Abstract
Sodium/potassium/chloride cotransporter (NKCC1) proteins play important roles in Na+ and K+ concentrations in key physiological systems, including cardiac, vascular, renal, nervous, and sensory systems. NKCC1 levels and functionality are altered in certain disease states, and tend to decline with age. A sensitive, effective way of regulating NKCC1 protein expression has significant biotherapeutic possibilities. The purpose of the present investigation was to determine if the naturally occurring hormone aldosterone (ALD) could regulate NKCC1 protein expression. Application of ALD to a human cell line (HT-29) revealed that ALD can regulate NKCC1 protein expression, quite sensitively and rapidly, independent of mRNA expression changes. Utilization of a specific inhibitor of mineralocorticoid receptors, eplerenone, implicated these receptors as part of the ALD mechanism of action. Further experiments with cycloheximide (protein synthesis inhibitor) and MG132 (proteasome inhibitor) revealed that ALD can upregulate NKCC1 by increasing protein stability, i.e., reducing ubiquitination of NKCC1. Having a procedure for controlling NKCC1 protein expression opens the doors for therapeutic interventions for diseases involving the mis-regulation or depletion of NKCC1 proteins, for example during aging.
Keywords: NKCC1, Na+-K+-2Cl−-cotransport protein, pathway regulation, steroid hormone, aging, physiological systems, therapeutics
proper regulation of ionic concentrations is necessary for optimal performance of the body's physiological systems. The Na+-K+-2Cl− cotransport protein (e.g., NKCC1) is a key membrane molecule that moves Na+, K+, and Cl− into and out of cells for proper physiological function (39). Its properties have been studied in the cardiovascular system, including regulation of salt concentration, cell volume, and maintenance of cellular homeostasis in response to osmotic and oxidative stress functions in cardiomyocytes and vascular smooth muscle (26). NKCC1 is also involved in regulation of blood pressure and left ventricular pressure (20, 34). In particular, Jiang et al. (28) found that aldoterone (ALD) increases NKCC1 activity in conjunction with heart failure, but the mRNA expression levels do not change, suggesting posttranslational modifications. The human gene locus for NKCC1 has been identified as 5q23.3 and is encoded by Slc12a2 in mouse (9).
NKCC1 also plays key roles in renal physiology and fluid ionic regulation. For instance, in response to reductions in intracellular chloride concentrations, Ste20-related proline-alanine-rich kinase (SPAK) phosphorylates NKCC1 to elevate cotransporter activity and raise chloride influx (12, 53). Oxidative stress response kinase 1 (OSR1) also phosphorylates and activates NKCC1 in the presence of oxidative stress (51). Therefore, because of its important physiological functions, mis-regulation or deficiencies in the expression or distribution of NKCC1 isoforms in kidney epithelial cells can have negative physiological consequences.
For sensory systems, the cochlea, a specialized organ of the auditory sensory system, critically depends on the presence of NKCC1 transporters in epithelial cells of its lateral wall, particularly in the basolateral plasma membrane of stria marginal cells (57, 59) where endolymph is made, an unusual, K+-rich fluid. The endocochlear potential (EP), of the endolymph is the physiological “battery” providing power to the auditory transduction receptors, or hair cells, epithelial cells of the inner ear that convert sound into the code of the nervous system (43, 45, 46). The critical physiological role of NKCC is supported by evidence indicating that furosemide blocks NKCC1 function in the cochlea, causing hearing loss or balance deficits, resulting from impaired endolymph production and EP declines (45–47). Furosemide is a loop diuretic used clinically for the treatment of congestive heart failure and edema by reducing NKCC activity in epithelial cells of the kidney. The findings of Schmiedt et al. (48) suggest that since the EP declines with age in the mammalian cochlea, reductions in the expression or functionality of NKCC1 proteins in epithelial cells of the cochlear lateral wall play a role in age-related hearing loss, presbycusis (10).
Initial studies report that NKCC1 proteins are also expressed in the nervous system. They can be found in the apical membrane of the choroid plexus, in perikarya of certain central nervous system (CNS) neurons, in oligodendrocytes, and in dorsal root ganglion sensory neurons of the peripheral nervous system (20, 25). It is known, for example, that the relative expression levels of NKCC1 and NKCC2 determine whether neuronal responses to gamma amino butyric acid (GABA), an important neurotransmitter, are excitatory (depolarizing) or inhibitory (hyper-polarizing) in the CNS (7). The relative protein expression levels and corresponding neurophysiological responses that they determine change during neuronal development, including olfactory bulb neuronal migration (25, 32), and during peripheral sensory nerve regeneration following sectioning of the mouse sciatic nerve in vivo (40). Also, since GABA is a prevalent neurotransmitter that modifies neuronal excitability, altered NKCC1 regulation has been implicated in cases of epilepsy (12).
A number of serious and prevalent diseases involve disorders and pathologies of epithelial cells. Specifically, in respiratory epithelial cells, NKCC1 resides in the basolateral membrane of salivary gland and epithelial cells lining the airways. In the gastrointestinal tract, NKCC1 is found in the inner medullary collecting duct cells, and rectal gland cells, thus allowing efficient salt and water secretion and reabsorption and volume regulation (26, 43). Disruption of the NKCC1 system can be significant for these physiological systems. For example, cystic fibrosis, a debilitating lung disease that also affects the kidneys, liver, and intestine, is characterized by abnormal transport of Na+ and Cl− across epithelial cells, leading to thick, viscous secretions and serious respiratory ailments, and its mechanisms have been investigated utilizing HT-29 epithelial cells (3, 4, 37).
Precise control of NKCC1 expression and function would have pharmaceutical and biotherapeutic implications, given the important roles that NKCC1 proteins play in key physiological systems, including cardiac, vascular, renal, hepatic, and sensory. Thus an understanding of NKCC1 regulatory pathways is significant, in light of potentially new treatments for the disorders described above.
Initial investigations have implicated the mineralocorticoid, steroid hormone ALD synthesized in the glomerulosa of the adrenal gland, as a regulator of NKCC1. For instance, when adrenalectomized rats received a multiday treatment of ALD, there was a 63% increase in NKCC1 activity as measured by bumetanide-sensitive efflux of 86Rb for vascular smooth aortic muscle (28). Interestingly, application of ALD did not elevate NKCC1 transcripts as determined by real-time polymerase chain reaction. Additionally, NKCC1 knockout mice have deficient or abnormal responses to ALD (30, 57). It not yet clear whether ALD regulates NKCC1, or vice versa, which is part of the motivation in carrying out the present investigation. ALD can exert its action by binding to mineralocorticoid receptors (type I) to form a complex that interacts with nuclear DNA to exert gene transcription and protein synthesis (40, 56). The present study tested the hypothesis that ALD can directly and precisely increase NKCC1 expression.
METHODS
Cell culture and buffers.
Human colon adenocarcinoma epithelial HT-29 cells (obtained from the laboratory of Dr. Edward Seto, Moffitt Cancer Res. Center, Tampa FL) were utilized in the present investigation, since they express NKCC1, which can be effectively detected in these cells using straightforward protein techniques (more complicated procedures involving manipulation of transfected constructs not necessary), and since previous reports have demonstrated HT-29 utility for being a representative epithelial cell line for investigations of cell signaling and transductional factors and pathways (7). These include, for example, studies of COMM domain-containing protein 1, which is involved in NKCC1 ubiquitination and transcriptional regulation of epithelial Na+ channels (ENaC) located in the apical membrane of polarized epithelial cells, in particular, cells in the colon, kidney, and lung, by ALD (6, 15, 52). HT-29 cells were grown in DMEM media (Invitrogen, Carlsbad CA) with 10% FBS and antibiotics. Cells were grown in a humidified 95%-5% CO2 incubator at 37°C. Experiments were performed at 70–80% confluence.
86Rb uptake.
