inert vehicle. Neurourol Urodyn 22: 353–355, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Parajuli SP, Petkov GV. Activation of muscarinic M3 receptors inhibits large-conductance voltage- and Ca2+-activated K+ channels in rat urinary bladder smooth muscle cells. Am J Physiol Cell Physiol 305: C207–C214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker H, Hoonpongsimanont W, Vaca F, Lotfipour S. Spontaneous bladder rupture in association with alcoholic binge: a case report and review of the literature. J Emerg Med 37: 386–389, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci 24: 8322–8332, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sui GP, Wu C, Fry CH. A description of Ca2+ channels in human detrusor smooth muscle. BJU Int 92: 476–482, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Sui GP, Wu C, Severs N, Newgreen D, Fry CH. The association between T-type Ca2+ current and outward current in isolated human detrusor cells from stable and overactive bladders. BJU Int 99: 436–441, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Sun GY, Sun AY. Ethanol and membrane lipids. Alcohol Clin Exp Res 9: 164–180, 1985 [DOI] [PubMed] [Google Scholar]
- 51.Treistman SN, Martin GE. BK channels: mediators and models for alcohol tolerance. Trends Neurosci 32: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utkan T, Erden F, Yildiz F, Ozdemirci S, Ulak G, Gacar MN. Chronic ethanol consumption impairs adrenoceptor- and purinoceptor-mediated relaxations of isolated rat detrusor smooth muscle. BJU Int 88: 278–283, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Wang G, Lemos JR, Treistman SN. Ethanol directly modulates gating of a dihydropyridine-sensitive Ca2+ channel in neurohypophysial terminals. J Neurosci 14: 5453–5460, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin W, Soder RP, Cheng Q, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin W, Soder RP, Cheng Q, Rovner ES, Petkov GV. Selective inhibition of phosphodiesterase 1 relaxes urinary bladder smooth muscle: role for ryanodine receptor-mediated BK channel activation. Am J Physiol Cell Physiol 303: C1079–C1089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yokoi K, Ohmura M, Kondo A, Miyake K, Saito M. Effects of ethanol on in vivo cystometry and in vitro whole bladder contractility in the rat. J Urol 156: 1489–1491, 1996 [PubMed] [Google Scholar]
- 57.Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 microM during a Ca2+ spark. J Gen Physiol 120: 15–27, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]