Similar to previous studies of NKCC1 activity (19), cells were plated on six-well dishes and incubated at 37°C/5% CO2 until confluent. For the uptake, cells were first washed twice with 1 ml of isosmotic saline (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, 5 mM HEPES buffered to pH 7.4, and 300.5 mosM). Cells were then preincubated for 15 min in 1 ml of the same isosmotic saline plus 1 mM ouabain (Sigma) ± 20 μM bumetanide (Sigma, St. Louis MO). The preincubation solution was then aspirated and replaced with an identical solution containing 1 μCi of 86Rb and 1 mM ouabain ± 20 μM bumetanide. Four 5-ul aliquots of flux solution were sampled at the beginning of each 86Rb uptake condition and used as standards. After a 20-min uptake, the radioactive solution was aspirated, and the cells were washed three times with 1 ml of ice-cold solution, lysed for 1 h with 500 μl of 0.25 N NaOH, and neutralized with 250 μl of glacial acetic acid. 86Rb tracer activity was measured by using 150 μl of lysate for y-scintillation counting. NKCC1 flux was expressed in millimoles of K+ per micrograms of protein per minute.
Relative quantitative RT-PCR.
Total RNA was extracted using the RNAeasy Mini Kit (Qiagen, Valencia CA). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using two methods: per manufacturer's suggestion using the Enhanced Avian HS RT-PCR-100 Kit (HSRT20; Sigma) and the Fast Real-Time PCR System with their SYBR Green PCR Master Mix (7900HT; Applied Biosystems, Carlsbad CA). Primers used for qRT-PCR were as follows: β-actin, 5′-CCTGGCACCCAGCACAAT (sense) and 5′-GGGCCGGACTCGTCATAC (antisense); and NKCC1, 5′-ACCTTCGGCCACAACACCATGGA (sense) and 5′-ACCACAGCATCTCTGGTTGGA (antisense). The semiquantitative qRT-PCR reaction took place at 45°C for 50 min. The competition between primer sets was excluded by adjusting the reaction condition. Then, the primer products were PCR amplified directly. A first cycle of 10 min at 95°C, 45 s at 65°C, and 1 min at 72°C was followed by 45 s at 95°C, 45 s at 65°C, and 1 min at 72°C for 25 cycles. The conditions were chosen so that the RNAs analyzed were in the exponential phase of amplification. Each set of reactions always included a no-sample negative control. We usually performed a negative control containing RNA instead of cDNA to rule out genomic DNA contamination.
The real-time RT-PCR reaction mixture was prepared using the SYBR-Green PCR Master Mix. Thermal cycling conditions were the same as in the semiquantitative method. Amplification specificity was checked using melting curves. Both negative and positive controls were included in each PCR reaction. All assays were carried out three times as independent PCR runs for each cDNA sample. Gene expression was referenced to the expression of β-actin as the housekeeping gene. Each gene expression level was normalized with respect to β-actin mRNA content. Calculations of expression were performed with the 2ΔΔCT method (5).
Western blot.
Cell lysates were prepared in RIPA buffer (Pierce 89901; Thermo Scientific, Waltham, MA) with protease inhibitor cocktail (78430; Thermo Scientific). Cell samples were homogenized in buffer, followed by centrifugation at 2,000 rpm for 10 min at 4°C. Supernatants were subjected to Western blot analysis by loading 200 μg of protein per lane, after the protein concentrations were determined by Bradford protein assay. Proteins were fractionated by SDS-PAGE gel electrophoresis and transferred to a PVDF blotting membrane (Whatman, Piscataway, NJ). The blot was incubated with primary antibodies against β-actin and Na-K-2Cl cotransport protein (Cell Signaling, Danvers MA); primary antibodies for p-SGK1, SGK1, pNedd4–2, and Nedd4–2 were utilized (concentration 1:1,000). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000; Cell Signaling).
Tl-uptake in single cells for the detection of NKCC1 activity.
To measure NKCC1 activity, we used a fluorescence assay to assess cotransporter activity in single isolated HT-29 cells (9, 21, 59). HT-29 cells were incubated at 37°C/5% CO2 for 24 h before use. For the uptake experiment, thallium was used as the tracer for the K+ (FluxORTM Thallium Detection Kits; Invitrogen). Cells were first loaded with the thallium-sensitive FluxORTM dye (1×) in a hypotonic (275.5 mosM) low chloride solution (125 mM NaMeSO3, 2 mM KCl, 2 mM CaCl2, 0.8 mM MgSO4, 5 mM glucose, and 20 mM HEPES) plus 1× PowerLoad concentrate and 2.7 mM probenecid (supplied by the kit) for 90 min. The loading solution was then aspirated, and cells were washed three times with the same solution to remove excess dye. Cells were then preincubated for 10 min using 1 ml of the same saline containing 1 mM ouabain and 2.7 mM probenecid in the presence or absence of 20 μM bumetanide (Sigma). For the detection of fluorescence, the preincubation solution was removed, and the cells were treated with a 340.5 mosM hypertonic stimulus solution containing the following (in mM): (2.8 TlSO4, 140 NaCl, 2 KCl, 2 CaCl2, 0.8 MgSO4, 5 glucose, 20 HEPES, 27 sucrose, 1 ouabain, and 2.7 probenecid) in the presence or absence of 20 μM bumetanide. Images were made using a ×40X objective inverted florescent microscope at 488-nm excitation wavelength. HT-29 cells were loaded with the thallium-sensitive dye FluxOR (Invitrogen). FluxOR fluorescence (excitation/emission: 488/525 nm) was recorded after 90 s upon addition of 2.8 mM TlSO4 to the external medium. The difference between the two measurements, made with and without bumetanide treatment, represents the bumetanide-sensitive component of the Tl+ uptake, mediated by NKCC1.
Immunoprecipitation (autoubiquitination assay) and western blot analysis.
An autoubiquitination assay was followed (20). Cell extracts were prepared in a modified radioimmunoprecipitation assay 1 (RIPA) buffer, containing the following (in mM): 50 Tris·HCl at pH 7.4, 150 NaCl, 1 EDTA, and 1 dithiothreitol with 1% Nonidet P-40, 0.1% SDS; in a 1:200-diluted protease inhibitor cocktail (Sigma), containing the following (in mM): 1 PMSF, 10 NEM, and 0.1 iodoacetamide. Immunoprecipitations were accomplished using a rabbit polyclonal anti-NKCC1 antibody (Cell Signaling). Antibody was bound to lysate with endogenous NKCC1, and then beads were added. Bound proteins were eluted in 1× SDS sample buffer, fractionated on an SDS-polyacrylamide gel, transferred onto a PVDC membrane (GE Healthcare, Piscataway, NJ), and immunoblotted with anti-ubiquitin antibody visualized using enhanced chemiluminescence detection reagents (Pears, Shelton CT) according to the manufacturer's instructions. Immunoblotting with anti-ubiquitin antibody was performed as described above.
Sources.
Chloroquine, an agent that impairs lysosomal acidification (lysosome inhibitor), MG-132 (proteasome inhibitor), cycloheximide (translation inhibitor), and GSK650394 (SGK inhibitor) were purchased from Sigma (St. Louis, MO). The polyclonal antibodies to NKCC1 and β-actin were purchased from Cell Signaling (Cambridge, MA); and the polyclonal antibodies to p-SGK (Thr256) and SGK from Santa Cruz (Dallas, TX). The polyclonal antibodies to p-Nedd4–2 (S328), Nedd4–2, and LAMP2 were purchased from Abcam (Cambridge, MA), and the polyclonal antibody to LC3 was purchased from Novus Biologicals (Littleton, CO).
Statistical analysis.
Images from films were imported into Adobe Photoshop (v 5.0), and further processed using Adobe Photoshop CS and ImageJ (National Institutes of Health) for the densitometry analysis. Data are reported as means ± SD. Statistical analyses were performed with PRISM 4.0 (GraphPad Software, San Diego CA). Differences were analyzed with a one- or a two-way repeated-measures ANOVA as appropriate or a two-way ANOVA followed by Bonferroni post hoc analyses that were corrected for multiple comparisons. Values of P < 0.05 were considered significant.
RESULTS
ALD induced a sustained induction of NKCC1.
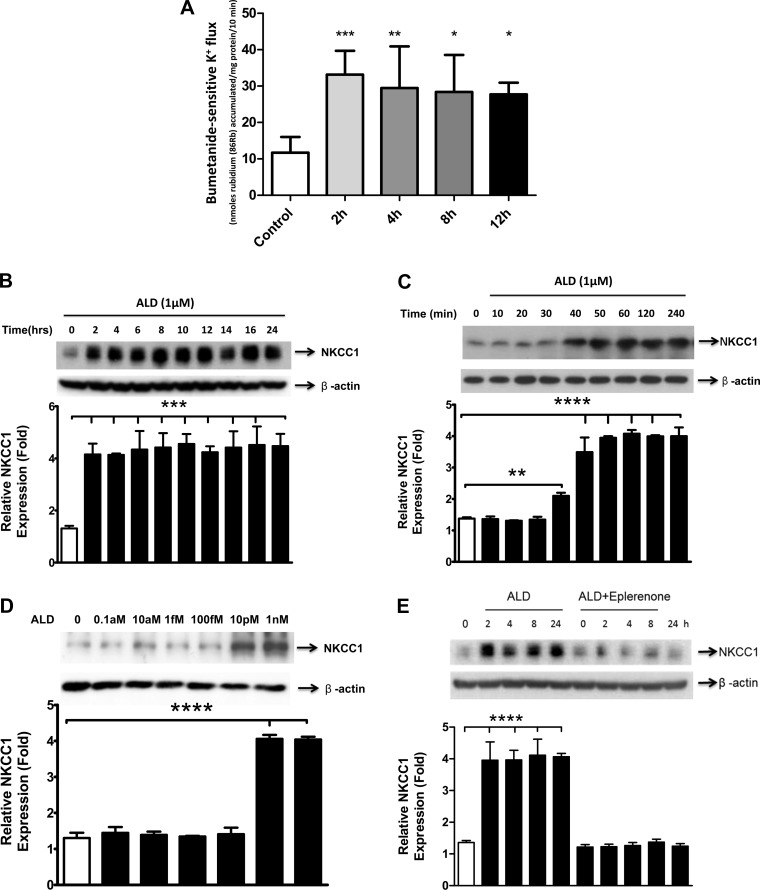
ALD treatment upregulated NKCC1 protein activity and expression levels, which remained relatively stable for over 12–24 h of treatment (Figs. 1, A and B). This upregulation took effect within the first 2 h of ALD treatment (1 μM; Fig. 1C). The threshold of NKCC1 induction was quite sensitive, as there was a significant response at ∼10 pM (Fig. 1D). Further, simultaneous treatment with ALD (1 μM) and eplerenone (20 μM), a mineralocorticoid receptor antagonist, prevented the ALD upregulation of NKCC1 protein expression (Fig. 1E).
Fig. 1.
Aldosterone (ALD) increases activity levels and protein expression of Na+-K+-2Cl− cotransport protein (NKCC1). A: NKCC1 activity was determined through 86Rb uptake, which was measured in an isosmotic solution containing 1 mM ouabain, at 2, 4, 8, and 12 h after ALD administration. B: HT-29 cells were treated with ALD for the times and doses indicated and lysed by RIPA buffer with cocktail protein inhibitors; proteins were resolved by SDS-PAGE. Western blot was probed sequentially with antibodies to NKCC1 and β-actin as the control. ALD treatment increases NKCC1 protein expression. The time frame of upregulation is from 2 h up to 24 h after application of ALD (1 μM). C: further investigation revealed that the induction of NKCC1 by ALD (1 μM) starts 30–40 min after treatment. D: immunoblot of NKCC1 protein expression for different doses of ALD. The threshold for an effective dose of ALD on NKCC1 protein expression is on the order of only 10 pM. Bar graph results are means ± SD from 3 independent experiments, the ordinate represents relative expression, defined as expression level relative to the time 0 or dosage point. E: increase in NKCC1 protein levels is mediated via the activation of mineralocorticoid receptors (MR). HT-29 cells were treated with or without a specific inhibitor of MR, eplerenone (20 μM), and then applied with 1 μM ALD for the indicated times. NKCC1 protein expression was detected in cell lysates by a Western blot, using antibodies to NKCC1, and β-actin as a loading control. Open bar: control, nontreated sample; the vehicle was 100% alcohol, in which the ALD and eplerenone were dissolved. Statistical significance: ****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05.
The increase of NKCC1 protein expression by ALD is not associated with mRNA induction but is mediated by mineralocorticoid receptors.
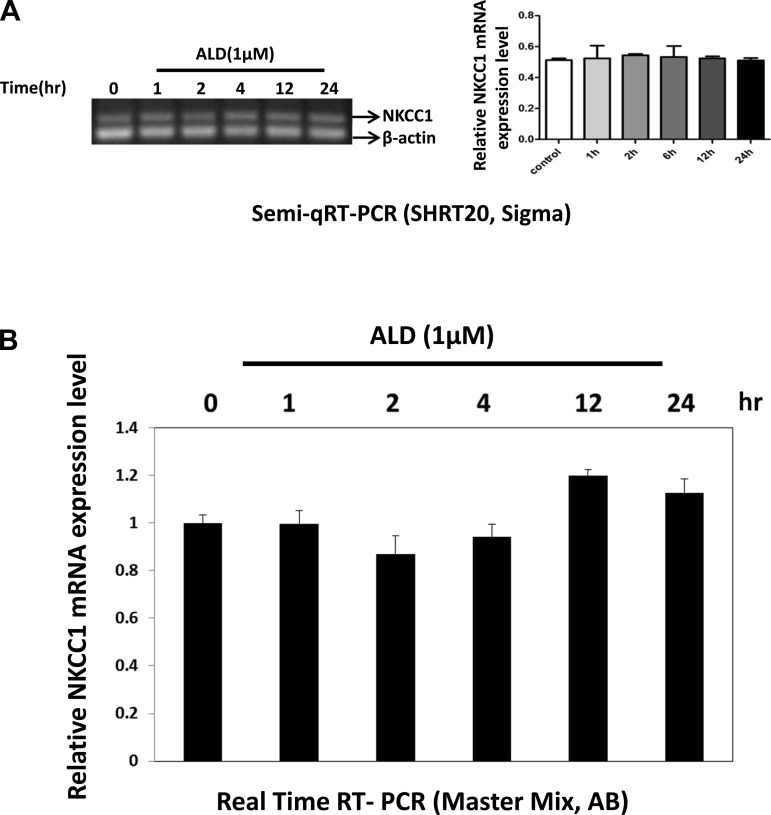
We investigated whether the increased protein expression of NKCC1 was tied to elevated mRNA levels by using RT-PCR. Both methods utilized here resulted in similar findings: there was no mRNA change with ALD stimulation (Fig. 2). These results indicate that the ALD-induced increase of NKCC1 protein expression is associated with the activation of mineralocorticoid receptors by ALD.
Fig. 2.
NKCC1 mRNA is not induced by ALD treatment (1 μM). Total RNA was isolated from HT-29 cells treated with ALD (1 μM) at the indicated times and reverse transcribed. A: semiquantitative RT-PCR analysis with primers representing the NKCC1 NH2 terminus was performed using 10 ng of the diluted RNA, analyzed on a 2% TAE agarose gel, and stained with GelRed Nucleic Acid Stain (left). Amplified products were resolved by agarose gel electrophoresis. β-Actin was included as a loading control. Bar graph results are means ± SD from 3 independent experiments, the ordinate represents relative expression, defined as expression level relative to the control (β-actin expression, right). B: 10 ng of RNA and primers were used for real-time quantitative (q)RT-PCR. Bar graph results are means ± SD from 3 independent experiments. There were no statistically significant differences.
ALD enhances the stability of NKCC1 protein.
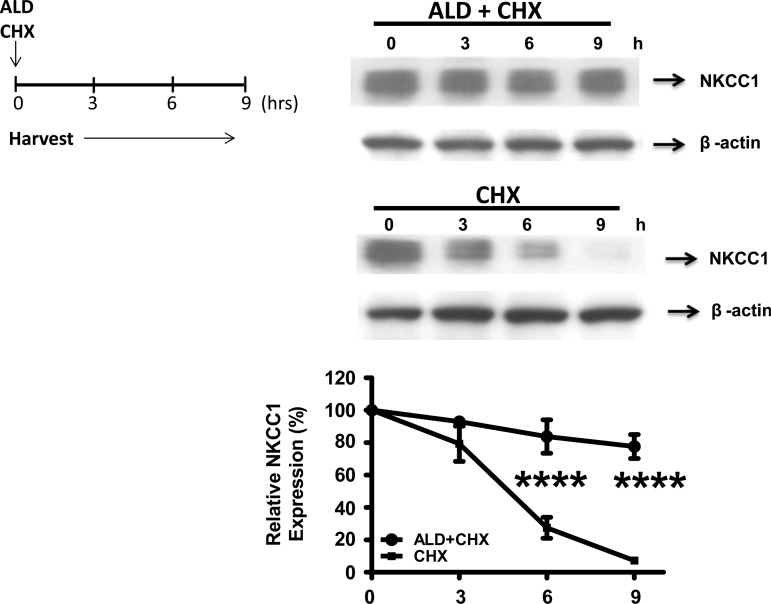
We discovered that ALD increases NKCC1 protein levels, whereas induction of NKCC1 mRNA synthesis is absent. The regulation of intracellular protein levels is highly dependent on two factors: ribosomal protein synthesis, which is associated with mRNA levels; and posttranslational modification, which is independent of transcriptional activation and translational regulation. To explore the possibility that posttranslational modification is the mechanism by which ALD exerts its effects, cells were treated with cycloheximide, a translation inhibitor, for 3, 6, or 9 h. The results show that in the presence of ALD (1 μM) the decline of NKCC1 protein levels was much slower in response to cycloheximide, relative to the nontreated cells (Fig. 3).
Fig. 3.
Role of ALD in NKCC1 protein induction and stability. Cell lysates were analyzed by gel electrophoresis and Western blot analysis. Blots were probed with antibodies against NKCC1, and β-actin served as the control. HT-29 cells were stimulated with ALD (1 μM) and then either treated or not treated with the protein synthesis inhibitor cycloheximide (CHX; 20 μg/ml) for 3, 6, or 9 h. Western blots (top) showing NKCC1 protein expression in HT-29 cells treated with the above protocol. The relative expression of NKCC1 as a function time since the ALD treatment (x-axis) is summarized by a line graph from 3 independent experiments (bottom). Line graph expression levels are normalized to 100%, relative to the vehicle alone. Statistical significance: ****P < 0.0001.
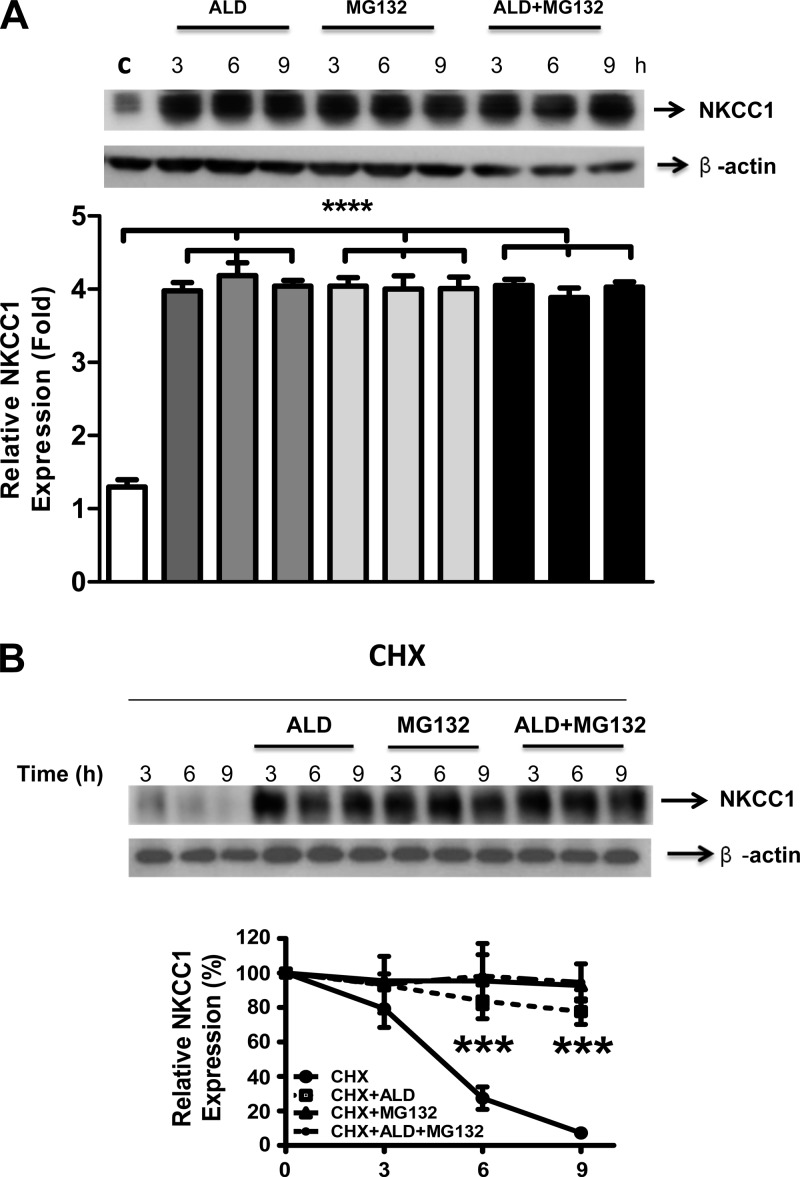
Cells were then treated with MG132 (20 μg/ml) to inhibit the ubiquitination of NKCC1 protein. Specifically, in the presence of MG132, proteins that are polyubiquitinated will accumulate intracellularly, often as aggregates. This accumulated pool is typically nonfunctional. The effect of ALD on NKCC1 expression was mimicked by MG132, suggesting that ALD reduces proteasomal degradation of NKCC1. Interestingly, when combining ALD with MG132 treatments, the increased level of NKCC1 protein expression was the same as the ALD or MG132 treatments alone (Fig. 4A).
Fig. 4.
Net result of inhibition of NKCC1 ubiquitination is an increase in its protein expression. A, top: cells were treated with 1 μM ALD or proteasome inhibitor MG132 (20 μg/ml), which prevents/reduces the degradation of proteins by ubiquitin-proteasome mechanisms, or the combination of ALD and MG132, for 3, 6, or 9 h. NKCC1 protein expression was detected by Western blot with β-actin as the control. B, top: cells were first treated with CHX (protein synthesis inhibitor, 20 μg/ml) and then ALD (1 μM) or MG132 (50 μg/ml) or the combination of ALD/MG132 for 3, 6 or 9 h. For both A and B, open bars are the control, medium-shaded are ALD alone, light shading indicates MG132, and the dark shading designates the ALD + MG132 combined condition. For each of these 4 conditions, the left bar represents 3 h, the middle bar represents 6 h, and the right bar designates 9 h. The relative expression of NKCC1 is plotted in the graphs for 3 independent experiments (bottom), with the same format as Fig. 1. Statistical significance: ****P < 0.0001, ***P < 0.001, indicating a significant difference between the control condition and the other conditions.
Next, global protein translation was inhibited with cycloheximide, while simultaneously treating with MG132 and ALD. In the presence of cycloheximide, the joint effect of ALD and MG132 did not increase the NKCC1 stability relative to either ALD or MG132 treatments alone (Fig. 4B). Apparently, ALD promotes NKCC1 protein stability and subsequently enhances its accumulation in cells by preventing posttranslational modifications involving ubiquitination of NKCC1.
Ubiquitination, but not the lysosome pathway, is involved in NKCC1 induction by ALD.
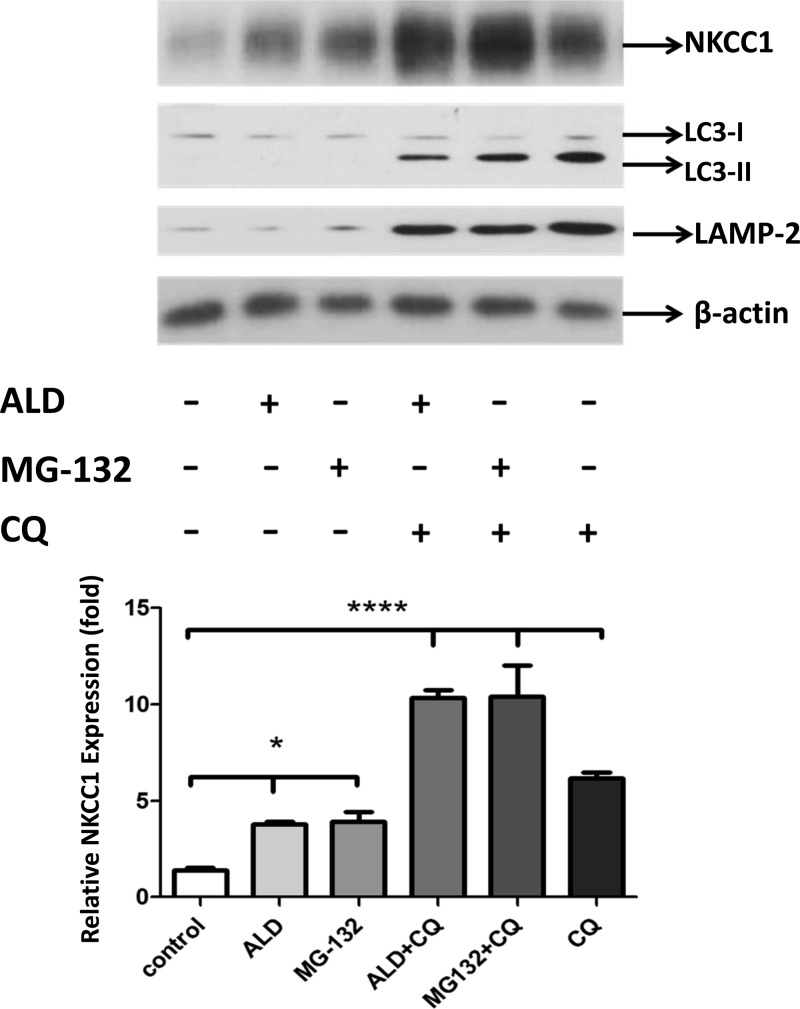
A parallel experiment was performed with chloroquine (CQ), a lysosome inhibitor, to exclude the possibility that prolonged MG-132 treatment depleted free ubiquitin by nonspecific inhibition of lysosome degradation of NKCC1 and to confirm whether or not ALD is involved in a lysosome pathway. In Fig. 5, the positive marks indicate application of ALD, MG-132, or CQ. Activation of lysosome pathway proteins is given in Fig. 5, top (LC3-I and -II and LAMP-2). As shown in Fig. 5, ALD and MG-132 treatments induce NKCC1 protein expression similarly [1st bar (control) vs. 2nd and 3rd bars], whereas NKCC1 protein expression increased when ALD (1 μM) was combined with CQ (50 μM) treatment (4th bar). The combined treatment with MG-132 (20 μM) and CQ (5th bar) on the induction of NKCC1 equals the effects of ALD and CQ together (4th bar) but exceeds those of ALD, CQ, or MG132 alone (2nd, 3rd, and 6th bars). Note that only when the lysosome pathway blocker CQ was used was their significant upregulation of LC3II and LAMP-2 expression levels. Taken together, these data suggest that ALD specifically suppresses the proteasomal degradation of NKCC1 without affecting its degradation via the lysosomal pathway.
Fig. 5.
Ubiquitination, but not the lysosome pathway, is involved in the regulation of NKCC1 by ALD. To exclude the possibility that depletion of free ubiquitin by the prolonged MG-132 treatment triggered nonspecific inhibition of lysosome degradation of NKCC1 and investigate whether ALD is involved in a lysosome pathway, a parallel experiment with chloroquine (CQ) was performed. Here, the positive marks (+) indicate application of ALD (1 μM), MG132 (20 μM), CQ (50 μM), ALD + CQ, and MG132 + CQ for 9 h. An autophagosome marker, autophagy-related gene 8 named as light chain 3 (LC3), and the lysosome-associated membrane protein 2 (LAMP-2) were used as indicators of lysosome pathway activation (phagocytic activity) under treatment with CQ. Activation of lysosome pathway proteins is indicated as the increased expression of lysosome markers, LC3-I, -II, and LAMP-2 vs. controls (1st bar). ALD and MG-132 treatments achieved some induction of NKCC1 protein expression [1st bar (Control) vs. 2nd and 3rd bars] without increasing lysosome pathway markers (rows 2 and 3). The induction of NKCC1 protein expression reached higher levels when ALD (1 μM) was combined with CQ (50 μM) treatment (4th bar). The effects of combined treatment with MG-132 (20 μM) and CQ, (5th bar) on the induction of NKCC1 equal the effects of ALD and CQ together (4th bar) but exceed those of ALD, CQ, or MG132 alone (2nd, 3rd, and 6 bars). Note that only when the lysosome pathway blocker CQ was used was their significant upregulation of LC3II and LAMP-2 expression levels. These data suggest that ALD specifically suppresses the proteasomal degradation of NKCC1 without affecting its degradation via the lysosomal pathway. Bottom: mean data ± SD bars; ****P < 0.0001, indicates the significance of control vs. 4th, 5th, and 6th bars; *P < 0.05, indicates the significance of control vs. the ALD and MG132 conditions alone.
ALD decreases the endogenous ubiquitination of NKCC1 and inhibition of SGK1 activation blocks this downregulation.
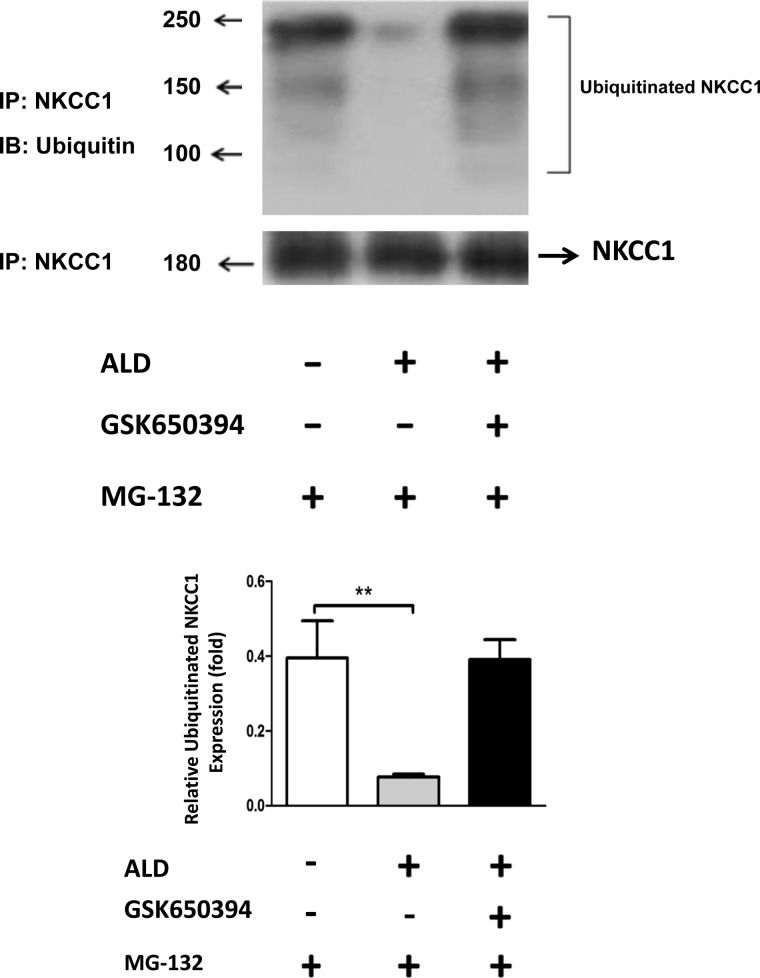
To confirm the role of ubiquitination of NKCC1 in the ALD mechanism, we blocked the endogenous ubiquitination of HT-29 cells by applying MG132 (20 μM). Two hours after the MG132 application, cells were treated with ALD (1 μM) or the SGK1 inhibitor GSK 650394 (50 μM) or ALD (1 μM) combined with SGK1 inhibitor GSK 650394 (50 μM) for 24 h. It was previously shown that SGK1 regulates Na+/Cl− cotransporter (NCC) and NKCC1 has structural similarities to NCC (2). As predicted from the results of Fig. 4, the NKCC1 expression in the three groups (with or without ALD treatment, or ALD + GSK 650394) was equal (Fig. 6, left and right bars). Correspondingly, endogenous ubiquitinated NKCC1 was significantly reduced in the ALD-treated cells (middle bar), because fewer mono- and polyubiquitin bands were seen in the ALD treatment group relative to the cells in the non-ALD group (Fig. 6, compare left bar to middle bar). Next, blocking SGK1 reduces the ability of ALD to suppress NKCC1 ubiquitination (Fig. 6, compare middle bar to right bar). These results, utilizing a complete endogenous system (autoubiquitination assay), confirm that ALD is responsible for the inhibition of the NKCC1 ubiquitination, likely mediated by SGK1-Nedd4–2 activation.
Fig. 6.
ALD blocks the ubiquitination of NKCC1. HT-29 cells were treated with 20 μM MG132 for 2 h with or without ALD (1 μM) or GSK650394 to the media for 24 h. Cells were collected in RIPA buffer and used for 2 assays: Western blot for the detection of NKCC1 protein expression, and immunoprecipitation with anti-NKCC1 antibody followed by immunoblotting with anti-ubiquitin antibody. Left: MG132 alone. Middle: MG132 plus ALD shows low levels of ubiquitinated NKCC1. Right: MG132 plus ALD and GSK650394 shows increased levels of NKCC1 ubiquitination. IB, immunoblotting; IP, immunoprecipitation. Top left: numbers indicate molecular mass in kDa. Bottom: bar graph summary from 3 independent experiments like that at top. Statistical significance: **P < 0.01.
ALD inhibits the degradation of NKCC1 through the SGK1-Nedd4–2 pathway.
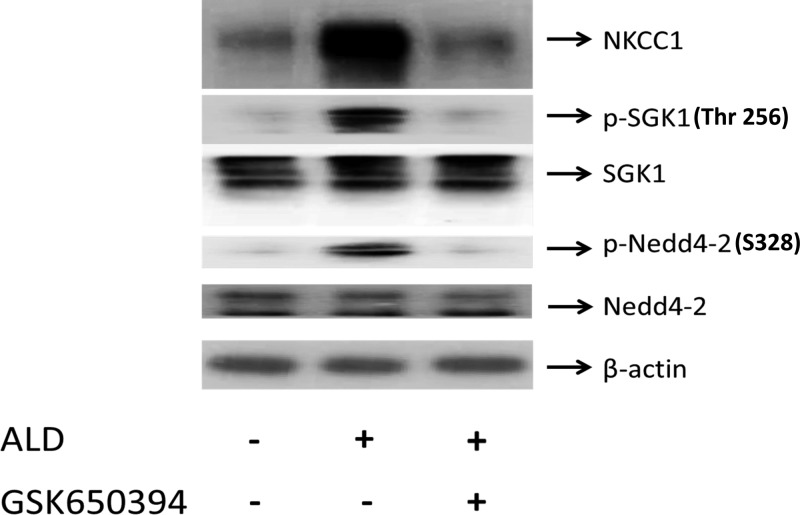
Interaction between ENaC (a Na+ channel) and Nedd4–2 is required for ENaC internalization and protein stability (2). Specifically, Nedd4–2 binds ENaC via a specific domain called the PY motif. Mutation of this domain induces a kidney disease called Liddle's syndrome. Since NKCC1 and NCCs belong to the cation Cl− cotransporters (CCCs), and the CCCs exhibit a common structure in their functional regulation domains, such as the membrane associated domain and the phosphoacceptor sites (42, 50), we hypothesize that there is a similarity in the regulation of NCC and NKCC1 inductions by ALD. To test this, we found that ALD increases the phosphorylation of SGK1 and Nedd4–2 and concomitantly there is an increased induction of NKCC1 protein expression (Fig. 7, middle bar). We also observed (Fig. 7, compare middle bar to right bar) that the SGK1 inhibitor GSK 650394 (50 μM) blocked phosphorylation of SGK1 and Nedd4–2 but the total protein expressions of SGK1 and Nedd4–2 showed no differences. Lastly, ALD in the presence of GSK 650394 did not increase protein expression of NKCC1 (Fig. 7, compare middle bar to right bar). These data suggest that the most likely target of ALD in regulation of NKCC1 is the ALD-SGK1-Nedd4–2 pathway.
Fig. 7.
ALD modulates NKCC1 via the ALD-SGK1-Nedd4–2 pathway. HT-29 cells were treated with ALD (1 μM) or ALD (1 μM) + GSK650394 (50 uM) for 9 h. Cell lysates were analyzed by Western blot as indicated. Blots are representative of 3 independent experiments. Left: controls. Middle: ALD increases the phosphorylations of SGK1 and Nedd4–2, concomitantly there is an increased induction of NKCC1 protein expression (1st row). Right: SGK1 inhibitor GSK 650394 (50 μM) blocked phosphorylation of SGK1 and Nedd4–2 (2nd and 4th rows), but the total protein expressions of SGK1 and Nedd4–2 showed no differences, and ALD did not increase the protein expression of NKCC1.
Influence of ALD on NKCC1 transporter activity.
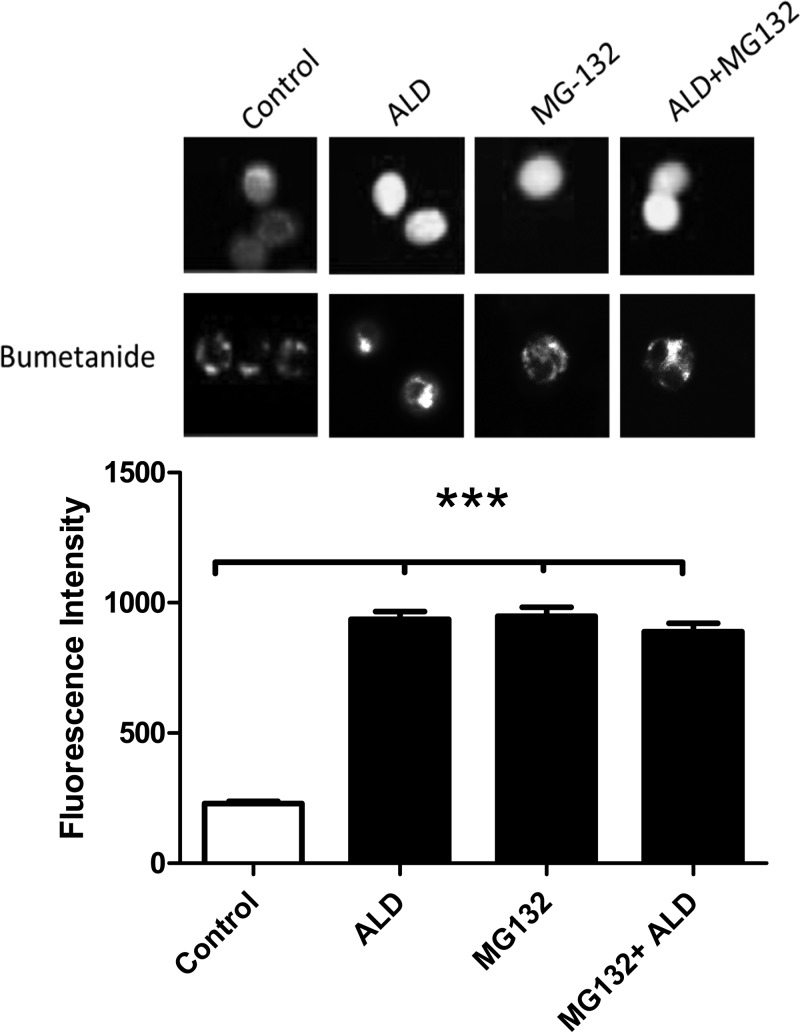
It is possible that increasing NKCC1 protein expression may not have a significant physiological functional role unless there is a parallel increase in potassium transport activity. We hypothesize that the overall activity levels of NKCC1 are associated with its expression levels in cells. To test this hypothesis, a more efficient and sensitive method, fluorescence Ion flux assays was adopted to measure NKCC1 transporter activity. The signal intensity was analyzed using TI+ as a surrogate of K+ and a TI+-sensitive fluorescent dye (FlixORTM) to visualize TI+ uptake through NKCC1 in single cells. The signal intensity corresponding to the relative K+ efflux was measured in a total of 24 cells. We found that when ALD was added to the culture medium, a marked increase in fluorescence was observed, reflecting upregulated TI+ uptake (Fig. 8). Adding MG132 and the combination of ALD with MG132 had identical enhancements similar to the ALD treatment alone (Fig. 8, 3rd and 4th bars), since the fluorescence intensities were identical among them.
Fig. 8.
Functional assays revealed that ALD increases the K+ efflux of NKCC1. The functionality of NKCC1 activity was analyzed in individual cells using bumetanide, an NKCC1 antagonist, combined with thallium (TI+) uptake, a physiological measure of NKCC1 activity. Cells were loaded with thallium-sensitive dye FluxOR. Fluorescence photos (fluorescence excitation and emission: 488/525 nm) were taken 90 s after addition of 2.8 mM TISO4 to the media. Top row: photomicrographs represent the fluorescence signal average after application of bumetanide, from left to right: 1) control: no bumetanide; 2) ALD: aldosterone alone, no bumetanide; 3) MG132: the proteasome inhibitor alone (no bumetanide); and 4) MG132 in combination with ALD (no bumetanide). Bottom row: photomicrographs represent the fluorescence signal average after application of bumetanide, from left to right: 1) control: with bumetanide; 2) ALD: aldosterone alone, with bumetanide; 3) MG132 alone (with bumetanide); and 4) MG132 combination with ALD (with bumetanide). Bar graph (bottom) represents the bumetanide-sensitive component of the TI+ uptake, as measured with densitometry, in 24 labeled cells for each condition. The intensity reported on the ordinate in the bar graph is normalized by subtracting the bumetanide fluorescence levels (bottom row) from the nonbumetanide conditions (top row). ***P < 0.001.
DISCUSSION
NKCC1 proteins play important roles in key physiological systems, including neural, cardiac, renal, and sensory systems. Correction of mis-regulation of the expression and functionality of NKCC1 that accompanies disease states and aging would have biotherapeutic and pharmaceutical implications. The present study demonstrates for the first time that this naturally occurring steroid hormone can precisely and sensitively regulate NKCC1 protein expression and accompanying functionality.
Additionally, the present investigation revealed that ALD exerts its regulatory effects on NKCC1 protein expression via mineralocorticoid receptors without altering mRNA levels.
Prevailing evidence suggests that the mechanisms underlying rapid ALD effects, such as actions observed in the present study, can involve activation of protein kinases and secondary messenger signaling cascades and also modulation of the transcriptional action of ALD through mineralocorticoid receptors (24). Additionally, rapid nongenomic effects of ALD have been recognized for some time, but whether or not mineralocorticoid receptors are involved remains controversial (14, 32, 55). For example, spironolactone can have nongenomic actions in cases of diabetic retinopathy. Also, in the RCCD2 rat cell line, early increases in transepithelial sodium transport elicited by ALD are not associated with transcriptional events but operate through the PKCα signaling pathway. This is accompanied by serine and threonine phosphorylation of the endogenous mineralocorticoid receptors. Interestingly, activation of this PKCα signaling cascade appears as a key event in the development of the later genomic response; blockade of this initial pathway prevents the late response to ALD (18). Additionally, nongenomic effects of ALD can be inhibited by specific mineralocorticoid receptor antagonists, such as eplerenone and water soluble RU28318 (31, 35). Grossmann and colleagues (22, 36) proposed three possible ALD signaling pathways: genomic (mineralocorticoid receptor dependent), nongenomic (mineralocorticoid receptor dependent), and nongenomic (mineralocorticoid receptor independent). Our study suggests that the NKCC1 protein induction resides on the rapid nongenomic, mineralocorticoid receptor-dependent ALD pathway, since the time to start the enhancement of NKCC1 protein expression in response to ALD treatments is relatively short, is blocked by eplerenone, and shows no changes in mRNA levels.
Previous studies implicate posttranslational phosphorylation associated with NKCC1 expression changes (1, 9, 23, 25, 29, 44, 50). In contrast, the present investigation is the first report that ALD exerts its effects on NKCC1 expression via prevention of posttranslational ubiquitination, e.g., reduces proteasome-dependent degradation of the NKCC1 protein.
Further, we explored the physiological action of NKCC1 in ALD induction. Stimulation of NKCC1 increases Na+, K+, and Cl− fluxes, as previously noted (16, 17, 23, 28, 49, 54). Ion flux assays represent functional assays that measure efflux of ions through cotransporters such as NKCC1. Radioactive 86Rb flux assays have been used effectively to study activity of a number of K+ channels and cotransporters and was employed in the present investigation. The cotransporter unidirectional efflux of Rb+ (as a tracer for K+) is an accepted method to measure cotransporter activity, despite it being bidirectional. Also, the net flux under physiological conditions is inward because of the inward gradients resulting from the equilibrium potentials for both Na+ and Cl− (28). Recently, a fluorescent assay for the measurement of thallium ions through potassium channels was used to measure NKCC1 activity (30). We utilized this methodology following the protocol of the Delpire group (21), since they and other groups have shown similar results between 86Rb and FluxOR. This method uses TI+ as a surrogate of K+, and a TI+-sensitive fluorescent dye (FlixORTM) to visualize TI+ uptake through NKCC1 in single cells (27, 38, 59). We found that addition of ALD to the media resulted in a marked increase in fluorescence of NKCC1 with both the 86Rb and Tl+ methodologies. MG132 and the combination ALD and MG132 treatments have identical enhancements to ALD treatment alone. Combining the data from the experiments with ALD and MG132 effects on NKCC1 protein expression and the 86Rb and TI+ uptake fluorescence studies, suggested that increasing NKCC1 protein expression involves increasing its functional activity levels.
Summary and conclusions.
The present investigation demonstrated that NKCC1 protein expression can be sensitively regulated by application of the naturally occurring hormone ALD. Further experiments suggested that this expression regulation occurs via mineralocorticoid receptors and takes place utilizing mechanisms involving protein stabilization, i.e., reduction of NKCC1 ubiquitination. Since mis-regulation of Na+ and K+, and/or declines in NKCC1 proteins are involved in many disease states and in aging, being able to precisely control NKCC1 expression levels and functionality has noteworthy biotherapeutic implications for improved clinical practice and drug development.
GRANTS
This work was supported by National Institute on Aging Grant P01-AG-009524 and National Institute on Deafness and Other Communication Disorders Grant R01-DC-004295.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.D., R.D.F., X.Z., Y.S., B.S., and J.P.W. conception and design of research; B.D., X.Z., and Y.S. performed experiments; B.D., R.D.F., X.Z., and J.P.W. analyzed data; B.D., R.D.F., X.Z., Y.S., B.S., and J.P.W. interpreted results of experiments; B.D. prepared figures; B.D., R.D.F., and J.P.W. drafted manuscript; B.D., R.D.F., X.Z., Y.S., B.S., and J.P.W. edited and revised manuscript; B.D., R.D.F., X.Z., Y.S., B.S., and J.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Margaret Harvey for technical assistance and Shannon Salvog for assistance in manuscript preparation. Special thanks to Dr. Syed Jalal Khundmiri at University of Louisville, who generously conducted the 86Rb experiments reported here.
REFERENCES
- 1.Akar F, Skinner E, Klein JD, Jena M, Paul RJ, O′Neill WC. Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl−-cotransporter in rat aorta. Am J Physiol Cell Physiol 276: C1383–C1390, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, Ruffieux-Daidié D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O. Nedd4–2 modulates renal Na+-Cl− cotransporter via the aldosterone-SGK1-Nedd4–2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudouin-Legros M, Brouillard F, Cougnon M, Tondelier D, Leclerc T, Edelman A. Modulation of CFTR gene expression in HT-29 cells by extracellular hyperosmolarity. Am J Physiol Cell Physiol 278: C49–C56, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Baudouin-Legros M, Hinzpeter A, Jaulmes A, Brouillard F, Costes B, Fanen P, Edelman A. Cell-specific posttranscriptional regulation of CFTR gene expression via influence of MAPK cascades on 3′-UTR part of transcripts. Am J Physiol Cell Physiol 289: C1240–C1250, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR–a perspective. J Mol Endocrinol 34: 597–601, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cohen E, Ophir I, Shaul YB. Induced differentiation in HT29, a human colon adenocarcinoma cell line. J Cell Sci 112: 2657–2566, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Cox SW, Ebersole LE, Carpenter GH, Proctor GB. Effects of autonomic agonists and immunomodulatory cytokines on polymeric immunoglobulin receptor expression by cultured rat and human salivary and colonic cell lines. Arch Oral Biol 52: 411–416, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol Sci 15: 309–312, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl− cotransport in primary afferent neurons. J Physiol 588: 3365–3373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl cotransporter. Nat Genet 22: 192–195, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Diaz RC, Vazquez AE, Dou H, Wei D, Cardell EL, Lingrel J, Shull GE, Doyle KJ, Yamoah EN. Conservation of hearing by simultaneous mutation of Na, K-ATPase and NKCC1. J Assoc Res Otolaryngol 8: 422–434, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 278: 27347–27353, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med 11: 1205–1213, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Eisen C, Meyer C, Christ M, Theisen K, Wehling M. Novel membrane receptors for aldosterone in human lymphocytes: a 50 kDa protein on SDS-PAGE. Cell Mol Biol 40: 351–358, 1994 [PubMed] [Google Scholar]
- 15.Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol Gastrointest Liver Physiol 278: G718–G724, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Flatman PW. Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta 1566: 140–151, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Forbush B. Regulatory activation is accompanied by movement in the C terminus of the Na-K-Cl cotransporter (NKCC1). J Biol Chem 287: 2210–2220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension 46: 1227–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gagnon KB, Delpire E. Molecular determinants of hyperosmotically activated NKCC1-mediated K+/K+ exchange. J Physiol 588: 3385–3396, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garg P, Martin CF, Elms SC, Gordon FJ, Wall SM, Garland CJ, Sutliff RL, O'Neill WC. Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol 292: H2100–H2105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng Y, Hoke A, Delpire E. The Ste20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem 284: 14020–14028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossmann C, Benesic A, Krug AW, Freudinger R, Mildenberger S, Gassner B, Gekle M. Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol Endocrinol 19: 1697–1710, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Grossmann C, Gekle M. New aspects of rapid aldosterone signaling. Mol Cell Endocrinol 308: 53–62, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Ramirez A, Waddell DS, Li Z, Liu X, Wang XF. Axin and GSK3-b control Smad3 protein stability and modulate TGF- β signaling. Genes Dev 22: 106–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas M, Forbush IIIB. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflügers Arch 447: 580–593, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hille B. Potassium channels in myelinated nerve: selective permeability to small cations. J Gen Physiol 61: 669–686, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang G, Cobbs S, Klein J, O'Neill CW. Aldosterone regulates the Na-K-2Cl cotransporter in vascular smooth muscle. Hypertension 41: 1131–1135, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kenneth BG, Delpire E. Molecular determinants of hyperosmotically activated NKCC1-mediated K+/K+ exchange. J Physiol 588: 3385–3396, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SM, Eisner C, Faulhaber-Walter R, Mizel D, Wall SM, Briggs JP, Schnermann J. Salt sensitivity of blood pressure in NKCC1-deficient mice. Am J Physiol Renal Physiol 295: F1230–F1238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Moëllic C, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004 [PubMed] [Google Scholar]
- 32.Marver D. Influence of adrenalectomy and steroid replacement on heart citrate synthase levels. Am J Physiol Endocrinol Metab 246: E452–E457, 1984 [DOI] [PubMed] [Google Scholar]
- 33.Mejia-Gervacio S, Murry K, Lledo PM. NKCC1 controls GABAergic signaling and neuroblast migration in the postnatal forebrain. Neural Dev 6: 4, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol 283: H1846–H1855, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Michea L, Delpiano AM, Hitschfeld C, Lobos L, Lavandero S, Marusic ET. Eplerenone blocks nongenomic effects of aldosterone on the Na+/H+ exchanger, intracellular Ca2+ levels, and vasoconstriction in mesenteric resistance vessels. Endocrinology 146: 973–980, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Mihallidou AS. Nongenomic actions of aldosterone: physiological or pathophysiological role? Steroids 71: 277–280, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Montrose-Rafizadeh C, Kole J, Bartkowski LM, Lee LH, Blackmon DL, Behnken SE, Gearhart JD, Cohn JA, Montrose MH. Gene targeting of a CFTR allele in HT29 human epithelial cells. J Cell Physiol 170: C299–C308, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. Novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol 73: 1213–1224, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Pedersen SF, O'Donnell ME, Anderson SE, Cala PM. Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl− cotransport in the heart, brain and blood. Am J Physiol Regul Integr Comp Physiol 291: R1–R25, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Phakdeekitcharoen B, Kittikanokrat W, Kijkunasathian C, Chatsudthipong V. Aldosterone increases Na+-K+-ATPase activity in skeletal muscle of patients with Conn's syndrome. Clin Endocrinol (Oxf) 74: 152–159, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Pieraut S, Laurent-Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, Mersel M, Delpire E, Valmier J, Scamps F. NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J Neurosci 27: 6751–6759, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salt AN, Thalmann R. New concepts regarding the volume flow of endolymph and perilymph. Adv Otorhinolaryngol 37: 11–17, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Schmiedt RA. The physiology of cochlear presbycusis. In: The Aging Auditory System: Perceptual Characterization and Neural Bases of Presbycusis, edited by Gordon-Salant S, Frisina RD, Popper A, Fay RR. New York: Springer-Verlag, chapt 2, 2010, p. 9–38 [Google Scholar]
- 47.Schulte BA, Schmiedt RA. Lateral wall Na,K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res 61: 35–46, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Schmiedt RA, Lang H, Okamura HO, Schulte BA. Effects of furosemide applied chronically to the round window. J Neurosci 22: 9643–9650, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sid B, Miranda L, Vertommen D, Viollet B, Rider MH. Stimulation of human and mouse erythrocyte Na(+)-K(+)-2Cl(-) cotransport by osmotic shrinkage does not involve AMP-activated protein kinase, but is associated with STE20/SPS1-related proline/alanine-rich kinase activation. J Physiol 588: 2315–28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simard CF, Daigle ND, Bergeron MJ, Brunet GM, Caron L, Noël M, Montminy V, Isenring P. Characterization of a novel interaction between the secretory Na+-K+-2Cl− cotransporter and the chaperone hsp90. J Biol Chem 279: 48449–48456, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Simard CF, Bergeron MJ, Frenette-Cotton R, Carpentier GA, Pelchat ME, Caron L, Isenring P. Homooligomeric and heterooligomeric associations between K+-Cl− cotransporter isoforms and between K+-Cl− and Na+-K+-2Cl− cotransporters. J Biol Chem 282: 18083–18093, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Smith L, Litman P, Liedtke CM. COMMD1 interacts with the COOH terminus of NKCC1 in Calu-3 airway epithelial cells to modulate NKCC1 ubiquitination. Am J Physiol Cell Physiol 305: C133–C146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith LP, Smallwood N, Altman A, Liedtke PKCdelta acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem 283: 22147–22156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J 441: 325–337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas W, Harvey BJ. Mechanisms underlying rapid aldosterone effects in the kidney. Annu Rev Physiol 73: 335–357, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Tsuchiya K, Giebisch G, Welling PA. Aldosterone-dependent regulation of Na-K-ATPase subunit mRNA in the rat CCD: competitive PCR analysis. Am J Physiol Renal Fluid Electrolyte Physiol 271: F7–F15, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Wangemann P. K+ cycling and the endocochlear potential. Hear Res 165: 1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Weaver CD, Harden D, Dworetzky SI, Robertson B, Knox RJ. A thallium-sensitive, fluorescence-based assay for detecting and characterizing potassium channel modulators in mammalian cells. J Biomol Screen 9: 671–677, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Weber PC, Cunningham IIICD, Schulte BA. Potassium recycling pathways in the human cochlea. Laryngoscope 111: 1156–1165, 2001 [DOI] [PubMed] [Google Scholar]